Mimicry is the adaptive resemblance in signal between several species in a locality. The most spectacular and intriguing cases are those of accurate resemblance between distantly related animals, such as spiders mimicking ants. Closely related species can also benefit from mutual resemblance, in which case mimicry results from selection against signal divergence.
The vast majority of the hundreds of thousands of insect species are described and identifiable on the basis of morphological characters. This bewildering diversity is, however, ordered because species share characters with their relatives—and one of the taxono-mist’s tasks is indeed to recognize, among the shared and divergent characters, a sign of the relatedness of the taxa. Nevertheless, some distantly related species may share a common morphology. Such resemblance may be the result of evolutionary convergence, that is, parallel lifestyles leading to the selection of similar morphological structures; in this case, resemblance per se is not under selection. On the contrary, when a character is taken as a signal between individuals, one species may benefit from bearing the same signal as the one already used by another species; then selection acts directly to favor increased resemblance.
AN INTERACTION BETWEEN THREE PROTAGONISTS
The Discovery of Mimicry and the Development of Evolutionary Hypotheses
Mimicry in insects has been a puzzle for entomologists long before the Darwinian concept of natural selection, but the explanations for mimicry are tightly linked to the development of evolutionary thinking. While he was traveling in the Amazon with Alfred
Russel Wallace in 1842, British entomologist Henry Walter Bates noted that distantly related butterfly species bore the same wing color pattern. Moreover, these communities of species changed their shared pattern in concert across localities. Among these species were the very abundant Ithomiinae (called Danaoid Heliconiidae then, now a subfamily in the Nymphalidae) and rarer Dismorphiinae (called Leptalidae then, now a subfamily in the Pieridae). Bates, as a pioneer evolutionist (but after Darwin published his On the Origin of Species), developed an adaptive explanation for the resemblance. Hypothesizing that ithomiines were inedible to most predators, he proposed that the edible pierids would benefit from being mistaken for their defended counterparts and would thus be selected to resemble them. Edward B. Poulton later named this kind of mimicry after him as Batesian mimicry, when an edible species mimics a distasteful one.
Bates also realized that some apparently inedible ithomiine species in the genus Napeogenes seemed to mimic other inedible Ithomiinae. He proposed that, in fact, rare species, whatever their palatability, should benefit from resembling defended common species. It was, however, more difficult to understand the resemblance of abundant and distasteful Melinaea, Mechanitis (Ithomiinae), Lycorea (Danainae), and some Heliconius (Heliconiinae) from Peru and Colombia, so he assumed the resemblance was the result of some inorganic or environmental factors. In 1879, German naturalist Fritz Muller was the first to develop a mathematical demonstration that two unpalatable prey could benefit from mutual resemblance. He understood that, if the community of predators had to kill a certain (fixed) number of prey to learn to avoid them, two indistinguishable distasteful species would together suffer this mortality and both reduce their death rate per unit time. Muller actually showed that this benefit was biased in favor of the rarer species, to a factor equal to the square of the ratio of the species’ abundance. Therefore, unequal population sizes translate into even more unequal, although still mutual, benefits: Mu-lerian mimicry, thus defined, could be beneficial for both species, and perhaps also for the predators, in contrast to parasitic Batesian mimicry.
Mimicry: An Interaction between Senders and Receivers
Mimicry typically involves at least three protagonists, two senders and one receiver, with the receiver judging the resemblance of the signals from the two senders (Fig. 1). Obviously, both the senders and the receiver should be found in the same locality for the mimicry to be possible, although time lags or geographic separation between senders may be plausible if receivers have a long-term associative memory and/or migrate. In a habitat, many senders will converge on the same signal, thereby forming what is called a mimicry complex, or mimicry ring. Signals may involve different sensory modalities, depending on the receiver’s sensory ecology: static visual signals (e.g., warning color patterns in butterflies, recognizable body shapes in ants), motion (flight behaviors), acoustic signals (clicking in many arctiid moths), olfactory/chemical signals (pherom-ones or the so-called cuticular hydrocarbon profiles by which social Hymenoptera recognize one another), or tactile signals (used by brood parasites of ants to be allowed to enter their nests). Signaling is indeed often multimodal.
Apparent complications may arise when, for example, one of the senders is also the receiver. For example, a predator may mimic the appearance of its prey when approaching it (aggressive mimicry in some spiders or chemical/tactile mimicry for brood parasites); the
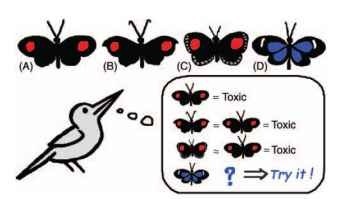
FIGURE 1 Conditioned predators and signaling prey. Predators are known to generalize their knowledge of distasteful prey to other resembling prey. Therefore, once predators recognize one prey as distasteful (prey A), other prey may gain from mimicry, whatever be their palatability (prey B and C). If the prey is palatable (prey C), its mimetic gain becomes limited by its abundance in the locality. Finally, a conspicuous prey with a (nonmimetic) pattern new to the predator should suffer higher mortality, making the evolution of diversity in warning color and mimicry a puzzle.
prey is thus fooled by the predator via its own conspecific signal. The two senders can also be the same species. This is sometimes the case in chemical-sequestering phytophagous insects when unpalat-ability varies among individuals in the same population (e.g., Danaus gilippus in Florida), leading to so-called automimicry of palatable toward unpalatable individuals in the same species. Similarly, male Hymenoptera do not have the defenses that females have.
However, the present article is not organized around these clas-sificatory distinctions, which are based on subtle differences in the identities of senders and receivers or ecological situations. Instead, it highlights the important evolutionary dynamics that arise from whether receivers are expected to try to discriminate or generalize on the senders’ signals or, in other words, from senders sending honest compared to dishonest signals. This should bring into perspective some of the main and still unresolved puzzles in mimicry theory, such as the rise and maintenance of diversity in mimicry signals. Most examples are chosen from the butterfly genera that represent today’s best known mimetic organisms, such as Papilio and Heliconius; indeed, our knowledge of the ecology and genetics of mimicry in these genera is unequaled by any other group of insects.
