Moths and butterflies make up the order Lepidoptera, and they are among the most familiar and easily recognized insects. The Lepidoptera is defined as a monophyletic lineage by a suite of more than 20 derived features, the most obvious of which are the scales and proboscis. The scales are modified, flattened hairs that cover the body and wings, shingle-like, and are the source of the extraordinary variety of color patterns typical of these insects. In all but the most primitive forms, feeding by adults is accomplished by pumping in liquid via a tubular proboscis (haustel-lum), which usually is elongate and coiled under the head. The sister group of Lepidoptera, the Trichoptera (caddisflies), lack this development of mouthparts and the covering of scales and possess caudal cerci on the abdomen, which are not present in Lepidoptera.
Like other holometabolous insects, lepidopterans pass through egg, larval, pupal, and adult stages. Mating and egg deposition are carried out by the adult moths and butterflies. Within the eggs, embryos develop to fully formed larvae. The larvae, commonly called caterpillars, feed and grow, which is accomplished by a series of stages (instars). At maturity they transform to pupae, usually within silken cocoons spun by the larvae, although many species pupate without a cocoon. Metamorphosis to the adult occurs during the pupal stage, and the fully developed adult breaks the pupal shell to emerge. Adults of most species feed, but they do not grow. Diapause, an arrested state of development, may occur in any of these stages, prolonging life and enabling the insect to bypass seasons that are unsuitable for growth and reproduction.
The Lepidoptera is one of the two or three largest orders of insects, with an estimated 160,000 named species. Based on specimens in collections and extrapolating from recent studies of Central American moths, we believe that fewer than one-half of the known species have been named by taxonomists; even in North America, an estimated one-third of the fauna is undescribed. Thus, a realistic projection of the total world Lepidoptera species number is not possible, but certainly it exceeds 350,000 and may be much larger. Much of this diversity can be attributed to the radiation of species in association with flowering plants. Lepidoptera represent the single most diverse lineage of organisms to have evolved primarily dependent upon angiosperm plants, and their numbers exceed those of the other major plant-feeding insects, Heteroptera, Homoptera, and Coleoptera (Chrysomeloidea and Curculionoidea). Figure 1 depicts the hypothesized evolutionary lineages and lists currently recognized superfamilies of Lepidoptera.
MORPHOLOGY
Adult
The body framework (Fig. 2) consists of a hardened (sclerotized) exoskeleton made up of a head capsule with appendages; three fused thoracic segments, each with legs, and two pairs of wings, on the middle (mesothoracic) and third (metathoracic) segments; and an abdomen, which has 10 segments, is less sclerotized than the thorax, and is movable by intersegmental membranes. Complex
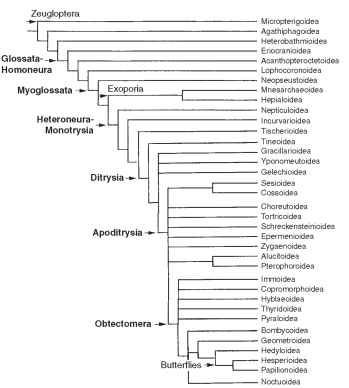
FIGURE 1 Hypothesis of phylogenetic relationships of extant lepidopteran superfamilies. Successively more derived clades representing major morphological changes are indicated in boldface to the left.

FIGURE 2 Schematic representation of the exoskeletal anatomy of a ditrysian moth, with prothoracic leg enlarged below. Head: an, antenna; eye, compound eye; oc, ocellus; l.p., labial palpus; ha, haustellum (proboscis); Thorax: pa, patagium; te, tegula; me, mes-oscutum; w.b., wing base; co, coxa; tr, trochanter; fe, femur; ti, tibia; t.s., tibial spurs; ta, tarsomeres; cl, tarsal claws; ep, epiphysis. Abdomen: tergites and sternites 1-7 and spiracles shown.
genital structures of external origin arise from abdominal segments A8-10, and often there are accessory structures (pouches, glands, hair brushes) associated with sound reception, courtship, or other functions.
HEAD Structures include paired simple eyes (ocelli) and scale-less, raised spots (chaetosema), which are unique to Lepidoptera, although one or both are lost in many taxa ( Figs. 2-4). There is enormous variation in the form of the antennae, often between the sexes of a species, being filiform or with the flagellar segments variously enlarged or branched. Antennae of butterflies are enlarged distally,
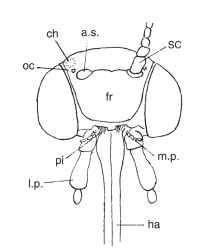
FIGURE 3 Descaled lepidopteran head, frontal aspect. ch, cha-etosema; oc, ocellus; a.s., antennal socket; sc, scape; fr, frons; pi, pilifer; m.p., maxillary palpus; l.p., labial palpus; ha, haustellum, consisting of fused galeae.
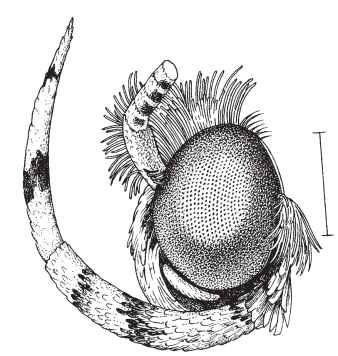
FIGURE 4 Head of ethmiid moth, showing the strongly upcurved labial palpus that is characteristic of most Gelechioidea. Scale bar = 1.0 mm.
forming apical clubs, while those of moths are not, although some moths have distally enlarged antennae that are tapered or hooked to the tip. The mouthparts of the most primitive moth families retain functional mandibles as in their mecopteroid ancestors, but in the majority of moths the mandibles are lost, and the maxillary galeae are elongate and joined to form a tubular proboscis (haustellum) with musculature that enables it to be coiled under the head when not being used to suck nectar from flowers or other fluids into the digestive tract by a pumping action. The maxillary palpi consist of one to five segments and in primitive moths are conspicuous, often folded. The labial palpi are more prominent in most Lepidoptera and vary in curvature and length, but they are not folded.
THORAX The pro-, meso-, and metathorax are fused, each consisting of a series of nonmovable sclerites (Fig. 2). In primitive groups the meso- and metathorax and their wings are similar in size, but in derived families the mesothorax is larger and has more powerful musculature, and the forewing has more rigid vein structure, especially on the leading edge. In the largest superfamily, Noctuoidea, the metathorax is modified posteriorly into a pair of tympanal organs. The tibia of the foreleg has an articulated epiphy-sis on the inner surface, a uniquely derived feature in Lepidoptera, usually with a comb of stout setae, that is used to clean the antennae and proboscis by drawing them through the gap between the comb and the tibia. The wings are tiny and soft at eclosion from the pupa, then rapidly expand by circulation of blood pumped into the flaccid veins, causing them to extend, stretching the wing membranes to full size, after which they rapidly harden, with the membranes pressed closely together, and the system of tubular veins provides structure. Homologies of the six vein systems are discernible across all families of Lepidoptera, and the configuration of veins has been used extensively in classification. In the most primitive moths the forewing (FW) and hind wing (HW) are similar in shape and wing venation (homoneurous) (Fig. 5) , while the more derived groups have lost parts of the vein systems and have fewer remaining in the HW than in the FW (heteroneurous) (Fig. 6). There are various wing-coupling mechanisms by which the FW and HW are linked to facilitate flight. Primitive homoneurous moths have an enlarged lobe at the base of the FW (jugum) that folds under the HW when the insect is at rest but extends over the HW in flight, which does not couple the wings efficiently. Most moths have the HW frenulum that hooks under the FW retinaculum, the development of which varies among taxa and between the sexes of many species.

