Mating behavior is typically viewed as comprising all events from pair formation through courtship to the final breakup of the mating pair. In most pterygote insects, sperm transfer is achieved through copulation. In contrast, in most apterygotes,including both Insecta (Archaeognatha and Thysanura) and Ellipura (Collembola, Protura, and Diplura), sperm transfer is indirect; the spermatophore is placed on the substrate and is picked up by the female either following a period of courtship or with the pair making no contact at all. This article focuses on events occurring after the male and female have made physical contact; pair formation in insects is covered elsewhere. The main theme here is the function and adaptive significance of mating behaviors, that is, how mating behavior increases reproductive fitness such as by increasing the number of offspring produced.
There is a vast amount of published information on the mating behaviors of insects. These behaviors have traditionally been viewed as relatively invariant within species. However, it is now evident that insect mating can show a great deal of adaptive variation and flexibility. As an introduction to this variation, consider the wide range of mating behaviors by insects that use carrion, a resource that can attract both males and females and thus serves as a location for mating. The complexity and plasticity of mating behavior observed in carrion insects easily rival those of other animals, including vertebrates. Indeed, Shakespeare’s Romeo felt that “more courtship lives in carrion-flies than Romeo.”
One courting carrion fly is attracted to the dry hide and bones of large old carrion sources, the main larval food for the species. The fly Prochyliza xanthostoma (Piophilidae) shows a remarkably complex courtship that can last for over 15 min (see Fig. 1 for another example). A courting male approaches the female while stepping rapidly from side to side and striking his abdomen downward. Males
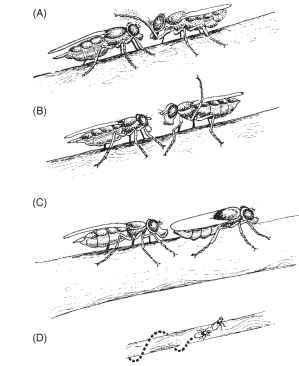
FIGURE 1 A fly similar to piophilids in exhibiting complex male courtship is the otitid, Physiphora demandata . The male first taps the female with a foreleg (A), then raises a middle leg (B), and turns and presents his abdomen to the female, who extends her proboscis to touch his abdomen (C). This can be followed by the female backing up in a spiral path, appearing to pull the male backwards (D).
TABLE IThe Functions and Context of Mating Behaviors |
|||
| Before copulating | While inseminating (copulating) | After insemination and copulation | |
| (1) To communicate information about sex (gender), possibly to suppress aggressive (in males) or cannibalistic tendencies (in predatory species). | X | ||
| (2) To synchronize mating behavior, such as when physiological mechanisms synchronize the behavior of the sexes. | X | X | |
| (3) To perform movements associated with positioning of genitalia, transferring ejaculates, and uncoupling. | X | X | X |
| (4) To communicate species information to prevent costly interactions (e.g., mate-finding movements or unviable offspring) with the wrong species. | X | X | |
| (5) To communicate information about direct benefits (for mates or offspring) supplied during or after mating such as: (a) fecundity or number of ejaculated sperm (fertility) and the ability to supply nutrients (nuptial meals) and (b) territory quality or level of parental care. |
X X | X X | X |
| (6) To communicate information about indirect benefits (i.e., for offspring) such as genetic quality (e.g. the ability to resist disease). | X | X | X |
| (7) To communicate competitive ability to rivals. | X | X | X |
| (8) To resolve struggles between the sexes that reflect conflict over whether to mate at all, when to terminate copulation, or whether the partner mates with another individual. | X | X | X |
can repeat these vigorous movements and vary the degree to which they display. If the female stops moving, the male stops courting, orients, and then slowly creeps toward her, occasionally repeating earlier parts of his routine. Individual males vary greatly in the vigor and length of the courtship display and this may represent variation in a signal of male genetic quality used by females to select mates that increase the fitness of her offspring (Table I, Nos. 5 and 6).