FREQUENCY-DEPENDENT POPULATION PROCESSES
Batesian Mimicry and Negative Frequency Dependence
THEORY AND CONSEQUENCES
In Batesian mimicry, one of the sender species, the mimic, sends a dishonest signal to deceive the receiver—for example, a predator. It is thought that deception is possible only if the receiver has previously inherited or acquired knowledge about this signal. There is ample evidence that (1) vertebrate predators (birds, lizards) can learn to recognize distasteful prey, (2) they can be deceived by mimicry, and (3) mimics gain from the resemblance. The most famous Batesian mimic is probably the viceroy butterfly, Limenitis archippus . which mimics the monarch D. plexippus, although this relationship is now questioned (because viceroys can be unpalatable). Hoverflies (Diptera: Syrphidae), diurnal moths (Sesiidae, Sphingidae), striped beetles (Cerambycidae), or crane flies (Tipulidae) are well-known Batesian mimics of wasps and
bees (Fig. 2 ).

FIGURE 2 Batesian mimicry. The day-flying moth Synanthedon tipuliformis (Sesiidae) (top) is a Batesian mimic of stinging wasps in Europe. The resemblance is very accurate, and the moth is very rare compared to its wasp models, so that it is not often observed. Similarly, but in a totally different group, the beetle Clytus arietis (bottom) mimics wasps and is sometimes seen on blossoms. These two examples illustrate how the same general appearance can be achieved by morphological changes of totally different nature in different groups of insects.
Clearly, the efficiency of the deception is directly linked to the probability that predators have knowledge of the prey. It thus depends on the ratio of models and mimics in the population of prey (Fig. 1). As in host-parasite systems, the fitness of Batesian (“parasitic”) mimics therefore depends negatively on their proportion in the prey community. Negative frequency dependence, the selective advantage to rare forms, is thought to be a strong force favoring and maintaining diversity in many ecological situations in nature. In Batesian mimics, any new (or rare) mutant resembling another protected model will be favored, leading to a balanced polymorphism between the two mimetic forms. Negative frequency dependence also predicts that the local number of Batesian species should be dependent on the abundance of the model(s).
Many, but by no means all, Batesian mimics are indeed polymorphic. Among the most famous is the African swallowtail P. dardanus, which may have three co-occurring forms that mimic different species of the Danainae genus Amauris. Hypolimnas mis-ipppus (Nymphalinae) is another African butterfly that has four forms mimetic of D. chrysippus. In South America, the swallowtail Eurytides lisithous has up to three forms that mimic the co-occurring Parides species (Papilionidae), whereas in Southeast Asia the famous Papilio memnon also mimics three or more different papilionid models. In the Diptera, the Old World hoverflies Volucella bombylans and Merodon equestris are examples of polymorphic species mimicking bumble bees.
EVIDENCE FOR NEGATIVE
FREQUENCY DEPENDENCE
Although experimental demonstration that Batesian polymorphisms stem from negative frequency dependence is still lacking, there is a lot of evidence for negative frequency dependence itself. A first line of evidence comes from the observation of patterns of abundance of models and mimics in nature. For example, the North American butterfly Battus philenor is known to be unpalatable to most birds and is believed to act as model for a number of edible mimics in the ” black” mimicry ring. In one of them, P. glaucus, females are found as a mimetic and a nonmimetic (male-like) form, and the proportion of the mimetic form tends to be higher where its model B. philenor is more abundant. Similarly, the resemblance of the mimic P. troilus to B. philenor is higher where the latter is abundant. These give an overall pattern of mimics’ occurrence consistent with negative frequency dependence. Moreover, field experiments directly showed a strong selective advantage to mimetic vs. nonmi-metic Callosamia promethea day-flying moths, another Batesian mimic of B. philenor.
Experimental approaches give more insight into the mechanisms involved in frequency dependence. In experiments, captive or wild predators can be tested with a variety of artificial or real prey, and the mimic/model proportions can be experimentally changed to explore how it affects the preys’ survival. Traditional experiments were carried out in the 1970s with mealworms or pastry baits colored with food dyes, and/or dipped in quinine to make them distasteful, and exposed to garden birds in suburban Britain. Such experiments do suggest that a rare mimic has an advantage over a common one if the “model” is slightly distasteful, which demonstrates frequency-dependent selection. However, if the “models” were made very distasteful, the advantage of being rare decreased and eventually vanished. Laboratory experiments can also be used to search for evidence of frequency dependence, while avoiding potential confounding effects of field experiments. Experiments with captive great tits as predators showed that the mortality of both mimics and models depended on the frequency of the model and that both models and mimics survived better when mimics were fewer.
These experiments tell us that the intensity of frequency-dependent selection in mimics is highly dependent on the palatability of the models. To see its selective advantage decrease, the palatable mimic must become very common, or the model must be not very distasteful. This suggests there is some kind of effective “equivalence” between relative numbers of prey encountered and their relative levels of toxicity.
Positive Frequency Dependence in Mullerian Mimicry
THEORY: THE DISADVANTAGE OF RARE FORMS
Warning signals, or aposematism, evolve because prey bearing signals that predators associate better with unprofitability (e.g., harmful prey) survive better. The evolution of warning signals brings some apparent paradoxes that are not treated in that entry. However, there is plenty of evidence that aposematic prey are easily learned and subsequently avoided by vertebrate predators. Both the warning prey and the learning predator benefit from a correct interpretation of the signal. Under such an “honest signaling” framework, rare or new variants within a prey population should not be recognized as distasteful and should suffer higher predation (Fig. 1). This selection against rare forms translates into positive frequency-dependent selection: rare mutants are removed, leading to monomorphism in all populations.