FIGURE 5 Wing venation of a homoneurous moth (Eriocraniidae). Vein systems: Sc, subcostal; R, radial; M, medial; Cu, cubital; A, anal.
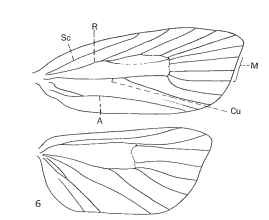
FIGURE 6 Wing venation of a heteroneurous moth (Tortricidae). Abbreviations as in Fig. 5 .
In a few groups (e.g., Psychidae, Lymantriidae) females of many species are flightless, having very reduced wings (brachypterous), or are apterous and may not even shed the pupal skin. Brachyptery has evolved many times independently, such as in high montane and winter-active species of various families in Europe, North America, and Australia. Both sexes are flightless in species of several families on remote southern oceanic islands and in one species of Scythrididae that occurs only on windswept coastal sand dunes in California.
ABDOMEN The abdomen has segments A7-10 or A8-10 modified to form external parts of the genitalia; the sternum of A1 in homoneurous families is small and is lost in other Lepidoptera. Articulation of the thorax and abdomen in derived families is accomplished by musculature attached to sclerotized struts (apodemes) that project from abdominal sternite 2. There are paired tympanal organs at the base of the abdomen in Pyraloidea and Geometroidea. Various male glandular organs associated with courtship occur on the abdomen in several families. Usually these are developed as expandable hair brushes or tufts, or as thin-walled, eversible sacs (coremata), from the intersegmental membrane at the base of the genitalia or on other segments.
The genitalia of Lepidoptera are highly complex and provide the basis for taxonomic species discrimination in most families and often generic or family-defining characteristics. In the male (Fig. 7) the valvae, which are thought to provide clasping stability during mating, usually are large, more or less covering the other structures in repose , and usually are densely setate on the inner surface, scaled exteriorly, and the most visible part of the genitalia externally. The phallus, which is separately articulated and passes through the dia-phragma, is sclerotized and contains the membranous vesica, the intromittent organ. The vesica often is armed with cornuti, which sometimes are deciduous and deposited in the female. Sperm are produced in paired testes and pass through a duct leading to the vesica and are deposited in a spermatophore produced by the male accessory glands during mating. The precise functions of most of the external, sclerotized parts of the genitalia are unknown, and they vary independently in form, being uniform in some taxa, variable in others, and thus of differing taxonomic value from one taxon to another.
I n the female there are three fundamental types of genitalia. Primitive moths possess a single genital aperture near the posterior end of the abdomen, through which both copulation and oviposition occur (monotrysian). Other Lepidoptera have separate apertures for copulation and oviposition; Hepialidae and related families are exoporian (i.e., the spermatozoa are conveyed from the gonopore, or
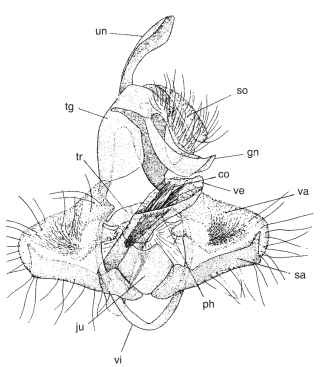
FIGURE 7 Male genitalia of a ditrysian moth (Tortricidae), ven-terolateral aspect with valvae reflexed. un, uncus; tg, tegumen; so, socii; gn, gnathos; tr, transtilla; ju, juxta; va, valva; sa, sacculus; vi, vinculum; ph, phallus (aedeagus); ve, vesica; co, cornuti.
ostium bursae, to the ovipore via an external groove). All remaining families are ditrysian (i.e., having internal ducts that carry the sperm from the copulatory tract to oviduct) (Fig. 8). This feature defines the Ditrysia, comprising most of the superfamilies and more than 98% of the species. The papillae anales typically are soft and covered with sensory setae but in many taxa are modified for various kinds of oviposition, such as piercing. Both the ductus and the corpus bursae are variously modified in different taxa, the corpus often with one or more thornlike sclerotized signa that may aid in retaining the spermatophore. Sperm are transported from the corpus bursae through the ductus seminalis to the bulla seminalis and ultimately to the oviduct. The musculature that controls the ovipositor and papillae anales, often involving extension and telescoping the abdomen, as well as the copulatory aperture, is inserted on the posterior and anterior apophyses.
INTERNAL ANATOMY
Lepidoptera possess the same fundamental internal systems for breathing, blood circulation, digestion, excretion, central nerves, and endocrine functions as do other holometabolous insects (see Diptera, Coleoptera, Hymenoptera, Neuroptera articles).
Egg
With few exceptions, female Lepidoptera produce eggs that are deposited externally after fertilization in the oviduct (Figs. 9 and 10 ). Moth and butterfly eggs vary enormously in size, shape, surface sculpture, and arrangement during oviposition. Within lineages such as families, larger species produce larger eggs, but depending upon the family, the sizes and numbers differ greatly. For example, females of hepialids, including some of the largest moths in the world, produce vast numbers of tiny eggs (20,000-30,000 or more by

FIGURE 8 Female genitalia of a ditrysian moth (Tortricidae), ventral aspect; broken lines represent segments of abdominal pelt. p.an., papilla anale; p.ap., posterior apophysis; a.ap, anterior apophysis; st, sterigma; o.b., ostium bursae; d.b., ductus bursae; c.b., corpus bursae; si, signum; d.s., ductus seminalis; b.s., bulla seminalis.
a single female) that are broadcast in the habitat. Conversely some small moths and butterflies produce few, relatively large eggs.
The shell (chorion) is soft during development and quickly hardens after oviposition, assuming a regular form consistent for the species and often characteristic for genera or families. The chorion may be smooth or strengthened by raised longitudinal ribs or transverse ridges or both. At one end there is a tiny pore (micropyle), through which the sperm enters, surrounded by a rosette of radiating lines or ridges. Two types of egg form are defined, those laid horizontally, with the micropyle at one end, which are usually more or less flat, and those that are upright, with the micropyle at the top. Flat eggs are prevalent in the more ancestral lineages, microlepidoptera, while most derived groups, larger moths and butterflies, have upright eggs with more rigid and ornamented chorion. Eggs of either type are laid singly or in groups; flat eggs are sometimes deposited shinglelike, with the micropylar ends protruding partway over the preceding row (Fig. 9), while upright eggs are arranged side by side, like rows of miniature barrels (Fig. 10). Usually the eggs are glued to the substrate by a secretion of the female accessory (colleterial) glands, applied within the oviduct, sometimes forming a thick, paint-like covering to egg masses. Eggs may be covered with debris collected by the female or hairs or scales from her abdomen or wings or may

FIGURE 9 Shells of the flat type ditrysian moth eggs (Amorbia, Tortricidae), which in this instance are deposited overlapping, in regularly arranged imbricate masses.

FIGURE 10 Eggs of the upright type of a ditrysian moth (Arctiidae).
be surrounded by fences of upright scales, but lepidopteran eggs are not tended or guarded by the adults.