Courtship by males of another temperate-zone carrion fly also appears to mediate female discrimination among males. Females of the fly Dryomyza anilis (Dryomyzidae) lay eggs on small carrion items such as dead fish. For this fly, courtship on the carrion occurs after copulation. A single courtship sequence consists of the male’s genital claspers tapping vigorously on the female’s external genitalia and then lifting and releasing her abdomen. Males vary in the number of genital tapping sequences performed and the number of sequences correlates with greater fertilization success. Bouts of tapping are followed by oviposition during which the male guards his mate from rivals. A male’s success in fertilization is apparently achieved by the female biasing the distribution of sperm within her sperm storage organs (see Box 1) . A similar influence on the success of courting male red flour beetles (Tribolium castaneum: Tenebrionidae) comes from a display in which the male rubs the female’s elytra.
In a beetle that buries carrion, considerable variation occurs in reproductive behavior after the sexes have paired up and mated. A male and a female of the beetle Nicrophorus defodiens (Silphidae) cooperate both to defend a mouse-sized carcass from intrusions by other Nicrophorus and to bury the carrion in an underground chamber where it becomes food for the pair’s offspring. However, the behavior of the sexes is quite different after interment of a rat-sized carcass, one large enough to support more offspring than can be produced by the initial pair. Here, conflict between the pair becomes evident when the male produces a pheromone to attract additional females. The male’s signal causes his resident mate to try and thwart
this signaling by mounting and biting the male (Table I, No. 8). Conflict stems from a potential sexual difference in success on larger carrion. On a larger food resource, the male stands to gain substantially from the increased number of larvae he fathers when mating several females, whereas his first mate can expect only decreased fitness owing to increased larval competition.
Sexual conflict and flexibility in mating behavior are also apparent in the postmating interactions of a neotropical rove beetle, Leistotrophus versicolor (Staphylinidae). Male and female L. versi-color are attracted to carrion (and occasionally dung) not as an ovi-position site but as a place to prey on flies. After a pair copulates, the male can be observed to attack and bite his mate, often running after her for up to half a meter. However, this behavior occurs only when there are a number of rival males present. Male aggression appears to serve in driving the female away from the carrion, thus preventing her from mating with other males (Table I, No. 8). An alternative possibility is that aggression toward females is a form of postcopula-tory courtship (see Box 1 and Table I, No. 6).
SEXUAL CONFLICT DURING MATING
These episodes of insect mating reveal how Darwinian selection theory can be used to understand variation in behavior. The basic underlying assumption of this theory is that individuals behave in such a way as to yield the greatest number of surviving progeny (fitness). This theoretical insight argues that courtship and copulation should be a purely cooperative venture only when the fitness interests of the sexes are completely aligned. Cooperation in courtship was the prevailing view among biologists at one time, in part because courtship was thought to synchronize mating events (Table I, No. 2). In contrast to this view, much research indicates that the sexes are often in conflict. Sexual conflict is expected to be common when the reproductive interests of male and female are at odds (Table I, No. 8).
Box 1 A Broader View of Courtship: The Concept of “Cryptic” Sexual Selection
Sexual selection, typically greater on males than females, is selection in the context of competition for more mates or the best mates. This form of selection explains many sexual dimorphisms, from peacock tails to elaborate cockroach genitalia. Competitive males typically maximize the number of surviving offspring. Therefore male success is best estimated as fertilization success rather than success in mating many females. Thus, both direct competition between males and female discrimination should not end at copulation. The full development of this insight has coincided with the advent of a number of molecular-genetics methods to assign paternity. In insects the potential for paternity competition is high because females typically mate with more than one male and store their long-lived sperm in specialized organs. Indeed, males have been found to possess adaptations that incapacitate, physically displace, or remove rival ejaculates, examples being copulatory movements in dragonflies, such as Calopteryx maculata. in which penis brushes remove virtually all rival sperm from the female sperm storage organs. In fact, male insects can enhance fertilization success even after sperm transfer has occurred. For example, males are known to transfer chastity-enforcing chemicals to females or substances that cause the females to increase the rate of laying eggs. Commonly, mechanisms that bias fertilization success are under female control and so are often cases of female discrimination, a phenomenon that can be revealed by experimentally removing “male-control” effects on the paternity of offspring. Female adaptations include fertilization biases caused by moving favored ejaculates. Also, in species with “last ejaculate stored is the first to be used” mechanisms, females mating high-quality males (representing genetic quality in species with no paternal care, Table I, No. 6) can simply increase the rate of egg laying (e.g., Oecanthus tree crickets and Hylobittacus scorpi-onflies) or differentially allocate more resources to these eggs, thereby increasing offspring fitness (no examples from insects, but this is known in birds). Finally, the consequences of these “cryptic” sexual selection mechanisms are: (i) a male’s courtship that signals his quality, that is, genital copulatory displays (Table I, Nos. 5 and 6), can occur at any point during mating until the female oviposits (thus making courtship synchronous with mating) and (ii) the Darwinian division between primary (e.g., penes and testes) and secondary (e.g., the peacock’s tail) sexual structures is blurred: male structures such as dragonfly penis brushes and large testes that are adapted to deliver large numbers of gametes into the sperm competition lottery are probably sexually selected devices. The vast diversity and complexity of insect genitalia may result from these processes: species with multiple-mating females are known to have more complex male genitalia than species in which females mate only once.