Because predators select only on prey appearance, the selective pressure does not stop at the species boundary: several protected prey species may be selected to use the same warning signal, that is, become Mullerian mimics. Although the phenomenon is not necessarily symmetrical, two or several defended species should all benefit
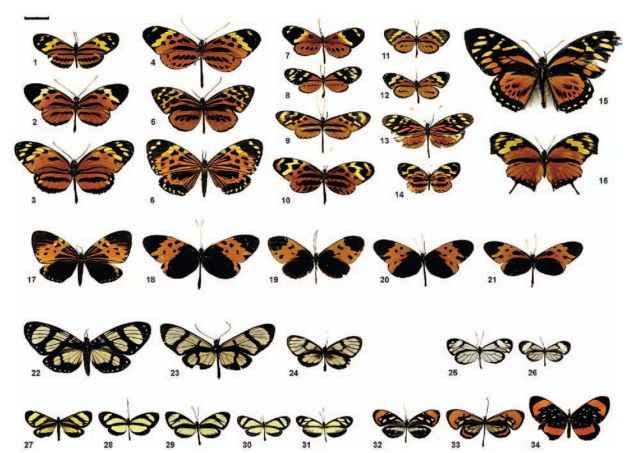
FIGURE 3 Six butterfly mimicry rings from eastern Peru. The mimicry rings (groups of mimetic species) presented here are dominated by butterflies in the Ithomiinae and occur in the forests around the city of Tarapoto. Following G. W. Beccaloni’s nomenclature, these mimicry rings are Tiger (1-16), Melanic tiger (17-21), Large transparent (22-24), Small transparent (25 and 26), Small yellow (27-31), and Orange-tip (32-34) mimicry rings. At least 5 other mimicry rings can be recognized involving Heliconiinae and/or Ithomiinae in this area, which brings the total to at least 11 mimicry rings for these two butterfly subfamilies. Many more species, not featured here, belong to these mimicry rings, particularly Ithomiines and especially in the Small transparent group. The Tiger mimicry ring involves a lot of species and the size distribution is almost continuous from small to very big. This may be because as more and more Mu-lerian mimics join the mimicry ring, predators might generalize more, and the selection for close resemblance could be somewhat relaxed. Note that some day-flying moths (6, 17, 22, 27) participate in these mimicry rings, probably as Mullerian mimics (they reflex-bleed bitter hemolymph when handled). Butterflies 13-16 and 31 are supposed to be Batesian mimics as they belong to palatable groups within their families. See more species belonging to these mimicry rings in Figs. 4 and 5. All butterflies are Nymphalidae: Ithomiinae, except 1-3 (Nymphalidae: Heliconiinae), 14 (Nymphalidae: Melitaeinae), 16 (Nymphalidae: Charaxinae), 15 (Papilionidae), 13 and 31 (Pieridae: Dismorphiinae), 34 (Riodinidae), and 6, 17, and 22 (Arctiidae: Pericopinae). Scientific names: 1, Eueides Isabella; 2, Heliconius pardalinus; 3, H. hecale; 4, Melinaea menophilus; 5, Tithorea harmonia; 6, Chetone histriona; 7, Napeogenes larina; 8, Mechanitis lysimnia; 9, Mec. polymnia; 10, Mec. mazaeus plagifera ssp.; 11, Ceratinia tutia; 12, Hypothyris cantobrica; 13, Dismorphia amphiona; 14, Eresia sp.; 15, Pterourus zagreus; 16, Consul fabius; 17, Chetone histriona; 18, Mel. marsaeus; 19, Hyposcada anchiala; 20, Hypot. mansuetus; 21, Mec. mazaeus deceptus; 22, Notophyson helico-nides; 23, Methona confusa; 24, Godyris zavaleta; 25, Greta andromica; 26, Pseudoscada florula; 27, Notodontid moth; 28, Aeria eurimedia; 29, Ithomia salapia; 30, Scada sp.; 31, Moschoneura sp.; 32, Hypos. illinissa; 33, Hypoleria sarepta; 34, Stalachtis euterpe. Scale bar, 2 cm.
from sharing a warning signal, which reduces their per capita pre-dation rate. As more and more individuals join in the mimicry ring, the protection given by the signal becomes stronger. Therefore, the direct, and naive, prediction is that all unpalatable prey of a similar size in a habitat should converge into a mimicry ring.
EVIDENCE FOR THE FREQUENCY-DEPENDENT BENEFITS OF MULLERIAN MIMICRY
Although comparative and/or biogeographical studies give strong support to the theory, the first convincing experimental evidence came from pastry-bait experiments with garden birds that tend to attack rare distasteful baits more often than common ones. Recently, laboratory experiments also showed strong selection against new rare warningly colored prey items. However, field evidence with free-living prey is crucial for a validation of these results. In one experiment, J. Mallet reciprocally transplanted Heliconius erato individuals between populations in which H. erato have different wing patterns, thus effectively releasing rare “mutant” and “control” butterflies into the host populations. A strong selective advantage of about 50% was calculated for the commoner form. More recently, to avoid the potential pathology of color patterns being adaptations to local habitat conditions in addition to mimicry, D. D. Kapan used a similar reciprocal release-recapture technique but used polymorphic populations of the butterfly H. cydno. In this species, two morphs coexist but participate in two different mimicry rings that differ in relative abundance in different locations in Ecuador. Life expectancy was 12 days for the locally common forms and only 2 days for the locally uncommon forms. These field data give unequivocal evidence for strong selection against rare forms in these Mullerian species.
CONSEQUENCES AND CHALLENGES
Strong purifying selection now seems well supported by theoretical, comparative, and experimental evidence. To evolve a new pattern, a toxic prey would have to pass an apparently impassable initial disadvantage, survive a transient polymorphism, and win the aposematic competition with alternative warning signals. It is therefore no surprise that most distasteful Mullerian mimics are indeed monomorphic in local populations (Fig. 3) and that polymorphisms are usually restricted to narrow hybrid zones between color-pattern races. In H. erato, in which two color races abut, frequency-dependent selection maintains a sharp boundary, alternative forms being positively reinforced on either side of a steep cline. Many species join Mu-lerian mimicry rings, which itself represents interspecific evidence for strong frequency-dependent selection.
However, in contrast with such extremely conservative forces, diversity is present at all levels in mimicry (Fig. 3). At a macroevolu-tionary level, aposematic and mimetic groups typically undergo rapid mimetic radiations into numerous species and races differing in color pattern, like heliconiine butterflies or pyrrhocorid red bugs. At the community level, many radically different mimicry rings coexist in the same habitat (e.g., five or six coexisting rings just within the Heliconius of Costa Rica, at least seven or eight rings just within the Ithomiinae of the Peruvian Amazon—Fig. 3). At the biogeographical level, many aposematic species show a bewildering diversification in more or less sharply defined mimetic races. Finally, at the population level, several chemically defended species show mimetic polymorphism. For instance, the bumble bee Bombus rufocintus has two mimetic forms in North America, the burnet moth Zygaena ephialtes has two sym-patric forms in Italy, the African monarch D. chrysippus has four main color forms coexisting in large areas in East Africa, and the Amazonian H. numata shows the most astounding polymorphic mimicry with up to 7-10 forms in the Andean foothills. In each of these cases, the different forms closely match the different local mimicry rings (Fig. 4).

