Specialized Terms
glycolysis The principal metabolic pathway responsible for
oxidation of carbohydrate (glucose) to pyruvate during cellular
respiration.
gluconeogenesis A metabolic pathway responsible for the
net synthesis of carbohydrate (glucose) from amino acids, lactate,
and glycerol.
respiration The collection of metabolic pathways responsible
for the oxidation of glucose, amino acids, and fatty acids,
with the production of energy involving an electron transport
chain.
lipid A chemically diverse group of molecules that are insoluble
in water and other polar solvents.
supercooling The absence of freezing at or below the normal
freezing point of water.
metabolome A quantitative metabolite profi le associated
with a cellular process.
The chemical reactions of cells, linked together in series to form pathways, are collectively referred to as metabolism. Metabolic pathways are interdependent and exquisitely regulated for efficient extraction of energy from fuels and for synthesis of biological macro-molecules. Cellular processes produce unique chemical fingerprints or metabolite profiles, and a complete quantitative set of metabolic intermediates associated with a cellular process is referred to as the metabolome. Metabolomics is the study of changes in the metabo-lome that may arise from metabolic regulation or alteration in gene expression, or a combination of both mechanisms. Studies of metabolism and metabolomics are subject areas of biochemistry, which also includes the structural chemistry of biological molecules and the chemistry of molecular genetics.
Metabolic studies with insects have focused on the biochemical bases for the unique physiological capabilities of insects and their arthropod relatives. Early studies considered chemical content, individual chemical reactions, respiration, and metabolic rate. Much of this was discussed in Sir V. B. Wigglesworth’s The Principles of Insect Physiology that first appeared in 1939. With advances, other comprehensive reviews appeared, including D. Gilmour’s 1961 The Metabolism of Insects, the 1964 edition of Physiology of Insecta and in 1978 The Biochemistry of Insects, both edited by M. Rockstein. More recently, insect metabolism was described in several volumes of Comparative Insect Physiology Biochemistry and Pharmacology, edited by G. A. Kerkut and L. I. Gilbert (1985). A recent update is Comprehensive Molecular Insect Science, edited by L. I. Gilbert, K. Iatrou, and S. Gill (2005), but the coverage of metabolism is restricted.
INTERMEDIARY METABOLISM
Insects share with other invertebrates the common pathways of carbohydrate, lipid, and amino acid metabolism. Although much has been presumed based on overt similarities to more extensive studies of mammals and other higher taxa, many aspects of intermediary metabolism have been examined in a number of insects and different insect tissues. Much of intermediary metabolism, including synthesis and storage of carbohydrate and fat, takes place in the fat body.
Metabolism and utilization of the glucose disaccharide trehalose as the principal hemolymph or blood sugar is unique to insects and some other invertebrates. First described from an insect by G. R. Wyatt in pupae of the silk moth, Antheraea polyphemus, trehalose, a non-reducing sugar, occurs in many insects at variable but high levels. In lepidopteran insects, trehalose concentrations are commonly between 25 and 100 mM, levels greatly exceeding those of glucose in the blood of mammals. Blood glucose in man typically is about 5 mM, a low value for trehalose in hemolymph. With few exceptions, glucose occurs in insect hemolymph at levels less than 5 mM, and often at less than 1 mM. Trehalose serves multiple functions, as a storage carbohydrate that serves as a fuel for flight and as a cryopro-tectant, protecting insects from damage during overwintering in cold climes. The hemolymph level of trehalose plays an important role in regulating carbohydrate intake and maintaining nutritional home-ostasis. Levels of trehalose in the hemolymph are maintained by a complex interaction of nutrient intake and metabolism.
Trehalose is synthesized in the fat body from two glycolytic intermediates, glucose-1-phosphate and glucose-6-phosphate. The reactions are catalyzed by trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Sources of glucose for trehalose synthesis include dietary sucrose, glycogen, and gluconeogenesis; dietary sugar being the sole source of glucose under fed conditions. Trehalose formation from glycogen has been described in several insects including the cockroach Periplaneta americana and moth Manduca sexta during starvation. The breakdown of glycogen to glucose is due to activation of the enzyme glycogen phosphorylase and is under endocrine control by a neurohormone released from the corpora cardiaca in the brain. Induction of a “hypertrehalosemic” hormone RNA transcript in the central nervous system of the cockroach, Blaberus discoidalis, in response to starvation was recently demonstrated. Glucose synthesis, followed by trehalose formation, via gluconeogenesis, has only been reported in M. sexta and was induced when larvae were maintained on low carbohydrate diets. Starvation did not induce gluconeogenesis.