Conversely, cooperation is expected in any case in which male and female interests are similar. For example, interactions between a pair of burying beetles are mainly cooperative after they have interred a mouse-sized carcass. In contrast, when a larger carcass is buried, conflict is evident because, unlike the situation with small carcasses, the male has an opportunity to increase his reproductive success by attracting additional mates, whereas any added larvae from such mat-ings probably decrease the initial success of the female. The latter situation exemplifies a type of sexual conflict expected in the reproductive activities of animals because of a sexual difference in reproductive strategy: males typically maximize the number of females mated so as to maximize fertilization success, whereas females maximize fecundity and offspring quality. This sexual difference also causes conflict when already-mated females are harassed by promiscuously mating males. Examples come from the precopulatory struggles often observed in insects. A well-studied case involves water striders (Heteroptera: Gerridae). When a male uses forelegs and genitalia to secure a female for copulation, a vigorous struggle ensues during which the female attempts to dislodge him. Superfluous matings can be costly to females in terms of increased predation risk (Box 2) and energetic cost. To reduce such costs, female Gerris incognitus have evolved upcurved abdominal spines that appear to function in thwarting male mating attempts. Another possible purpose for precopulatory struggles is that they test male quality (Table I, No. 6; see also the example of seaweed flies considered under “Genetic Quality and Mate Choice”).
Precopulatory struggles appear to be a result of sexual conflict in species in which males feed their mates, because females pay a cost if the size of their meal is reduced in any way. Thus at the end of mating, a male and female scorpionfly (Mecoptera: Bittacidae) can both be seen to pull on the prey offering (Fig. 2). Conflict comes from male attempts to recover the prey in order to conserve food for copulations with other mates and the female maximizing how
Box 2 Risk of Predation and Mating Behavior
Insects engaged in mating activities are known to assess the risks of predation and to adaptively change their behavior. For example, the typical song preference shown by female crickets, Gryllus integer (Gryllidae), can be overcome if the female can safely approach a less preferred song. In water striders, Aquarius remigius (Hemiptera: Gerridae), high predation risk appears to reduce male activity, thus decreasing their tendency to harass females. This in turn allows large males to achieve high mating success (possibly because females can be more selective or can more easily avoid mating with smaller males). The threat of predation from fish, insect, and spider predators of this species and other gerrids can cause a decrease in mating frequency as well as in the duration of copulation.
long she feeds on the prey. Conflict in some mate-feeding insects is particularly evident in the struggle between the sexes when a male attempts to force a copulation without providing the beneficial meal to his mate. To overcome female resistance, males of both panorpid scorpionflies and haglid orthopterans have specialized abdominal organs that function in holding onto females during forced matings.
A widespread form of sexual conflict arises from multiple mating by a female, which increases her success while compromising her mate’s confidence of paternity. This conflict is evident when male rove beetles and tree weta Hemideina crassidens (Orthoptera: Anostostomatidae) drive their mates away from rivals. Striking examples also occur in male adaptations that not only enforce chastity in the female but also reduce her survival. Examples include toxic chemicals in the seminal fluid of fruit flies, Drosophila melanogaster (Drosophilidae), and damaging

FIGURE 2 Sexual conflict behavior in insects can be seen in mating struggles between a male and a female. Here a pair of scorpionflies, Hylobittacus similis, are using their hind tarsi in a tug-of-war over a nuptial prey (a blowfly) item captured by the male.
spines on the penis of cowpea weevils, Callosobruchus maculatus (Bruchidae), both of which decrease female life span. In the beetle, females vigorously kick males in order to terminate copulation; a shorter copulation probably decreases the severity of wounding (Table I, No. 8).