FIGURE 4 Polymorphic Mullerian mimicry. The Amazonian butterfly H. numata (Nymphalidae: Heliconiinae—right column) is a Mu-lerian mimic in a variety of tiger-pattern mimicry rings. Each population (here around the city of Tarapoto in Eastern Peru) is polymorphic and up to seven forms may coexist, each being an exceptionally accurate mimic of species in the genus Melinaea (Nymphalidae: Ithomiinae—left column). Spatial variation in selection pressure is probably what maintains the polymorphism, by a balance between local selection for mimicry of the commonest Melinaea species and movement of individuals (gene flow) between neighboring localities selected for different wing patterns. From top to bottom (left column): Melinaea ludovica ludovica, Mel. sat-evis cydon, Mel. marsaeus mothone, Mel. marsaeus phasiana, Mel. menophilus ssp. nov., Mel. menophilus hicetas. and Mel. marsaeus mothone. (Right column) H. numata forms silvana, elegans, aurora, arcuella, tarapotensis, timaeus, and bicoloratus. Scale bar, 2cm.
This rampant diversity does not question the existence of frequency dependence itself, but the details of how purifying selection may or may not prevent the evolution of diversity. It may also question the validity of the two classical categories of protective mimicry (Batesian and Mullerian) and the existence of a sharp divide between them along the spectrum of prey palatability. Explaining these unexpected cases is therefore central to our understanding of signal evolution in distasteful insects.
The Palatability Spectrum and Predator Psychology
MODELS OF MIMICRY EVOLUTION
Case studies and experiments on mimicry are practically difficult, time consuming, and inform us only on potential processes in particular cases. They are thus not always very informative as to which processes are generally important in the evolution of mimetic diversity. For these reasons, mathematical models simulating mimicry evolution have been widely used. Models of mimicry evolution have been traditionally of two different types: “evolutionary dynamics” models have concentrated on trait evolution in the prey populations, underestimating the effects of the details of predator behavior; ” receiver psychology” models have concentrated on the effect of predator cognitive abilities in driving the costs and benefits to mimetic prey, but largely ignored evolutionary processes in the prey populations, particularly frequency or density dependence. The second category of models are those that apparently pose a threat to the validity of the Batesian/ Mullerian distinction, and M. P. Speed even coined the new term ” quasi-Batesian mimicry” for the strange, though possibly common, intermediate dynamics that his model highlighted.
The main discrepancies lie in the way predators are thought to respond to prey palatability and density. Speed’s models assumed that predators attack a fixed fraction of a prey in a population, irrespective of their total number (linear frequency dependence), and that this fraction depends on the palatability of the species. In a mixture of prey of differing palatability, the resulting fraction killed would be intermediate between the fractions lost in each prey in the absence of mimicry, leading to one prey species benefiting and the other suffering from mimicry. This view, however, leads to the strange prediction that as more mildly unpalatable prey are present, the attacked fraction (per unit time) can increase. In contrast, J. Mallet and M. Joron argued that predators are unlikely to be sensitive to frequency per se and should instead need only to attack a fixed number of prey before learning, making the “attacked fraction” a decreasing function of the total number of prey bearing the pattern. This should lead to a strongly nonlinear, effectively hyperbolic frequency dependence. The attacked fraction (per unit time) should always decrease when the total number (and therefore the density) of unpalatable prey increases, whatever be their relative unpalatability.
The debate is still very much active, and decisive data are surprisingly scarce. In an experiment with pastry baits and wild passerines, Speed showed that the attack fraction of a mimetic pair was indeed intermediate between that of either ” species ” alone. Furthermore, birds seemed to learn only to a certain extent; that is, they never completely stopped attacking the unpalatable items. Despite some potential problems in the experimental design (especially, the artificially high prey density in a time of scarce prey density), these data remain a puzzle and may hint at more complex learning processes than a pure number-dependent dose response. More decisive evidence came from L. Lindstrom’s study, in which novel toxic prey were introduced into a great tit’s foraging arena at varying frequencies (= densities in this setting). Although the total number of attacked toxic prey increased with their initial frequency, the attack fraction decreased. Her data support the validity of nonlinear frequency dependence, although the idea of a strictly fixed number of prey killed could be an oversimplification. Absolute numbers of prey attacked may increase with warning signal density, but proportion will inevitably decrease, which should lead to a traditional Batesian-Mullerian distinction. More recently, in an experiment where the total number of signaling prey individuals was allowed to increase with the addition of a mimetic species, thereby approaching more natural conditions for the evolution of mimicry, Speed’s group provided long-awaited evidence that mimicry unpalatable prey of varying strength benefited mutually from the mimicry due to an increased density of the signal in the prey population. The mutual benefits may, however, be modulated by the availability of alternative prey to predators in the habitat, as this and theoretical models have suggested. It appears optimal foraging, in which predators tune their discrimination abilities to the availability of prey and their pal-atability distribution, should be taken into account to understand the interplay between density-dependence and the unpalatability spectrum in the evolution of mimicry.
More intriguing prospects have come from the recent discovery by J. Skelhorn that the benefits of mimicry tend to be enhanced when mimics use different types of chemical defenses for protection. Although the mechanisms underlying this are unknown, the potential consequences for speciation and the community ecology of insect species assemblages may be large if selection facilitates the recruitment of distantly related mimics more easily than close relatives.
THE STRENGTH OF THE SELECTION
Muller’s number-dependent model also leads to a prediction that has hitherto been largely overlooked. At low densities, selection should act strongly against any transient polymorphism, but at higher densities, selection quickly becomes weak at intermediate form frequencies. This leads to effective neutrality of polymorphism once it is established in abundant species. Kapan’s field experiments, in which H. cydno were released at varying density, showed precisely this trend. Polymorphism could therefore be nonadaptive but very weakly selected against by predators.
Numerical Mimicry and Density-Dependent Processes
The studies of J. Allen and his collaborators, and others, show that prey selection by predators can be frequency dependent in palatable, cryptic prey, that is, even in the absence of mimicry of unprofitable prey. This is probably caused, in part, from predators using search images when foraging. For instance, at low densities of a particular kind of (palatable) prey, predators usually prey on the more common form, which corresponds to their search image, imposing a negative frequency dependence. Cryptic prey may be globally numerous in a habitat, but because they are camouflaged, their apparent density to predators is bound to be low. This leads to the diversification of cryptic patterns, and perhaps the selection of plastic (partly environmentally induced) color-pattern genetic control, in prey. In contrast, at high density, predators usually prey on the odd phenotypes preferentially, even among perfectly palatable prey, effectively leading to a positive frequency-dependent selection on morphology.