Insects obtain energy principally from aerobic respiration, but many species have some capacity for anaerobic energy metabolism when exposed to hypoxic or anoxic conditions. This is best known in aquatic insects such as midge larvae where the fermentation products may include lactate, ethanol, and acetate. The midge Chaoborus crystallinis accumulates succinate, suggesting that this species is able to use anaerobic respiration for ATP production.
CHEMISTRY AND METABOLISM
OF SCLEROTIZATION
The hardness of cured exocuticle is the result of polymerization due to cross-linking between molecules of the protein sclerotin and/or cross-linking between sclerotin and chitin. Because of the resilience of cuticle, the chemical composition of sclerotin and of the chemistry of the polymerization process was first understood from organochemical analyses of the products resulting from chemical degradation of cuticle. Current knowledge of cuticular structure comes about through non-destructive NMR analysis of intact cuticle. Although sclerotiza-tion is principally the result of the reaction of cuticular proteins and chitin with quinones derived from N-acetyldopamine, the metabolism of sclerotization is complex and sclerotized cuticles vary greatly in their physical and chemical properties because of metabolic variation.
The metabolism principally involves reactions of quinone methide intermediates that produce so-called (3 sclerotin, with cross-linkages involving the (3 carbon of the catecholamine side chain, as well as dehydrodopamine intermediates producing linkages to the benzoyl-ring. The metabolism of the quinone and dehydrodopamine intermediates is integrated, such that both the side chain and the ring structure may be involved in an individual cross-linked structure. Phenoloxidases, quinone isomerases, and quinone tautomerases are the principal enzymes involved in this metabolism.
The structure of the cross-linkage between sclerotin and chitin in the pupal cuticle of M. sexta was established through use of solid-state NMR spectroscopy. The structure demonstrates cross-linkage of phenolic and quinone intermediates with the imidizole ring of histidine residues of the protein and the ( hydroxyl group on C4 of N-acetylglucosamine units of chitin (Fig. 1).
CHEMISTRY AND METABOLISM OF PIGMENTS
The diversity of insect coloration is in large measure because of an abundance of pigments. Combinations of pigments together with effects of light diffraction, refraction, and interference involving various anatomical structures produce the array of exotic colors familiar to insect observers. Many insects synthesize melanins, ommochromes, porphyrins, pteridines, and/or quinones. Other pigments such as flavonoids and carotenoids, although not synthesized, are often sequestered by insects from plants and contribute to coloration. Two pigment groups are notable. Ommochromes, first reported from the eyes of insects, and papilochromes, a unique group that occur in the bodies and wings of the butterfly family Papilionidae.
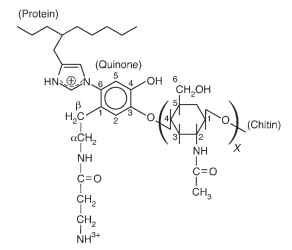
FIGURE 1 Structure of the protein-chitin cross-linkage in the pupal cuticle of Manduca sexta (adapted from Schaefer et al., 1987). Protein may be linked through the 1 or 3 nitrogen of the imidizole ring to the 2, 5, or 6 ring carbon of the quinone derivative, and carbon 4, or other carbons of chitin may be linked to phenoxy carbon 3 or 4 of the quinone.
Ommochromes are polymers of heterocyclic phenoxines, distributed among a variety of different insect tissues, producing yellow, red, and brown coloration. They are synthesized from tryptophan in a metabolic pathway involving kyneurenine derivatives. In the compound eye, ommochromes form the principal masking pigments that surround and isolate the individual ommatidia and thus the origin of the name. Several eye-color mutants, described in several insect species, result from the absence of enzymatic function at specific steps in the synthetic pathway. Identification of these steps in Drosophila was one of the early confirmations of A. Garrod’s one gene-one enzyme hypothesis. The ommochrome biosynthetic pathway in the coloration of M. sexta larvae is hormonally regulated.