Embryonic development is related to temperature, proceeding more rapidly under warmer conditions, but the rate is physiologically and hormonally controlled in many instances. It requires 7- 14 days in most Lepidoptera but may be delayed for many weeks or months in species that overwinter in the egg stage.
Larva
The head (Figs. 12 and 14) is sclerotized, usually rounded (flattened in leaf-mining species, Fig. 11), with large lateral lobes, each bearing an ellipse of usually six simple eyes (stemmata) ventrolaterally
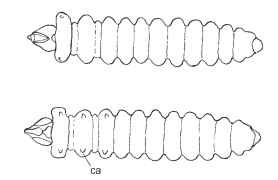
FIGURE 11 Flattened body form ofa leaf-mining larva (Tischeriidae), dorsal aspect above, ventral below. ca, ambulatory calli that represent vestigial remnants of the thoracic legs.
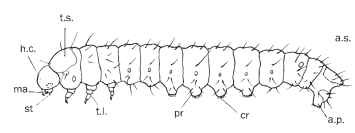
FIGURE 12 Typical form of a ditrysian caterpillar (Cossidae), lateral aspect. h.c., head capsule; ma, mandible; st, spinneret; t.s., thoracic shield; t.l., thoracic leg; sp, spiracle; pr, abdominal proleg; a.s., anal shield; a.pr., anal proleg; cr, crotchets.

FIGURE 13 Body form of Geometridae larva (inchworm), lateral aspect, lacking prolegs on abdominal segments A1-5.
and systematically arranged primary setae and are joined by a median suture, which is flanked by two narrow adfrontal sclerites. The mouthparts may be directed downward (hypognathous) or forward (prognathous). The labium is weak but carries a spinneret behind the mouthparts ventrally, which distributes the silk produced by modified salivary glands. The thorax has spiracles on the meso- and metatho-racic segments, except in some aquatic pyraloids that have external gills. The abdomen usually has spiracles on segments A1-8, restricted to segments A1-3 or absent in some aquatic pyraloids. There are paired, ventral, fleshy, and nonsegmented leglike organs on all segments in the most primitive moths, while on others they are restricted to segments A3-6 (ventral prolegs) and 10 (anal prolegs), equipped with circles or bands of tiny hooks (crotchets) that aid in grasping and walking. The prolegs are fewer in Geometridae (Fig. 13) and some other groups and are lost in some borers (e.g., Prodoxidae), leaf miners (e.g., Eriocraniidae, Nepticulidae), and sand-dwelling larvae (a few Noctuidae). In some groups, A10 has a musculated anal fork used to flip frass away from the larval shelter.
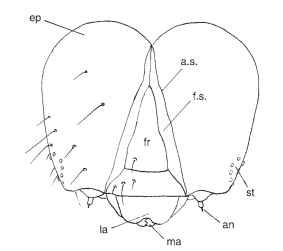
FIGURE 14 Schematic representation of the head capsule of a larval ditrysian moth, frontal aspect. ep, epicranial lobe; st, stem-mata; a.s., adfrontal suture; f.s., frontal suture; fr, frons; la, labrum; ma, mandible; an, antenna.
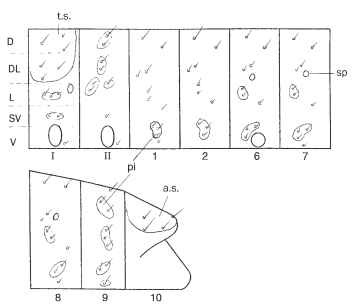
FIGURE 15 Chaetotaxy (setal map) of a larval ditrysian moth (Tortricidae); each rectangle represents one body segment from mid dorsum (upper border) to mid venter (lower border). I, II: pro- and mesothoracic segments; 1, 2, etc.: abdominal segments. Setal groups: D, dorsal; DL, dorsolateral; L, lateral; SV, subventral; V, ventral; t.s., thoracic shield; a.s., anal shield; sp, spiracle; pi, pinacula, which are raised and often pigmented.
There are sensory setae on the head and body integument, and the homology of their primary arrangements (chaetotaxy) (Fig. 15) can be compared in all but the few most primitive families. Their patterns have been valuable to understanding evolutionary trends and to identification of larvae, although the primary arrangement is lost or replaced by numerous secondary setae in many taxa, at least in later instars. The adfrontal sutures, arrangement of stemmata, and crotchet-bearing abdominal prolegs distinguish Lepidoptera from other insect larvae.
Pupa
The head, thorax, and abdomen of the pupa resemble those of the adult and can be recognized externally (Fig. 16). The mandibles of the most primitive families are functional and used to cut open the cocoon preceding eclosion of the adult. In other moths the head is sometimes provided with a beak or other armature that assists in the eclosion process. The appendages of the head and thorax are each encased in cuticle and in most Lepidoptera are fused to the venter of the body, with the wing cases wrapped around, adjacent to the antennae and mouthparts. Abdominal segments A7—10 are fused. In the more ancestral families some of the other segments are movable (Fig. 16A), usually provided with backwardly directed spines or spurs, and the pupa wriggles forward to protrude from the cocoon or burrow just before moth eclosion. Gelechioidea and derived moths (Obtectomera, Fig. 1) and butterflies are obtect, with fused abdominal segments (Fig. 16B and 16C). They remain in place, and adult eclosion occurs along a silken track or other means prepared by the larva or directly from the pupa, in butterflies and some moth groups that do not spin cocoons. Many species have a cremaster, hooked setae at the tip of the abdomen that anchor the pupa inside the cocoon or at the terminus of a silk emergence track, enabling pressure from the emerging adult to break the pupal shell. Others lack the cremaster but are held within a tight cocoon, in an earthen cell, or by a silk girdle. The integument is soft, smooth, and green or whitish when first formed but soon hardens and turns brown in most Lepidoptera. Those that pupate exposed, including butterflies, Pterophoridae, and some Gelechioidea, are mottled green or brownish and often have prominent spines or ridges that aid in camouflage.
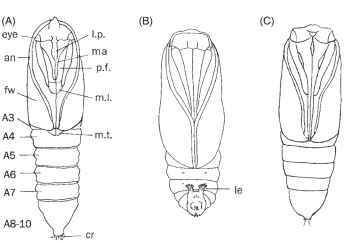
FIGURE 16 Pupae of ditrysian moths, ventral aspect. (A) Tortricidae, with abdominal segments A4—7 movable, enabling pupal movement forward at emergence. (B) Ethmiidae, with pupal movement restricted to flexible segments A5—6, and the pupa remains in place at emergence, a characteristic of Gelechioidea. (C) Noctuidae (Obtectomera) with all segments immobile. l.p., labial palpus; ma, maxilla including galeae (haustellum); p.f., prothoracic femur; m.l., mesothoracic leg; m.t., metathoracic tarsus; an, antenna; fw, forew-ing; A3—10, abdominal segments A3—10; cr, cremaster; le, leglike extensions of the 9th abdominal segment bearing hooked setae that anchor the pupa in lieu of a cremaster.