Gregarious palatable prey that are at locally high density and that presumably rely on predator satiation to escape predation, might then be selected for mutual resemblance. Such a prey might be called warn-ingly colored, whereas the appearance itself is not protective. This idea led to the supposition that several prey species that co-occur at unusually high densities, like mud-puddling butterflies or schooling fishes, might evolve “numerical” or “arithmetic” mimicry by simple frequency-dependent predation unrelated to unprofitability. Prey traits like color, shape, and especially locomotor behavior are therefore thought to be under purifying selection in mixed-species aggregations. This attractive idea remains largely untested in insects, although R. B. Srygley proposed the pair of bright orange butterflies Dryas julia (Heliconiinae) and Marpesia petreus (Nymphalinae) as a potential candidate.
Female-Limited Mimicry
Some of the most spectacular and best studied cases of Batesian polymorphism are found in swallowtails, and in some species only the female is mimetic (see an example in Fig. 5). This peculiar tendency to sex-specific polymorphism seems to be restricted to butterflies (Papilionidae and Pieridae), and virtually no other case of sex-limited mimicry seems to be reported for other insects (except for male-limited mimicry in some moths). Female-limited mimicry was often viewed as a result of negative frequency dependence: if mimicry is restricted to one sex, the effective mimetic population size is only about half that of a nondimorphic species, reducing deleterious effects of parasitism onto the warning signal. But this group-selection argument cannot in itself explain why females tend to become mimetic more often than males and why mechanisms arise that restrict the mimicry to one sex. However, more proximal, individual-selection arguments are not lacking. First, mimicry may be more beneficial to one sex than to the other. For instance, female butterflies have a less agile flight because of egg load and a more “predictable” flight when searching oviposition sites, and they suffer higher rates of attacks by visual predators. Second, male wing patterns can

FIGURE 5 Female-limited mimicry in Perrhybris pyrrha (Pieridae), Eastern Peru. The female (top) is a Batesian mimic of the tiger-patterned Ithomiines and Helicomiines (see Fig. 3- , while the male (bottom) has retained a typical pierid white coloration. Scale bar, 2 cm.
be constrained by sexual selection, via either female choice or male-male interactions: males could not evolve Batesian mimicry without losing mating opportunities. In experiments with North American swallowtails (of which only females mimic B. philenor), male P. glaucus painted with the mimetic pattern had a lower mating success than normal yellow males; similarly, painted P. polyxenes males had a lower success in male-male fights and therefore held lower-quality territories around hilltops. In these insects, the wing coloration appears to bear signals directed either to conspecific males or to predators, which creates a potential conflict leading to sex-limited polymorphism. It is interesting to note that Papilio and Eurytides species that mimic Parides (Papilionidae) in South America do not exhibit female-limited mimicry; different modes of sexual selection (e.g., absence of territoriality) may operate in the forest understory habitat. In a different ecological setting, diurnal males of the North American silkmoth C. promethea are exposed to visual predators, and mimicry of B. philenor is limited to males; female Callosamia fly at night and benefit more by crypsis during the day.
MIMICRY AND THE EVOLUTION OF SIGNAL FORM
Resemblance and Homology
Mimicry can arise as soon as the signal is effectively copied, that is, as soon as superficial resemblance is attained. Therefore, mimics usually bear characters similar to those of their models, but these are often clearly nonhomologous in terms of genes and mechanisms of development. For instance, red spots near the base of the wing in P. memnon mimic the spots on the bodies of their models. The trans-lucency and iridescence of distasteful Ithomiinae clearwing butterflies is mimicked by white raylets in dioptine and pyralid day-flying moths and provide the same impression in motion. Similarly, the black-wing patterning of some flies seems to mimic the superposition of wings over the abdomen in their wasp models. Therefore, mimics from distant phylogenetic groups are certainly under very different functional and developmental constraints to create a mimetic impression. Selection will retain the first characters that suddenly increase overall similarity. The initial step made may therefore strongly influence the route selected to achieve mimicry.
The Genetics of Mimicry: Polymorphisms and Supergene Evolution
THE DEBATE
The genetical study of the evolution of mimicry was first dominated by a debate between gradualists (Fisher) and mutationists (Goldschmidt). Goldschmidt proposed that ” systemic” mutations could affect the whole wing pattern of butterflies in one step and that models and mimics, although not using the same genes, were using at least the same developmental pathways. Because this view could not account for the obvious nonhomologies, like those pointed out above, Fisher and others claimed that mimicry was achieved by slow microevolutionary steps and the gradual accumulation of resemblance alleles.
Decisive steps toward a resolution of the debate came principally from the study, by C. Clarke and P. Sheppard in the 1960s, of Batesian butterfly mimics in which color pattern is easy to define and analyze and gene effects are straightforward to identify. Polymorphic mimics, particularly Papilio species, of which different forms could be crossed by breeding experiments (including hand pairing), were particularly useful. It appeared that color pattern is mainly inherited at one or few major loci, affecting the whole pattern. From rare recombinants, it could be shown that these loci were in fact super-genes, that is, arrays of tightly linked small-effect genes. Several additional unlinked “modifier” loci were also shown to increase resemblance via interaction and epistasis with the supergene. Goldschmidt’s ideas seemed refuted.
However, although supergenes seem to be a necessary condition for the evolution of polymorphism (otherwise numerous nonmimetic, unfit recombinants would be produced), how they evolve is another issue. Theoretical models suggested that supergenes could not be achieved by simple gradual reduction in recombination. In the absence of spatial variation in selection pressures, tighter linkage cannot evolve by small steps via Fisherian gradual evolution, because good combinations of alleles are immediately broken up by recombination. Instead, gene clusters should preexist the evolution of polymorphism.