Papiliochromes are novel white, yellow, and red pigments whose synthesis intersects the well-known metabolic pathways, the melanins and ommochromes. For butterflies of the genus Papilio, the precursors are (3 alanine, tyrosine, and tryptophan. Papiliochromes accumulate in the wing scales, and their distribution varies with the butterfly species. Recent studies on papiliochrome synthesis demonstrated that, as in the case of sclerotization, quinone methides derived from tyrosine are intermediates. The synthesis involves the non-enzymatic condensation of N-( -alanyldopamine quinone methide with L -kynurenine to produce a mixture of two diastereoisomers of papilochrome II, a white pigment. Papiliochrome II is a peptide in which the two aromatic rings are linked by a bridge between the aromatic amino group of kynurenine and the catecholamine side chain of norepinephrine derived from the quinone. Papiliochrome synthesis is regulated by the activation of ( -alanyldopamine synthase that shifts dopamine derived from tyrosine away from melanin synthesis and into papiliochrome synthesis.
ENERGY METABOLISM DURING FLIGHT
Insect flight muscles are obligately aerobic, deriving energy from O2-dependent substrate oxidation to CO2 and H2O. Small insects in flight achieve the highest known mass-specific rates of aerobic metabolism among animals. Of the estimated one-half million insect species capable of flight, the metabolism of only a few have been subjected to detailed examination. Insect species differ in the extent to which carbohydrates (principally trehalose), fats (mainly diacylg-lycerol), and proline (an amino acid) are used to fuel flight. The relevant pathways are shown in Fig. 2 .
The evolution of fuel use by insect flight muscles remains poorly understood. In some species of locusts and moths, flight is initially fueled by carbohydrate and prolonged flight is accompanied by activation of fatty acid oxidation and inhibition of carbohydrate oxidation. The switch from one fuel to another allows the use of a more energy-dense fuel (fat) while sparing carbohydrate stores and is triggered by adipokinetic hormones. Bees are unable to make use of fats to fuel flight, but instead oxidize carbohydrate, while using proline as a carbon source for augmenting the concentration of Krebs cycle intermediates. Pyruvate carboxylation serves a similar, “anaplerotic” role in these species. Carbohydrate and proline are oxidized simultaneously to support steady-state flight by flies and some beetles, but there is considerable inter-specific variation as to which fuel is used as the primary carbon source. Proline, for example, is the principal flight fuel for a few beetle species, whereas tsetse flies use proline exclusively.
A huge increase in the rate of ATP utilization in muscle occurs during the transition from rest to flight. In species possessing synchronous flight muscles (e.g., locusts, butterflies, and moths), this is the result of increased activities of actomyosin-ATPase, Ca2+-ATPase, and Na+-K+-ATPase. In insects with asynchronous muscles, actomyosin ATPase accounts for most of the ATP hydrolyzed, and the energetic cost of excitation-contraction coupling (involving Ca2+-ATPase and Na+-K+-ATPase) is much lower in comparison with synchronous muscles. Contraction frequencies in asynchronous muscles are not limited by maximum rates of Ca2+ cycling. This advantage, as well as the lower energetic cost of excitation-contraction coupling, may explain why asynchronous muscles, which are unique to insects, are found in 75% of insect species, including flies, beetles, and many species of wasps and bees.
ATPase activities increase upon the initiation of flight, and metabolic control mechanisms activate pathways of substrate catabolism, mitochondrial respiration, and oxidative phosphorylation to maintain ATP concentration and muscle function. Cellular rates of ATP hydrolysis and synthesis in insect flight muscle are so exquisitely matched that ATP concentrations are maintained within narrow limits, despite up to 100-fold increases in the rate of ATP turnover during the transition from rest to flight. The regulatory mechanisms that make possible these large flux changes between rest and flight remain poorly understood. The extremely high, steady-state rates of metabolic flux during flight are made possible by high concentrations of cata-bolic enzymes in the flight muscles, the operation of some of these enzymes at high fractional velocities, high mitochondrial content, large inner membrane surface areas per unit mitochondrial volume, and unusually high rates of electron flow between respiratory chain enzymes. Evolution has produced no locomotory muscles capable of higher rates of aerobic metabolism than those found in flying insects.