BIOLOGY
Success of Lepidoptera populations is dependent upon several factors in the climatic and biotic environment, interrelated with the insects’ behavior. First, larval foods, and for most species adult nourishment, must be available. Climatic conditions suitable for mating and oviposition, larval feeding, and pupation are necessary. Females must find appropriate places for deposition of eggs. Larvae must sense proper foods, eat, molt, grow, and pupate. Pupae need to avoid desiccation and other factors that might prevent successful adult eclosion. Finally, egg, larval, and pupal parasites and predators have to combine to take all but two of the offspring of each female (whose eggs may number 200—600 or more) that survive physical dangers, but on average they cannot exceed that, in order to maintain stable population levels.
Adult Behavior
Males usually begin emergence and peak in numbers a few days ahead of females. Both are sexually mature upon eclosion, and males of nearly all moths are attracted by chemical signals (pheromones) emitted by “calling” females. Hence, in most Lepidoptera mating takes place soon after female eclosion, and she has mature eggs ready to be fertilized and deposited within the first 24 h. Mate seeking involves primarily visual cues in most butterflies, although there may be short-range pheromones produced by one or both sexes that mitigate courtship. Males, and females too in most species, mate more than once. It is assumed that sperm precedence prevails, wherein the most recent male’s sperm is effective.
Adults of both sexes of most Lepidoptera feed and in confinement die quickly if water is not available. Feeding on honey-enriched fluids extends the life of some moths and increases fecundity. Most macromoths and butterflies feed at flowers, imbibing nectar, whereas most micromoths do not and apparently gain nourishment from extrafloral nectaries, sap flows, and honeydew secreted by aphids or other Homoptera. Exceptions occur in diurnal microlepidoptera (e.g., Adelidae, Sesiidae, Heliodinidae, Scythrididae, Plutellidae, and Tortricidae, but not nocturnal species of the latter three families), which visit flowers, often other than the larval hosts. The mouthparts are nonfunctional in a few families (e.g., Lasiocampidae, Lymantriidae) and in specialized species such as winter-active Geometridae and Ethmiidae, and females possess mature eggs upon eclosion.
Host-plant selection is made primarily by the female, which seeks by chemical and tactile cues the proper substrate or habitat for ovi-position. This choice is made by instinct, inherited genetically, and the newly hatched larvae also require specific stimuli, detected by chemoreceptors on the antennae and mouthparts; in host-specific species, they starve if the proper plant is not available, ignoring plants or synthetic diets that are quite acceptable and sufficient for nourishment of generalist species.
Most butterflies and moths live only a few days, until mating and egg laying are accomplished, but some are active for several weeks, or they may overwinter as adults and become active on warm days. Some adult microlepidoptera enter a prereproductive state lasting through summer and winter, followed by mating and oviposition in early spring.
Larval Development
The newly formed larva, or caterpillar, first bites its way out of the eggshell, leaving a crescentic slit or ragged hole at the micropylar end. Some species then eat the reminder of the eggshell. All growth takes place during the larval stages, so caterpillars consume enough nutrients to carry through cocoon formation, pupation, and metamorphosis to the adult. It must be sufficient for the moth or butterfly to move to its first feeding or, in species with nonfeeding adults, enough to provide for complete egg development of the next generation. To accommodate growth, the larva molts its skin (cuticle) several times, through successively larger stages (instars). Most Lepidoptera undergo five or six instars, but many larvae that feed on detritus or dry plant material undergo indeterminate numbers of instars.
Silk is produced by paired labial glands. It is composed of two proteins secreted in a viscous fluid in two strands, which consolidate as they leave the spinneret and contact the air. Its functions are many: first instars of many species are dispersed by air currents on silk strands; many or most species lay down a silk line as they move, enabling them to cling to substrates; silk is used by most external-feeding micromoths to form shelters in foliage or other food sources, and some construct portable cases from which they feed; others line tunnels with silk in fruits, stems, roots, or soil from which they forage to feed. Finally, silk is used in cocoon formation preceding pupation, within the larval shelter or gallery or separately, sometimes as a characteristically shaped structure.
Larval habits vary widely and often are quite specific for a family, genus, or species. These include leaf mining, in which a larva spends its entire life within a leaf, and the depth and form of the mines are consistent such that the moth family or genus often is recognizable from the mine (Figs. 17-22). Other types of internal feeding include stem mining; boring in seeds, stems, and roots (Figs. 25 and 26); or

FIGURES 17-22 Leaf mines. (17) Stigmella variella (Nepticulidae) on Quercus agrifolia; (18) mature larvae of Coptodisca arbu-tiella (Heliozelidae, Incurvarioidea) and their abandoned mines, on Arctostaphylos; (19) Cameraria gaultheriella (Gracillariidae) on Gaultheria shallon; (20) Marmara arbutiella (Gracillariidae) on Arbutus menziesii; (21) Phyllocnistis populiella (Phyllocnistidae, Gracillarioidea) on Populus tremuloides; (22) Epinotia nigral-bana (Tortricidae) on Arctostaphylos.
feeding in galls developed by plants, stimulated by the larvae (Figs. 27 and 28). Many external-feeding caterpillars avoid adverse conditions by seeking shelter in leaf litter at the base of the plant or in tunnels during the day and emerge at night to feed, when temperatures are cooler, humidity is higher, and diurnal predators are not active. Many macromoth and butterfly larvae remain exposed, motionless, protected by cryptic coloration, body form, and behavior (Figs. 29-32), or even camouflaged by a coat of flower bits or debris that collect on hooked body setae. Larvae of a few genera live gregariously in silken tents that shield them from climatic extremes (Fig. 33). Many others are protected from vertebrate predators by toxic chemicals they sequester, and advertize their presence by bright colors (aposematic) (Fig. 34).
The duration of larval development varies greatly with the feeding and life cycle types, even within families and genera. The time required to reach maturity also is dependent upon temperature within species, such as between seasonal generations. Most Lepidoptera grow slowly in early instars, increasing in size much more rapidly in later instars, particularly the last. Growth after eclosion from the egg to maturity usually takes 30-50 days, but sometimes is more rapid, as few as 18 or 19 days. Larval life can extend much longer, particularly in species that enter quiescent phases at lower temperatures, intermittently feeding when warmer, or in detritus feeders, which can simply wait long periods when food

FIGURES 23-28 Case-bearers, borers, and gall inducers. (23) Thyridopteryx meadii (Psychidae, Tineoidea), case on Larrea tri-dentata; (24) Coleophora species (Coleophoridae, Gelechioidea) on Malus; (25) larva of Synanthedon sequoiae (Sesiidae, Sesioidea) under bark of a conifer; (26) larva of Grapholita edwardsiana (Tortricidae) in stem of Lupinus arboreus; (27) stem galls induced by Gnorimoschema baccharisella (Gelechiidae) on Baccharis pilularis; (28) stem galls caused by Epiblema rudei (Tortricidae), with newly emerged moth and its pupal shell on Gutierrezia.

FIGURES 29-34 Cryptic and aposematic caterpillars. (29) Oidaematophorus species (Pterophroidae) on Petasites palmatus; (30) sticklike larva of Sicya macularia (Geometridae) on Ceanothus thyrsiflorus; (31) Schizura unicornis (Notodontidae, Noctuoidea) on unidentified tree; (32) Catocala species (Noctuidae) on Quercus kel-loggii; (33) tent caterpillars, Malacosoma californicum (Lasiocampidae, Bombycoidea), on Q. agrifolia; (34) Battus philenor (Papilionidae) on Aristolochia californica. is not suitable. Such species may live 100—140 days before pupation, and those that enter obligate diapause, usually as first or last instar, typically spend 9 or 10 months as inactive larvae in addition to their feeding and growth period.