THE TWO-STEP HYPOTHESIS
These results led to a unifying, now widely accepted two-step mechanism of mimicry evolution: (1) mutations at genes of major effect first allow a phenotypic leap achieving an approximate resemblance to a particular model. (2) Once these mutations have increased in the population, resemblance can be enhanced through the gradual selection of epistatic modifiers. This two-step mechanism is supported by three lines of evidence. First, empirical evidence from butterflies suggests the existence of a small number of major-effect genes and numerous small-effect modifiers. In fact some of these genes of major effect could even include a series of regulatory upstream elements and transcription factors, now known to be involved in the development of butterfly color patterns. Pigment pathway genes and scale maturation regulators can also have very dramatic effects on the color patterns. Second, population genetics and dynamics models support the prediction that a major phenotypic jump is necessary to cross the deep fitness valleys in a rugged fitness landscape, after which gradual, Fisherian evolution may proceed to enhance resemblance. Finally, experiments show that birds associate cryptic patterns with edibility and generalize those in such a way that only profoundly deviant prey are treated as separate cases by the birds and memorized as warning patterns when appropriate. These experiments also indicate that increased resemblance is still significantly advantageous in imperfect mimics, supporting the second step of the two-step scenario.
LARGESSE OF THE GENOME
Another, but not exclusive,route to supergenes for mimicry is called largesse of the genome, put forward by J. R. G. Turner. Under this scenario, it is believed that the modification of a trait can be achieved by so many different genes that some of them will inevitably happen to be linked. Among the many possible combinations of loci, selection could simply sieve out the ones that involve linked genes. This hypothesis is particularly likely for loss-of-function phenotypes that can be achieved by mutating any step in the development, like the loss of tail in the African swallowtail P. dardanus. Similarly, that different mimetic species use nonhomolo-gous supergenes can be viewed as indirect evidence for the validity of the largesse of the genome hypothesis in the broad sense.
SUPERGENES IN MULLERIAN MIMICS: A PUZZLE
Mullerian mimics being usually monomorphic locally, supergenes are not expected to control wing patterns, and multilocus control was hypothesized to be the norm. This basic prediction has, however, constantly been challenged by Heliconius color-pattern genetics, which show that a limited set of genes of large effect and supergenes control most of the racial color-pattern variation. In the polymorphic H. numata, one single gene seems to control the entire wing pattern, with as many as seven alleles, each allele bringing resemblance to a specific mimetic pattern (Fig. 4). Tight gene clusters are also found, to a lesser extent, in polymorphic H. cydno, in H. melpomene, and in H. erato. The existence of these supergenes seems puzzling. It is possible that butterfly color patterns in general are under the control of relatively few conserved genes, at least in some lineages, such as developmental regulatory genes involved in eyespot formation.
In toxic prey, strong selection against any new form and the impossibility of gradual color-pattern changes have been theoretically and empirically demonstrated. It follows that, like Batesian mimics, Mullerian mimics seem to need an initial phenotypic leap, perhaps involving multimodal signal modifications, to jump either to an already protected pattern or away from predators’ generalization of cryptic prey. Therefore, it is perhaps no surprise that most exaggerated signal forms studied are under the control of relatively few genes, following the same two-step scenario as in Batesian mimicry. Moreover, switches from one mimetic pattern to another are likely selected only if the new mutant’s mimetic characters are not randomly recombined in its descendants. This imposes another constraint (or “sieve”) on the genetic architecture for new mimetic patterns to be selected out of a transiently polymorphic population. It is therefore remarkable to note that although Batesian and Mullerian evolutionary dynamics are radically different, and are even perhaps engaged in an evolutionary arms race, the evolution of their signals might require a similar (though nonhomologous) genetic predisposition.
Recent comparative mapping, by M. Joron and colleagues, of the genetic architectures in H. numata and H. melpomene has uncovered a remarkable level of positional genetic homology between those species for the major loci controlling mimicry, despite the fact that H. numata and H. melpomene cover very different mimicry patterns, and are involved in totally disjunct mimicry rings (see Fig. 4). This suggests that the few genes involved are remarkably flexible in the array of possible phenotypes and that selection for ecological adaptation, as opposed to genetic constraints, is the chief determinant of mimicry diversity.
Myrmecomorphy
Ants represent the most abundant group of organisms in most biota and have powerful multimodal defenses such as acid taste, aggressive biting, painful sting, and social defense. For these reasons, foraging ants are generally little subject to predation and act as ideal models in mimicry rings. Many insects and spiders indeed have an altered morphology and resemble ants, a phenomenon called myrmecomorphy. For instance, several salticid spider genera such as Myrmarachne or Synemosyna are bewilderingly good ant mimics. It is also common to spot ant-like mirid nymphs (Heteroptera) running among leafcutting Atta ants or Ecitomorpha staphilinid beetles among Eciton army ant columns. The adaptive significance of antlike morphology has been the subject of considerable debate. For instance, several ant-like spiders are believed to mimic ants as a trick to approach and prey on their ant models (“aggressive mimicry”); some ant-like bugs use the same trick to approach and prey on ant-tended aphids. However, most ant-like insects are phytophagous, do not prey on foraging ants, and usually mimic the locally abundant ant species. They are therefore good Batesian mimicry candidates. The interesting aspect of ant mimicry is that, although small birds, lizards, or amphibians may be important predators on ant-sized insects, there are grounds to think that arthropod predators with developed visual skills could be the prime receivers selecting for ant mimicry. For instance, wasps in the Pompilidae are known as important predators of jumping spiders, but ignore ants, thus potentially selecting for ant-like morphology and behavior. Jumping spiders themselves are visual predators hunting insects and also tend to avoid stinging ants as prey. Although the cognitive abilities of arthropods are not well researched, several studies using mantids, assassin bugs Sinea sp., or crab spiders show that they are capable of associative learning and discriminate against ant-like prey. Despite the difference in visual acuity and cognitive abilities between vertebrates and arthropods, it is interesting to note that arthropod predators are likely responsible for visual mimicry that is very accurate to our eyes.