METABOLISM OF COLD HARDINESS
Many insects that exhibit arrested development during periods of cold, or routinely live in cold environments, display a resistance or tolerance to freezing. The phenomenon of supercooling was described for various insects over 100 years ago, and supercooling temperature may approach —50°C. In most cases, low-molecular-weight cryoprotectants, including some amino acids, mannitol, treha-lose, sorbitol, and particularly glycerol, accumulate in the hemolymph
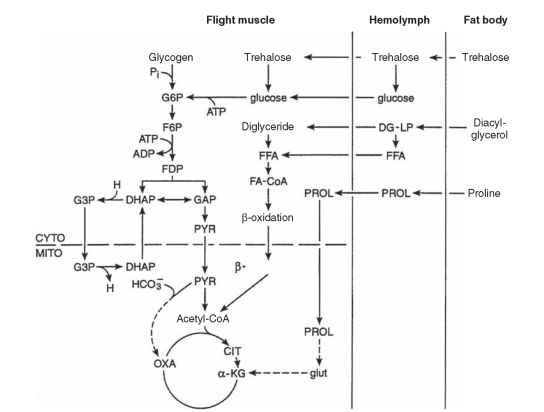
FIGURE 2 Metabolic scheme showing pathways of carbohydrate, fat, and proline oxidation in insect flight muscles. Included are the anaplerotic roles of pyruvate carboxylation and proline oxidation in some species, as well as the a-glycerophosphate shuttle (involving G3P and DHAP) for transferring reducing equivalents from cytoplasm to mitochondria. The contribution of each pathway varies according to species. In some species, substrate use may change with time in flight (adapted from Storey, 1985). ACETYL-CoA = acetyl-coenzyme A, ADP = adenosine diphosphate, ATP = adenosine triphosphate, CIT = citrate, CYTO = cytosol, DG-LP = lipoprotein-bound diacylglycerol, DHAP = dehydroxyacetone phosphate, F6P = fructose-6-phosphate, FDP = fructose bisphosphate, FA-CARNITINE = fatty acid-carnitine, FA-CoA = fatty acid-coenzyme A, FFA = free fatty acids, G3P = glycerol-3-phosphate, G6P glucose-6-phosphate, GAP = glyceraldehyde-3-phosphate, glut = glutamate, HCO3- = bicarbonate ion, H = nicotinamide adenine dinucleotide, a-KG = ketoglutarate, MITO = mitochondrion, OXA = oxaloacetate, PROL = proline, PYR = pyruvate.
and act as antifreeze. Cryoprotectants can reach levels of 20-30% fresh body weight with the level of cryoprotectant inversely related to the supercooling point. In freeze-tolerant species ice-nucleating proteins accumulate.
Among the first studies of cryprotectant synthesis was a demonstration of glycerol and sorbitol formation from glycogen by dia-pausing Bombyx mori silkworm eggs. The formation of polyols is an example of anaerobic fermentation. Their synthesis provides cryo-protection, but also maintains redox balance in response to a reduced level of oxidative respiration. Regulation of polyol formation is poorly understood, but cold-induced activation of glycogen phosphorylase is involved. Studies with Eurosta solidaginis fly larvae demonstrate independent control over glycerol and sorbitol formation, and hormonal cues may be important.
In insects that accumulate trehalose for cryoprotection, inhibition of phosphofructokinase has been suggested as the mechanism for shifting metabolism in the direction of sugar formation. A temperature-dependent change in the balance between glucose oxidation by glycolysis and the pentose phosphate pathway may also affect the balance between trehalose, sorbitol, and glycerol.
Metabolomic studies have characterized distinct changes in metabolite profiles associated with cryoprotection and diapause in the fly Sarcophaga crassipalpis . These confirm the accumulation of glycerol and sorbitol during rapid cold hardening (Fig. 3). Additional metabolites are also formed while others exhibit decreased concentration. Although cold hardening and diapause produce distinct metabolite profiles, insects in either of these states accumulate several of the same metabolites.
CONCLUSION
Insects exhibit many unique metabolic characteristics. The brevity of this article only allows discussion of a few select examples. Relatively few investigations have been conducted on molecular-genetic aspects of metabolism and metabolic regulation. Available techniques in cell and molecular biology, including differential display and microarray analysis, offer marvelous opportunities to examine the “transcriptional physiology” of hormonal and nutritional regulation of intermediary metabolism.
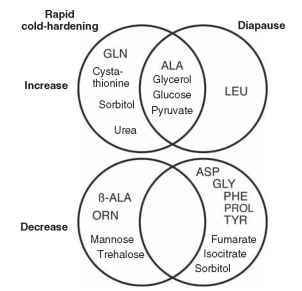
FIGURE 3 Summary of a metabolomic study (Venn diagram) illustrating the increased and decreased levels of metabolites associated with rapid cold hardening and diapause in the flesh fly Sarcophaga crassipalpis (adapted from Michaud and Denlinger, 2007). GLN = glutamine, ALA = alanine, ( ALA = ( eta alanine, ORN = ornithine, LEU = leucine, ASP = aspartate, GLY = glycine, PHE = phenylalanine, PROL = proline, TYR = tyrosine.