Larval Foods
The nutritional requirements of many caterpillars are generally similar. Synthetic diets that contain the same basic elements, casein, sucrose, salt, cellulose, wheat germ, amino acids, and vitamins, incorporated in an agar base, are successfully used for rearing many kinds of Lepidoptera. However, sometimes species that are specific to particular plants do not accept a synthetic diet. Hence, nutritional value alone may not be sufficient to elicit feeding, and natural plant chemicals act either as cues for feeding or as deterrents, often the same chemical in both roles with different larval species.
The majority of Lepidoptera caterpillars are phytophagous, consuming living plants, almost exclusively flowering plants, and primarily angiosperms. All parts of plants are eaten, each kind of caterpillar specializing on its particular niche, leaves, flowers, fruit, stems, or roots. Some species feed internally (endophagous) as leafminers and seed or root borers, others externally (exophagous), either concealed in shelters constructed with silk or exposed. Larvae of the most primitive family, Micropterigidae, consume liverworts and mosses or are general feeders on green plants, fern sporangia, or fungal spores in moist habitats. Some other groups of moths do not feed on flowering plants (e.g., Tineidae), but specialize on wood-rot fungi (Polyporaceae) or are detritivores on the ground, under bark of dead tree limbs, or in abandoned insect and spider nests or feed on animal products in mammal burrows, bird nests, or scats, and a few can digest wool. Many species feed on fallen leaves, notably Oecophoridae and Tortricidae on Eucalyptus (Myrtaceae) in Australia, and several groups of Noctuidae in wet forest habitats. Some Lepidoptera specialize on lichens (lithosiine Arctiidae, some Psychidae and Xylorictidae), mosses (some Crambidae), or ferns (unrelated species, mainly on oceanic islands). A few Lepidoptera are predaceous on scale insects or other Homoptera or in ant nests. A Hawaiian geometrid moth (Eupithecia) is predaceous on adult flies, which it catches by seizing the fly with elongate prolegs. Other members of the worldwide genus Eupithecia are plant feeders.
Virtually every kind of flowering plant is eaten by one or more species of caterpillar. Food preferences vary enormously among families; they are summarized in the accounts of the major families that follow. Nearly all internal feeders, such as leafminers, stem and root borers, and gall inducers, and most other microlepidoptera are specialists on one or a few related plants, whereas perhaps half or more of external-feeding macromoth species are generalists within habitats, such as ground-dwelling cutworms feeding on low-growing herbaceous plants or shrub- and tree-feeding species. Most butterfly species are specialists.
Pupal Development
The duration of pupation during which metamorphosis to the adult occurs varies with temperature, usually requiring about 10—12 days, but many species require several weeks or hibernate as pupae, often for 10 months or more.
Pupal movement is an important adaptation in primitive moths and basal Ditrysia. The pupa moves forward just preceding adult eclosion and either anchors by the cremaster to silk or wedges in the emergence aperture, which is prepared by the larva to be slightly narrower than the pupal abdomen. This movement is aided by rows of dorsal, backwardly projecting spines. Gelechioidea and the Obtectomera (Figs. 1, 16B, 16C) have independently derived fusion of abdominal segments that restricts movement, enabling turning within the cocoon but not forward movement, and the adult emerges directly from the pupation site. Pupae respond to tactile stimuli, including potential predators and probing by a parasitoid wasp ovipositor, by turning or wriggling. Some moth pupae have special structures on the abdomen that produce clicking or rattling sounds when the wriggling abdomen strikes the walls of the pupal cells or parchment-like cocoon, or sounds are produced by rubbing fine pegs or rasp-like surfaces on adjacent segments. Such sounds may aid in pupal defense.
Life Cycle
Most Lepidoptera in temperate climates undergo a single annual generation (univoltine), although many have two discrete seasonal broods (bivoltine), and some produce continuous generations as long as favorable temperature conditions prevail (multivoltine). Diapause, a state of arrested development regulated by hormones, controls the life-cycle pattern and enables populations to survive during unfavorable times (winter, dry season, etc.) when necessary resources are not available. Diapause may be the single most important adaptation leading to species radiation of Lepidoptera in northern climates and high mountains, in the world’s deserts and tropical dry season habitats, and in other places where insects could not grow and reproduce continuously. In Lepidoptera, diapause occurs primarily in eggs, in first or last instars, in pupae, or as a reproductive delay in adults, depending on the species. In Mediterranean climates, larval feeding typically occurs in spring when foliation peaks, and diapause lasts through the dry season in summer and hibernation in winter. Some species estivate in diapause as prepupal larvae or pupae, fly in autumn, and then hibernate as adults or eggs. Multivoltine species enter diapause at the end of the growing season, often triggered by decreasing day length, or the larvae simply wait in a quiescent state, feeding slowly on warm days through winter, and metamorphose, and adults eclose with warmer temperature in spring.
Most tropical Lepidoptera are too poorly documented to estimate the proportion of multivoltine to other life-cycle patterns. Some species migrate from wet regions to dry forest habitats at the beginning of the rainy season to take advantage of the newly available resources, but others undergo diapause through the dry season.
Many Lepidoptera are capable of maintaining the diapause to a second or later season if appropriate climatic conditions do not occur. This happens as a regular phenomenon in species adapted to seed feeding on plants with biennial crops such as conifers or sporadically in species that depend upon resources that are limited to a specific season but are erratic in abundance, such as flowering and fruiting by desert plants. Numerous prepupal larvae of yucca moths (Prodoxidae) have metamorphosed synchronously after 8-30 years in diapause under experimental conditions.
SIGNIFICANCE IN NATURAL AND HUMAN COMMUNITIES
The major role of Lepidoptera in natural communities is primary consumer of plants. Moths and butterflies make up the largest single evolutionary lineage adapted to depend upon living plants, in terms of species numbers and, in many communities, in biomass as well. Females of most species produce 200-600 eggs within a few days, vastly more in some species (1000-30,000), releasing a potentially enormous load of caterpillars onto particular plant species or plant groups such as herbs or woody shrubs and trees. Therefore, an important food resource is available for specialized parasitoid wasps and flies, general invertebrate predators such as spiders, mites, ants, and social wasps, and vertebrate predators, especially birds. There have been estimates of 80,000 caterpillars of several species feeding on a single oak tree and many times that number during outbreaks of single species that defoliate forest trees. Thus caterpillars comprise a major component of biological communities, affecting foraging by birds, buildup of yellow jacket colonies, and insect disease epidemics. A secondary role as decomposers also is filled by Lepidoptera. Tineidae, several groups of Gelechioidea (particularly Oecophoridae in Australia), and some Noctuidae and other moths are detritivores and assist in reducing fallen leaves and fruit, fungi, and animal products (hair, feathers, predator scats) to humus. Finally, a few species are secondary consumers, predaceous on scale insects or other Homoptera in natural communities.