The Importance of Behavior and Motion
Myrmecomorphy highlights a crucial aspect of mimicry: the importance of behavior. Predators integrate many aspects of prey appearance when making a decision of whether to attack, and behavior is an important part of multimodal signals. Ants are characterized by jerky (e.g., Pseudomyrmex spp.) or zigzag (e.g., Crematogaster spp.) movements that their mimics adopt. The rapid movement of antennae is a common feature of ant behavior which mimetic species copy by waving their front legs (e.g., ant-mimicking Salticidae spiders, and spider-wasp-mimicking leaf-footed bugs Coreidae). Because motion considerably enhances visibility, it is hardly surprising that details of the behavior make important identification cues for the predators. For instance, although slow flight in aposematic butterflies may save energy, slowness itself is certainly recognized as such by predators that can select on extremely minute details of flight unnoticeable to the human eye. R. B. Srygley’s work on locomotor mimicry has shown that the two butterflies H. erato and H. sapho differ in the asymmetry of the upward and downward wing strokes, which their respective (Mullerian) mimics H. melpomene and H. cydno copy accurately in Panama. Batesian mimics usually retain escape behaviors characteristic of their groups: the lazily flying Neotropical butterfly Consul fabius (Nymphalidae: Charaxinae) (see Fig. 3) can start rapid escape flight when detected; ant-mimicking salticid spiders are also usually reluctant to jump unless attacked.
The tendency for predators to generalize the characteristics of palatable prey, on which they actually feed, probably selects apose-matic signals away from these morphologies, and behavioral signals are no exception. Rapid jerky flight is usually characteristic of a tasty prey, a profit that predators have to weigh against the time and energy costs associated with catching the prey. Unconventional behaviors like the flight of Heliconius butterflies or the looping of honey bees make them highly noticeable to predators. This imposes an additional visibility cost on incipient mimetic prey; for the resemblance to be selected, such cost has to be offset by a significant reduction in predation. These considerations suggest that mimetic behavioral change probably evolves in much the same way as morphological characters do, that is, a two-step process.
Escape Mimicry
Unpalatability is not the only way to be unprofitable to predators. Fast, efficient escape is another way for preys to teach predators that pursuit is useless and will bring no reward: predators unable to consume the desired prey may associate this frustration with the prey appearance and reduce their attacks on this prey altogether. Even if the prey can be seized, predators probably trade off the energy spent and the (often low) nutritional reward. In several experiments birds were shown to be able to decrease their attack rates when the presented prey would quickly disappear (” escape ” ) during their attacks, and conspicuousness of the prey tended to enhance the response. Therefore, evasive prey could advertise their escaping abilities by color patterns, which other prey may mimic. At least three kinds of characters may enhance the difficulty of catching an evasive prey: erratic flight (like that of pierids), fast and maneuver-able flight (like that of charaxine butterflies), or high reactivity (like that of syrphid hoverflies). Typically, these escape specialists are all palatable to predators. Some species of the Neotropical butterfly genera Adelpha (subfamily Nymphalinae) and Doxocopa (subfamily Charaxinae) show convergent appearance and exhibit extremely quick escape when slightly disturbed, followed by very fast flight. Their resemblance is hypothesized by R. B. Srygley to be a case of escape mimicry. The poor resemblance of some hoverflies to their purported hymenopteran models has also led to the hypothesis that groups of syrphid species could represent an escape mimicry ring on their own.
Poor Mimicry
At least to our eyes, the model’s color pattern is not always copied very accurately. Many syrphid flies, for instance, are difficult to assign to particular mimicry rings, although they seem to mimic the general appearance of Hymenoptera. The heterogeneity in mimetic accuracy has led biologists to propose adaptive and nonadaptive hypotheses, none of which seems very strongly supported at present. (1) The null hypothesis is that poor mimics are no mimics: many mimicry associations have been claimed on the general appearance of an insect, whereas careful examination of the geographic covariation of purported models and mimics may reveal evidence against them. In the case of inaccurate mimics, this method is not very powerful because the mimetic association itself is hard to define; so such covariation is difficult if not impossible to judge. (2) Another nonadaptive scenario is that accurate mimicry may not always be possible, either because of functional constraints/trade-offs on the modified organs or because of genetic or developmental constraints on the variation available in populations. Mimicry may then asymptotically reach a maximum level of resemblance, contingent on the route followed in the initial stages of the mimetic change. Again, this is theoretically plausible, but difficult to test. (3) Among the adaptive explanations for inaccurate mimicry is the hypothesis that these species are in the initial stages of their mimetic change and that our instantaneous view of evolution doesn’t show us the complete picture. (4) Another adaptive scenario is that predators have biases and perceptions different from those of humans and are likely to generalize more in some directions than in others, leading to the possibility that mimics that look very inaccurate to us are in fact very good mimics for a predator. Generalization is also dependent on the strength of the harmfulness of the models, perhaps allowing lower levels of accuracy. This may be the case for poor mimicry in some hoverflies. The ultimate adap-tationist hypothesis is that inaccuracy itself may be beneficial. (5) It could either allow the mimic to benefit from the protection of several different models, perhaps in a heterogeneous environmental context, or—a related hypothesis—create conflict in the predators’ recognition, which may give the mimic more time and chances to escape.
MIMICRY, COMMUNITY ECOLOGY, AND MACROEVOLUTIONARY PATTERNS
Habitat Heterogeneity, Spatial Dynamics, and the Coexistence of Mimicry Rings
The efficiency of a warning pattern depends on the abundance of that pattern in the habitat. Therefore, as new species join a particular mimicry ring, the protection given by the pattern increases, and more species should converge on this best protected pattern.
Ultimately, all species should converge on a single mimicry ring. But nature seems to behave in a totally different way. In any one habitat, particularly in tropical environments, aposematic insects of similar size and shape usually cluster into a number of distinct mimicry complexes or mimicry rings.
MULTIPLE MIMICRY RINGS IN THE COMMUNITY
One possibility is that different mimicry rings are found in different microhabitats. If predators do not move between microhabitats, or retain microhabitat-specific information, insect species in different microhabitats could converge on different adaptive peaks. Flight height has been invoked as a possible explanation, following the rainforest stratification paradigm, but evidence from butterflies is rather equivocal. However, host-plant stratification and different nocturnal roosting heights in Neotropical butterflies have received empirical support. Forest maturity and succession stage influence the host-plant composition and may allow the maintenance of multiple mimicry rings in a mosaic habitat.
MULTIPLE MIMICRY RINGS WITHIN A SPECIES
If some species are patchily distributed because of their microhabitat requirements, each “subpopulation” may be particularly sensitive to genetic drift and allow the local predators to learn and select a different color pattern in different patches. Once locally stabilized, the new pattern may be hard to remove. Indeed, local positive frequency dependence is both very efficient at stabilizing patterns around fitness peaks and slow at removing already established suboptimal patterns. Any slight difference in microhabitat quality or patchiness of the species involved will increase the local apparent abundance of particular patterns to particular predators, further decreasing the power of selection to achieve ultimate convergence.