SEXUAL DIFFERENCES IN MATING BEHAVIOR
Advantages that females might obtain from choosing to mate with more than one male include acquiring goods and services—such as nuptial meals—or enhancing offspring quality by remating when a male of high genetic quality is encountered (Box 1). This point highlights the basic sexual difference in mating behavior: typically females are choosy when it comes to the males that father their offspring, whereas males compete and display as a way to obtain multiple matings.
The factors controlling these typical sexual differences in behavior stem from the basic difference in the way males and females maximize reproductive success. Females usually invest more in individual offspring than males by providing materials for egg production and, in some species, caring for progeny. These maternal activities mean that fewer females than males are available for mating, thus causing males to compete for the limiting sex. Therefore sexual selection (Box 1) is greater on males than on females. This theory predicts that in species in which males invest more in offspring than females, sexual selection on the sexes will be reversed, causing a reversal in the mating roles, that is, competitive females and choosy males. This prediction has been upheld in experiments with several katydid species (Orthoptera: Tettigoniidae). These species are useful experimental organisms because mating roles are flexible; when food in the environment becomes scarce, females compete for mates and males are choosy. Hungry females fight to obtain matings because each copulation comes with a nuptial meal, a nutritious spermato-phore [in contrast to sexual selection on males—to increase fertilizations (Box 1)—the sexual selection on meal-seeking females is to increase number of matings]. In support of the theory, food scarcity causes an increase in relative investment in individual offspring (eggs) because there is an increase in the material in eggs derived from males—their spermatophore nutrients. The degree of choosi-ness shown by a sex should also be influenced by variation in the quality of the sex being chosen. Members of a sex are expected to be choosy if variation in the quality of potential mating partners is high.
SIGNALS TO MATES DURING MATING
Material Benefits and Mate Choice
Rejection of a mate (usually of males by females) is only one of the explanations for the failure of a pair to mate successfully. Other causes of breakup of pairs are certain changes in the physical environment and a threat of harm from predators (see Box 2) or rival males. A number of studies have ruled out these alternatives and have thus shown that certain mating behaviors function in choosing mates, for example individuals noted to move between signaling or swarming members of the opposite sex before mating with one of them and individuals pulling away from their mates after the mating sequence has begun.
As predicted by theory, female choice of mates is more widespread than male choice. Some of the clearest examples of mate choice come from species in which females obtain material benefits from males (Table I , Nos. 5a and 5b). One case involves scorpionflies (family Bittacidae), of which females attracted to males presenting food gifts of prey will make tarsal contact with the offering (Fig. 2) and reject males presenting small prey. Female scorpionflies also discriminate against such males by breaking off copulation prematurely. Only a male that transfers a large prey will complete copulation by supplying both a full complement of sperm and chastity-inducing substances.
Most cases of male choice in insects appear to involve the acquisition of material benefits from large females, specifically the large number or size of eggs possessed by heavyweights. This has been noted in a number of insects, including tettigoniid orthopterans, cer-ambycid and brentid beetles, and empidid flies. There are very few cases of males choosing female displays. Indeed, despite instances of sexual selection on females, there are few examples of female ornaments, even in cases where the mating roles are reversed. One hypothesis for this is that investment in elaborate displays probably trades off with reduced fecundity. Consequently selection on males to mate with the most fecund individuals led to avoidance of ornamented females. There are, however, ornamented females in certain empidid flies. For example, female Rhamphomyia longicauda displays inflated abdomens and fringed legs to choosy males while flying in all-female swarms during twilight. Poor light conditions may have selected for ornaments as an indicator of female size and thus fecundity. Less likely is the hypothesis that these ornaments advertise genetic quality. As noted earlier, male choice is expected when the mating roles are reversed or when there is a high degree of variance in the quality of females. Examples of the former include male Mormon crickets (Anabrus simplex: Tettigoniidae) pulling away from mounted lightweight females—apparently after weighing them—and male empidid flies choosing large, fecund individuals from within all-female swarms. In contrast to male mate choice in role-reversed systems, male choice that evolves in response to a high variance in female quality typically is often found with a high degree of male—male competition, that is, sexual selection on males. Indeed, male choice in this situation can be caused by sexual selection among males to mate with the highest quality females. An example of male choice when females vary in quality includes winter moths, Operophtera brumata (Lepidoptera, Geometridae), and red flour beetles. Finally, local population variation in the primary sex ratio can affect the likelihood of male choice; a scarcity of males is associated with a higher degree of male choice in red milkweed beetles, Tetraopes tetrophthalmus (Cerambycidae); and in a butterfly Acraea encedon many males are killed by Wolbachia bacteria thus inducing a reversal in the mating roles.