Lepidoptera larvae damage plants grown for human use (food, lumber, cotton, garden ornamentals) and our stored products (grain, flour, nuts, woolen clothes and carpets). Most agricultural damage occurs because monoculture crops are grown in places distant from the natural enemies of the pest species, which themselves usually have been introduced by human activities to a new region. Wide-scale insecticide suppression of pest species has further increased problems because local parasites and invertebrate predators are eliminated, and the pest species become resistant to the insecticides by selection for survivors of repeated treatments. Similarly, pests of stored food and wool products have been transported worldwide by human activities. Lepidoptera probably are the most important insect group as plant defoliators (e.g., spruce budworm, the economically most important insect in Canada; larch budworm in Europe) and they cause huge losses by damage to fruits (e.g., codling moth, the “worm” in apples), corn (corn earworm, European corn borer), potatoes (potato tuberworm), cotton (pink bollworm), and many other crops and garden plants. They are a major problem in stored meal, grain, and nuts (Angoumois grain moth, Indian meal moth, Mediterranean flour moth) and woolen products (casemaking clothes moth, webbing clothes moth, tapestry moth, and others). Still others infest bee nests, eating the combs (greater and lesser wax moths).
Conversely, some moths are believed to play significant roles in pollination in natural communities, especially Sphingidae and Noctuidae, and they may aid in crop pollination in some instances. Several Lepidoptera have been purposefully introduced to act as biological control agents against noxious plants. Notable examples include a pyralid, the cactus moth, from Argentina used to successfully suppress millions of acres of introduced prickly pear cactus in Australia; an arctiid, the cinnabar moth from Europe, on tansy ragwort in the Pacific states of North America; and several Mexican species against lantana in Hawaii.
FOSSIL RECORD AND EVOLUTION
A widely accepted phylogenetic hypothesis of relationships among lepidopteran evolutionary lineages, based on morphological characteristics in living forms, primarily of the adults, is shown in Fig. 1. The problem in such analysis is that we do not know what kinds of species might have preceded and interceded with the primitive extant lineages, each of which is now represented by one or a few relict genera that have divergent larval features not shared with other Lepidoptera. Moreover, the fossil record is of little use in revealing clues to “missing links,” and the preservation usually fails to provide information on critical characteristics, particularly those of the larvae and pupae.
Fossil Record
There are fossils of Triassic age assigned to Trichoptera (caddis-flies), the presumed sister group of Lepidoptera, and so branching of the two lineages could have occurred in the early Mesozoic (Fig. 35). The earliest fossil recognized as lepidopteran is a small-scaled wing from the Lower Jurassic of Dorset, England. It was placed in a separate family, Archaeolepidae, suggested as a sister group to the Micropterigidae, but without characters known that might establish its relationships. Four genera were described from Upper Jurassic tuffites from Russia. Among these, two were assigned to Micropterigidae and two to Glossata and Ditrysia, but only one of them, Protolepis, possesses visible mouthpart structures. They were interpreted as a siphon formed of maxillary galeae, which would imply existence of Glossata, 20-30 mya, prior to the radiation of angiosperm plants during the early Cretaceous. That interpretation has been questioned, the structures possibly being maxillary palpi, and therefore the fossil may represent an extinct lineage of Aglossata. By the early Cretaceous there are well preserved Micropterigidae and an incurvariid (Heteroneura) in amber, and by the late Cretaceous several kinds of leaf mines representing modern families and host-plant associations, both heteroneuran (Nepticulidae) and ditrysian (Phyllocnistidae, Gracillariidae), as well as a ditrysian larval head capsule of a free-living form such as Tineidae. That is,
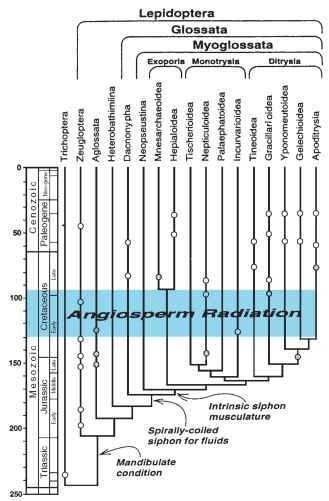
FIGURE 35 Phylogenetic hypothesis of major lepidopteran lineages superimposed on the geologic time scale, with fossil occurrences indicated. Open dots, reliable identifications; shaded dots, questionable assignments. Angiosperm radiation spans 130-95 mya from the earliest recognized occurrence of pollen to the time when angiosperms became the dominant vegetation.
the fundamental clades of Lepidoptera are all represented before the beginning of the Tertiary. Hence, although Lepidoptera is the most recently evolved major insect order, its radiation was relatively rapid, paralleling that of the angiosperms, the major lineages having evolved between ca. 140 and 90 mya.
Morphological Evolution
Major changes in morphological adaptation in adult feeding, ovi-position mode, wing structure, and larval locomotion are indicated by Figs. 1 and 35. The relict moths of ancient lineages (Micropterigidae, Agathiphagidae, Heterobathmiidae) share features of ancestral mecopteroids, functional mandibles in adults and pupae, similar fore-and hind wings with complete venation, and a single female genital aperture. However, larvae of their extant species differ greatly from one another, each adapted for a particular lifestyle. Micropterigid larvae are free-living ground dwellers in moist environments, with well-developed thoracic legs, no crotchet-bearing abdominal prolegs, and
fluid-filled chambers in the cuticle. Agathiphagids are legless borers in primitive gymnosperm seeds with reduced head sclerotization and sutures and few stemmata. Heterobathmiids are flattened leafmin-ers of southern beech, having a prognathous head with prominent adfrontal ridges, as well as seven stemmata laterally and thoracic legs with large, subdivided trochanters (unique in Lepidoptera), but no abdominal prolegs.
Adult Glossata (Eriocraniidae and all subsequent lineages) lack functional mandibles and feed by a proboscis formed of the maxillary galeae. Basal glossatan lineages have a piercing ovipositor and retain functional mandibles in the pupa, used to cut the cocoon at eclosion. The larvae have a spinneret. Several derived features occur beginning with the Exoporia (Mnesarchaeidae and Hepialidae): The ovi-pore and gonopore are separate, connected by an external groove for sperm transfer; the larvae have differentiated prolegs on abdominal segments A3-6 and A10, with circles of crotchets; and silk is used for various activities, not just cocoon formation, the ancestral condition in Lepidoptera. Functional pupal mandibles are lost and there is no piercing ovipositor. Differentiated size, shape, and venation between fore- and hind wings appear in the Heteroneura. The thoracic legs, crotchet-bearing larval prolegs, and silk webbing are lost by larvae of Nepticuloidea, which are severely modified for leaf mining. An independently derived piercing ovipositor occurs in Incurvarioidea, some of which have secondarily legless larvae.
The last fundamental change, leading to the Ditrysia, is the internal system for storage and transfer of sperm from the gonop-ore to oviduct. Evidently this had evolved by the mid-Cretaceous, when larval mines of Gracillarioidea appear in the fossil record. The most successful lineages, in terms of extant diversity, Pyraloidea, Geometroidea, and Noctuoidea, which are defined by independently derived tympanal organs, presumably originated coincident with radiation of the bats during the late Paleocene and early Eocene. The earliest butterfly fossils also date from late Paleocene-Eocene times.