This “mosaic mimetic environment” theory can help explain some problematic cases of Mullerian polymorphism. For instance, Laparus doris is a Heliconiine butterfly (Nymphalidae) that has up to four coexisting forms in some populations, some of which are probably mimetic and others are not. The maintenance of polymorphism in this species could be attributed to its high larval and pupal gregari-ousness (several hundreds of individuals), which results in a patchy distribution of the adults. When hundreds of butterflies suddenly emerge from one single vine, they make up their own local mimetic environment, and the mimetic environment prior to the mass emergence might be effectively neutral to L. doris.
If the species composition and the resulting mimetic environment are spatially variable, polymorphism can evolve in microhabitat gen-eralists, with gene flow across these microhabitats. For example, the Amazonian polymorphic species H. numata is selected toward different mimetic patterns in different localities that may represent different microhabitats for their more specialized models in the genus Melinaea (subfamily Ithomiinae) (Fig. 4). The balance between local selection and gene flow in a mosaic habitat (and perhaps weak selection against polymorphism as suggested earlier) can therefore maintain a nonadaptive, although widespread, polymorphism in H. numata.
Coevolution in Mimicry
EVOLUTIONARY RATES AND THE COEVOLUTIONARY
CHASE Despite many potential sieves constraining mimicry, several to many edible species can end up mimicking a particular warning pattern in a parasitic way. In such cases, is it possible that a “Batesian-overload” threshold is reached, beyond which the efficiency of the signal is severely lowered? Batesian mimics are indeed parasites of the honest signals of their models, and so the models should escape their mimics by evolving a new warning pattern. However, this escape would be transient because the new pattern would soon attract new Batesian mimics, resulting in an evolutionary arms race, or coevolutionary chase, between the model and its mimics. Some authors suggested that this chase could be a cause of the mimetic diversity in both models and mimics and that cyclical interactions could arise in some cases. However, first, theory has shown that mimics always evolve faster than their models, because they gain a lot more from mimicry than models lose from being mimicked. Any gradual move of the model should be quickly matched by a similar evolution in the mimic. Second, the models, which are the prime educators of local predators, are under strong purifying selection against any new warning pattern. This strong intraspecific conservative force should in the vast majority of cases be stronger than the deleterious effects of being mimicked and preclude pattern change in the models. Coevolutionary changes between Batesian mimics and their models should therefore be stopped in their early stages by a stronger selection for the status quo, and both the models and their mimics should be trapped in the same warning pattern. Only by a phenotypic leap toward an already established warning pattern (Mullerian mimicry) or by crossing a fitness valley thanks to local genetic drift could the model ever escape its mimics.
MUTUALISM AND COEVOLUTION IN MULLERIAN
MIMICRY In contrast with the unilateral Batesian evolution in which mimics outrun their models, Mullerian mimicry was traditionally thought to involve mutual resemblance of the species involved, as if all had moved toward some halfway phenotype. Of course, Muller himself and others were quick to point out that the mutual benefits were not even, but lopsided, that is, typically the rarer or the less distasteful species would benefit more than the more common or better defended one (respectively). However mutualistic the relation is, coevolution has often been assumed in Mullerian associations, and the protagonists are usually called comimics just because it is difficult to know if one species is driving the association. Coevolution also predicts that geographic divergence and pattern changes should be parallel in both species of comimics, like in the mimetic pair H. erato and H. melpomene in tropical America, presumably leading to parallel phylogenies. However, DNA sequences from mito-chondrial and nuclear genes show distinct phylogenetic topologies in these two species and distinctly nonparallel evolution.
In fact, there are a number of grounds on which to believe that the asymmetrical relationship leads to one-sided signal evolution even in Mullerian mimicry, one species being a mimic and the other a model. First, because of number dependence, mimetic change of a rarer species toward a commoner species will be retained, but the reverse is not true: by mimicry of a less common species, the commoner species would lose the protection of its own ancestral pattern, and a change toward a rarer pattern would be initially disadvantageous. The commoner species is therefore effectively locked in its pattern, and initial changes are only likely in the rarer species. Second, given the selection against nonmimetic intermediates, the mutants in the rarer species will have to be roughly mimetic of their new model to be selected, thus bringing the ultimate shared signal closer to that of the common species. Once this initial step is made by the mimic, there could be gradual “coevolution” to refine the resemblance, but the resulting change in color pattern will inevitably be more pronounced in the mimic, the model remaining more or less unchanged. Because Mullerian pairs are of a mimic-model nature, even with mutual benefits, the prediction for parallel evolution is therefore not likely to be valid. Indeed, in the mimetic pair H. erato/ H. melpomene, the phylogeography suggests that H. melpomene has radiated onto preexisting H. erato color-pattern races, thus colonizing all color-pattern niches protected by H. erato in South America.
Mimicry, Speciation, and Radiations
Racial boundaries in mimetic butterflies are usually very permeable to genetic exchange, since selection acts primarily on color-pattern genes. However, because clines moving geographically are likely stopped at ecological boundaries, the resulting racial boundaries are likely to rest on ecological gradients. Racial boundaries between mimetic color patterns could therefore be reinforced by adaptation to local ecology on either side of the cline, leading to spe-ciation. Color-pattern diversification could then accelerate specia-tion by allowing both postmating reproductive isolation, because of a higher mortality of nonmimetic hybrid offspring, and premating isolation if color pattern itself is used as a mating cue by the insect. For these reasons, mimicry has the potential to accelerate specia-tion. The pattern of mimetic associations in Heliconius butterflies seems indeed to indicate that speciation and mimetic switches are usually coincident: sister species usually differ in their mimetic color pattern. Direct evidence of the role of color pattern in mate choice has been gathered for the sister species pairs H. erato/H. himera and H. melpomene/H. cydno . The first two species are geographically separated across an ecological gradient in the Andes. The second pair is sympatric, although the species also differ in ecological requirements in a patchy distribution. In both pairs, therefore, color-pattern and mimetic switches probably accelerated speciation initiated by ecological adaptation. It is unknown how general this mimicry-based speciation is in mimetic insects but it could be an important consequence of the rampant and apparently easy diversification of mimetic patterns at the intraspecific level. The genetic predisposition of mimetic species to evolve polymorphism—the first stage toward speciation—might explain why mimetic lineages are usually very speciose and undergo rapid radiations, both geographically and phylogenetically.