Genetic Quality and Mate Choice
In theory, females are expected to show choice to obtain indirect benefits, that is, benefits that enhance the genetic quality of offspring. Female Dryomyza flies appear to do this by biasing fertilization after evaluating male copulatory courtship. But what sorts of indirect benefits do choosing females obtain? In yellow dung flies, Scathophaga stercoraria (Scathophagidae), females can favor the stored sperm from males with genotypes likely to enhance offspring growth. One allele in males is better when larvae feed in shaded dungpats (where the environment is relatively stable) and another is better in the sun (where temperature fluctuates greatly between day and night). In other insects, the cue to a male’s genetic quality is consistent. For example, following a courtship consisting of wing-flicking and pheromone displays, older females of Colias butterflies (Pieridae) show mating preferences for genotypes that fly well and are long-lived. Male and female calopterygid dragonflies also court using wing displays and the size of wing spots. The latter is a sexually dimorphic trait in European Calopteryx splendens and is correlated with several aspects of male quality, including the level of immuno-competence, developmental stability, and resistance to gut parasites. In a dipteran, the seaweed fly, Coelopa frigida (Coelopidae), mating interactions involve prolonged premating struggles in which a mounted male can be dislodged as a result of kicking and shaking by the female. Such struggles favor matings with large males and this female bias enhances the genetic fitness of her offspring: progeny of large males tend to be heterozygous for a chromosomal inversion that increases offspring viability. However, female choice in this system may be maintained by more than good-genes sexual selection. Females carrying the inversion genotype show a strong preference for large males. Genes for preference thus appear to be linked with genes for the male display trait, suggesting a form of female-choice sexual selection, termed “runaway” or “Fisherian” sexual selection (after the originator of this idea, R. A. Fisher), in which mothers gain by producing “sexy sons,” those that are highly attractive to females.
Mating Preferences for the Correct Species
One result of the expected rapid evolutionary change from runaway sexual selection may be speciation through behavioral isolation. Speciation results when there is sufficient between-population divergence in the female preference and the linked male display that a side effect of intraspecific mating preferences is discrimination against males from other populations (see also Box 1). This “effect” hypothesis for species discrimination differs from the hypothesis that certain female mating preferences have evolved to function in avoiding costly interactions with other species. An example of the latter involves the fruit flies Drosophila pseudoobscura and D. persimilis, in which hybrid matings result in decreased reproductive success because sons are sterile. Female Drosophila assess the wing-vibration displays of males, and D. pseudoobscura females collected from areas where the two species co-occur (sympatry) reject courting D. persimilis males more frequently than females collected from areas with no species overlap (allopatry). This result was not the result of differences in courtship by the males with the two types of females. These findings indicate that female discrimination against heterospe-cific male courtship has been reinforced in areas where maladaptive hybridization is likely to occur.
A high degree of discrimination against courtship by heterospe-cific individuals in sympatry has also been noted in Calopteryx dragonflies in which both sexes display patterned wings in precopulatory courtship. Compared with areas of allopatry, male C. maculata in sympatry discriminate more against the wing patterns of C. aequa-bilis females, and mate preferences during courtship appear to have reinforced wing-pattern differences between the species. For example, in a north-south transect in eastern North America, the proportion of pigmented wing area of both sexes is greater in areas of sympatry than in areas of allopatry.