Ecological Scenario
Questions remain concerning the origins of angiosperm feeding in basal lepidopteran lineages that led to major radiations of Lepidoptera. The ground-dwelling larvae of Micropterigidae are generalists, either detritivores or fungivores in leaf litter or feeding on low-growing green plants in moist habitats, including bryo-phytes and soft angiosperm leaves. Similar habits occur in Exoporia (Mnesarchaeidae and Hepialidae, except that many hepialids feed on roots or burrow into stems of woody angiosperms) and in basal Ditrysia (Tineidae, except that none feeds on green plants). By contrast, extant larvae of the other lower Lepidoptera are endopha-gous feeders that specialize on particular flowering plants (larvae of Lophocoronidae and Neopseustidae are unknown, but their ovipositor types indicate that at least early instars are internal feeders). We assume ground-dwelling, generalist habits are similar to those of mecopteroid ancestors of the Trichoptera-Lepidoptera clade, but we do not know if that mode of life persisted in basal members of all lineages through to the Ditrysia. If so, adaptation to endophagy and to specialist angiosperm feeding might have occurred at least four times, in heterobathmiids, in an eriocraniid + acanthopteroc tetid + lophocoronid + neopseustid lineage, in nepticuloids, and, probably independently, in incurvarioids, when a piercing ovipositor reappears, and finally in a palaephatid + tischeriid lineage. If an unknown angiosperm-feeding lineage was the common ancestor, at least two reversals to ground-dwelling, external-feeding, generalist caterpillars characterized by multiple morphological reversals must
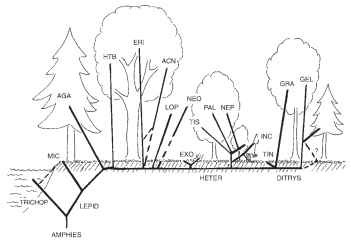
FIGURE 36 Cartoon representing a theoretical scenario of the origins of angiosperm feeding that led to the radiation of Lepidoptera during the Cretaceous. Ground-dwelling mecopteroid-like ancestor gave rise to the Trichoptera—Lepidoptera split, then successively to the ancestor of extant Micropterigidae (MIC) and several specialized, radically differing, angiosperm-feeding lineages. The ancestral ground-dwelling caterpillar form is presumed to have been retained in Exoporia (EXO, Mnesarchaeoidea, Hepialoidea) and basal Ditrysia (Tineoidea). AGA, Agathiphagoidea; AMPHIES, Amphiesmenoptera HTB, Heterobathmioidea; ERI, Eriocranioidea; ACN, Acanthopteroctetoidea; LOP, Lophocoronoidea; NEO, Neopseustoidea; HETER, Heteroneura; TIS, Tischerioidea; PAL, Palaephatoidea; NEP, Nepticuloidea; INC, Incurvarioidea; DITRYS, Ditrysia; TIN, Tineoidea; GRA, Gracillarioidea; GEL, Gelechioidea.
be postulated for exoporians and again for Tineidae. In either scenario, there were independent origins of a piercing ovipositor (at least twice) and endophagous larval feeding accompanied by numerous derived morphological specializations in larvae (several times). Repeated shifts to angiosperm feeding (Fig. 36) may have been facultative, as it is in extant micropterigids, and multiple adaptations to endophagy imply parallel evolutionary trends, a more parsimonious scenario than multiple reversals to an ancestral morphological and behavioral ground plan.
CLASSIFICATION
Historically the Lepidoptera have been classified in four or five suborders, all but one of which are primitive moths that retain ancestral characteristics as relict, morphologically dissimilar groups. All the more derived moths and butterflies, more than 98% of the described species, comprise one evolutionary lineage, or clade, the Ditrysia. In recent decades, much progress has been made in detailed analyses of the relationships of the primitive groups, aided by discoveries of new taxa and previously unknown larvae and pupae. Phylogenetic analyses have shown the primitive lineages to be paraphyletic with respect to the rest of the Lepidoptera (Fig. 1), and consequently, the use of suborders and other ranks between order and superfamily has been abandoned by lepidopterists. In contrast, we continue to recognize the obligate categories (family, genus, species) for purposes of names and communication across related lineages. Historically, the family has been the common denominator level for communication among entomologists, including for Lepidoptera, but in recent decades there has been a proliferation of both family and superfamily divisions such that the superfamily has become a commonly used and understood rank for lepidopterists. Recent authors have treated more than 120 families of Lepidoptera, and there is considerable discrepancy between analyses within some of the 45—48 superfamilies. Morphological and biological traits of the larger, worldwide super-families and families are summarized in the text that follows.
Primitive Lineages
ZEUGLOPTERA—MICROPTERIGOIDEA
Micropteri gidae are the most primitive lepidopterans, living fossils. There are micropterigids recognizable as modern genera preserved in amber dating back to dinosaur times in the early Cretaceous (125 mya). Adults (Fig. 37) are small (FW length 3—6 mm), often colorful, with metallic sheens of bronze or purple and yellow forewing markings, usually active in the daytime. They are characterized by numerous ancestral traits not shared by other moths, most notably retention of functional mandibles, which are used to feed on pollen of various trees in Europe, more primitive plants, sedges, Winteraceae, and fern spores in New Caledonia and Madagascar. A more complete, Mecoptera-like wing venation led to proposal of this group as a separate order, the Zeugloptera, but overall evidence indicates the combined Zeugloptera + other Lepidoptera as a sister group to the caddisflies (Trichoptera). The larvae are wholly unlike caterpillars of other Lepidoptera; they are plump, somewhat hexagonal in cross section, with long antennae and short thoracic legs, and they lack the abdominal prolegs with crotchets typical of most Lepidoptera. The larvae live in moist leaf litter among mosses or in rotting wood, habitats with high moisture conditions; the cuticle has specializations unique among arthropods, with exo- and endocuticle separated by a fluid-filled space leading via pores to chambers in the exocuticle, overlaid by sticky pellicle to which particles of debris adhere. The pattern of primary setae on the body is unlike that of other moth larvae. Larvae of some species feed on liverworts, but most micropterig-ids are generalists, feeding on detritus, fungal hyphae, or angiosperm leaves. About 120 species are known worldwide, in a disjunct, relict-ual distribution pattern. More than half the named species are in the genus Micropteryx in the Palearctic region, while only 2 are known in North America (Epimartyria); there is a greater diversity of genera in the Orient and southwest Pacific, particularly New Zealand, eastern Australia, and New Caledonia, which has about 50 species.
There are two other families of Aglossata: the Agathiphagidae (two species), caddisfly-like moths whose larvae are legless borers in the seed of primitive gymnosperms (Agathis) in Australia and south Pacific Islands, and Heterobathmiidae (nine species), which are similar moths to micropterigids but their larvae are leafminers in southern beech (Nothofagus) in Chile and Argentina.
GLOSSATA—HOMONEURA
The majority of Lepidoptera comprise the Glossata, the monophyly of which is well supported by a suite of derived characters. The most obvious traits that distinguish glosssatans are the adult mouthparts: the mandibles are nonfunctional and maxillary galeae elongated, forming a proboscis that is coiled in repose, accompanied by reduction of the head capsule and its cuticular thickening associated with mandibular musculature. The basal lineages retain ancestral features of the wings: similarly shaped fore- and hind wings with relatively complete venation (homoneur-ous) and the jugal lobe at the base of the forewing. Females in these families have a flattened, sclerotized abdominal apex with serrate edges, forming a “saw,” which is everted to cut into host-plant leaves to deposit the eggs.