SIGNALS TO RIVALS DURING MATING
In another North American calopterygid, Hetaerina americana, variation in wing-spot displays reflects an evolutionary history of competition between males; males with larger wing spots are more successful in defending mating territories than males with smaller spots. As males with experimentally enhanced spots suffer a cost (increased mortality), these signal patterns appear to have evolved as honest indicators of fighting ability. These signal indicators may convey to rivals information about male ability displayed during wing-waving directed at females. Other behaviors taking place during mating appear to function in a competitive context (Table I , No. 7). For example, males of the carrion beetle mentioned previously (L. versicolor) will occasionally mimic female behavior during interactions with potential mates (Fig. 3). Pseudofemale behavior reflects a remarkable plasticity in the way the male beetles obtain matings. The most profitable way to gain access to females is to fight to defend the carcass that attracts them. However, males can obtain some matings subversively by mimicking female behavior and thus avoiding costly fighting. This form of behavior is conditional on the relative size difference between opponents: a male will engage a smaller male in a fight but will switch to pseudofemale behavior if a subsequent rival is larger. There are other mating behaviors that appear to reflect male-male competitive interactions. For example, after attracting females, males of some singing insects switch from song to a more reclusive signal such as substrate vibration, apparently as a way of avoiding courtship behavior that attracts rivals.
HOMOSEXUAL BEHAVIOR AND MATING MISTAKES
A male L. versicolor beetle can be duped into courting a small female-mimicking rival (Fig. 3) and there are a few other examples, such as in some butterflies and dragonflies, of homosexual mating mistakes when certain males adaptively resemble females. Homosexual mounting can also occur among insects of which the males have not evolved to mimic females. This male behavior is widespread in animals and appears to be simply an effect of poor sex recognition; strong selection on males to mate frequently causes them to mount any object that resembles a female. Examples of mating mistakes can even include inanimate objects, such as in the case of Julidomorpha bakewelli, an Australian buprestid beetle, the males of which attempt to copulate with beer bottles with a coloration and reflection pattern resembling the female’s elytra (Fig. 4).
Poor sex recognition appears to be the explanation of why males of another beetle, Diaprepes abbreviatus, mount conspecific males. A difference between this species and others, however, is that females also perform homosexual mountings. In this case, however, mounting appears to be an adaptive reproductive strategy rather than a mating mistake. Laboratory experiments with this species reveal that a
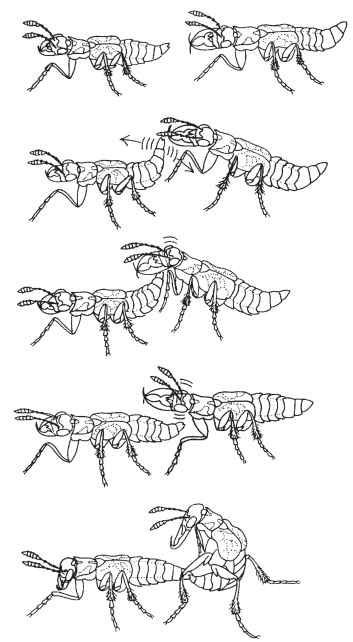
FIGURE 3 A male staphylinid beetle, L. versicolor, can avoid being chased by a rival from the carrion source by mimicking female behavior. The mimic male turns and presents his abdomen to the approaching rival, which antennates the abdominal tip and taps it with his head. Copulation (bottom) is the only stage of a heterosexual encounter that is not represented in these homosexual encounters (because the mimic male breaks up the encounter by walking away).
mounted pair of females attracts males. In fact, large males attempt to mate more often with paired females than with single large or small females. As both the mounting and the mounted females had similar probabilities of copulating with the attracted male, it appears that the mounted pair mimics a heterosexual pair in order to incite the attraction of large, competitive (i.e., high-quality) males.

FIGURE 4 A mating error by a male: a J. bakewelli male mounts a beer bottle. Note how the aedeagus (penis) is extended.
CONCLUSIONS
Observations of insect mating behaviors reveal a great diversity, some of which is a result of plasticity within species. The examples discussed here show how an understanding of the function of both inter- and intraspecific variation in mating behaviors can be gained by examining the consequences of behavior for the reproductive success of the mating male and female. Functions of insect mating that were proposed before the widespread use of the “selectionist” approach (Table I, Nos. 1 and 2) can be subsumed into this framework. For example, movements involved in delivering sperm are undoubtedly subject to sexual selection. And any observations of synchronized courtship in a species inevitably lead to the question of how such synchrony enhances the reproductive success of the male and female. There is a wealth of behavioral diversity for future research, including apterygote insects, a virtually unstudied group and one of great interest because many species lack copulation.