FIGURES 37-64 Adults and larvae of microlepidoptera. Micropterigoidea: (37) Epimartyria pardella (Micropterigidae) (California). Incurvarioidea: (38) C. arbutiella (Heliozelidae) ovipositing into leaf of A. menziesii (California); (39) Adela septentrionella (Adelidae) ovipositing into buds of Holodiscus discolor (California); (40) Greya reticulata (Prodoxidae), ovipositing into bud of Sanicula (California); (41) Tegeticula maculata (Prodoxidae) ovipositing into ovary of Yucca whipplei (California). Tineoidea: (42) Tinea pellionella (Tineidae) (Texas); (43) Larval cases of T. pellionella on wool fabric (Texas). Gracillarioidea: (44) Caloptilia reticulata (Gracillariidae) (California). Yponomeutoidea: (45) Atteva punctella (Yponomeutidae) nectaring (Illinois); (46) Ypsolopha maculatella (Plutellidae) nectaring at flower of Asteraceae, whereas the larval host is Ephedra (California). Gelechioidea: (47) Antaeotricha species (Stenomatidae) (Illinois); (48) Ethmia arctostaphylella (Ethmiidae), bird dropping-like resting posture on Eriodictyon, the larval host (California); (49) Larva of Ethmia delliella (Ethmiidae), which feeds on Cordia (Costa Rica); (50) Arotrura longissima (Scythrididae) nectaring at flowers of Senecio, whereas the larval host is Lycium (California); (51) Esperia sulphurella (Oecophoridae, Oecophorinae) (California); (52) Callimima lophoptera (Oecophorinae) (Australia); (53) Coleophora species (Coleophoridae) (California); (54) Holcocera species (Blastobasidae) (California); (55) Telphusa latifas-ciella (Gelechiidae) (Illinois). Choreutoidea: (56) Tebenna gemmalis (Choreutidae) nectaring at flowers of Achillea, whereas the larval host is Wyethia (California). Sesioidea: (57) S. sequoiae (Sesiidae) (California); (58) Castnia species (Castniidae) (French Guiana). Cossoidea: (59) Acossus species (Cossidae) (California). Tortricoidea: (60) Argyrotaenia citrana (Tortricidae, Tortricinae) (California); (61) Synnoma lynosyr-ana (Tortricidae, Tortricinae), flightless female in calling posture on Chrysothamnus, the larval host plant (California); (62) Pseudatteria leop-ardana (Tortricidae, Chlidanotinae), a diurnal and presumed distasteful species (Costa Rica). Alucitoidea: (63) Alucita species (Alucitidae) (Colorado). Pterophoroidea: (64) Platyptilinae species (Pterophoridae) (Costa Rica). (Photographs by: I. Common, 52; C. Covell, 58;R. Coville, 45, 47, 51, 53, 54, 55, 56, 57, 60, 64; H. Daly, 50; J. Hafernik, 42, 43, 59; P. Opler, 62, 63; J. Powell, 38, 39, 40, 41, 44, 46, 48, 49, 61; D. Wagner, 37.)
Eriocranioidea Eriocraniidae form a Holarctic counterpart to the South American Heterobathmiidae, resembling them superficially as adults and larvae, mining primarily in birch and oak (Fagales) in early spring. Adults are small moths (FW length 4-6.5mm) with relatively narrow wings covered by iridescent, simple scales and hairs, often golden with purplish markings. Most are diurnal and fly in early spring just as the host trees are beginning to leaf out. The larvae are legless miners in newly expanded leaves, forming “baggy” full-depth mines. They mature quickly and enter the soil for pupation, and the mines dry and deteriorate after the leaf hardens. The pupae are man-dibulate, and the emerging pharate adult uses the mandibles to cut through the cocoon and reach the soil surface the following spring. Larval foods are birch (Betulaceae), oak (Fagaceae), and other Fagales, or Rosaceae (1 species). There are about 20 species assigned to 5 genera, with about half the species in Europe and Asia and half in North America.
A related family, Acanthopteroctetidae, with two species in the western United States, formerly was included in the Eriocranioidea, has been given superfamily status based on its more derived type of scales and first thoracic spiracle. The larvae of one species are miners in Rhamnaceae.
EXOPORIA
Within the homoneurous Glossata, two super-families comprise the Exoporia, the Mnesarchaeoidea (15 species), a relict group of small, eriocraniid-like moths in New Zealand, and the worldwide Hepialoidea. Monophyly of the two is established by the unique configuration of the female genital system, which is shared by and interpreted as homologous in these otherwise quite dissimilar moths. The copulatory orifice is separate from the ovipore, but there is no internal connection between the two. Sperm is transferred via a groove in the body wall below the ovipore.
Hepialoidea The Hepialoidea is the most successful of the Homoneura and more primitive lineages in terms of extant diversity. The superfamily is characterized by having reduced mouthparts, with the proboscis absent or short and evidently nonfunctional. Hepialidae are large moths, even enormous in some genera, well represented on all nonpolar continents. Four other hepialoid families, Anomosetidae, Neotheoridae, Palaeosetidae, and Prototheoridae, are Southern Hemisphere relicts represented by one to a few species and are smaller moths.
Adults of the Hepialidae are large to very large (FW 10-120 mm), including some of the largest Lepidoptera in the world, Trichophassus in South America and Zelotypia in Australia, with a 10-in. wing span, and often beautifully colored in greens and pinks. The females carry enormous numbers of eggs—one female of Trictena in Australia laid 29,000 eggs and had another 15,000 in her ovaries when dissected— and therefore are bulky, heavy-bodied creatures, surpassing in weight the largest sphingid and saturniid moths. Hepialid males form groups, or leks, that fly together at dusk, as a ritual of courtship behavior; especially suggestive of the common name “ghost moths” is one European species that has white forewings and forms ghostlike clouds. The larvae are elongate, cylindrical, with fully developed thoracic legs and abdominal prolegs that bear rings of crotchets. They have primary setae distributed in patterns that are homologous with those of ditrysian larvae, and they lack secondary setae. Hepialid larvae are concealed feeders, living in silken galleries in leaf litter and grasslands, in tunnels in roots or trunks, feeding indiscriminately on pteridophyte, gymnosperm, or angiosperm plants. Early instars of some species feed on decaying wood and fungi associated with it, and then bore into tree trunks in later instars. There are about 550 named species in 50+ genera worldwide, best developed in Australia and Africa.
HETERONEURA—MONOTRYSIA
All the remaining Lepi-doptera have different fore- and hind wing shapes and venation, with reduced radial system in the hind wing, and the hind wing usually is smaller. They possess a frenulum-retinaculum wing-coupling mechanism, and they have lost the first abdominal sternite. The five most basal superfamilies of the Heteroneura retain the ancestral monotrysian female reproductive system, but they share no derived characteristics that would unite them as monophyletic. The three most diverse of these are Nepticuloidea, Incurvarioidea, and Tischerioidea.
Nepticuloidea These are tiny moths whose larvae are leaf and stem miners. Specialization on diverse flowering plants has led this group to become the most speciose of the primitive Lepidoptera.
Nepticulidae Adult nepticulids include the smallest Lepidoptera (FW length 1.5-4.5mm), characterized by having the basal an-tennal segment (scape) usually greatly enlarged, forming a cap over the upper half of the relatively large eye. The head is rough-scaled, and the mouthparts are primitive, with long, folded maxillary palpi, and rudimentary proboscis with galeae not joined, used to lap up moisture and honeydew secreted by aphids. The FW is relatively broad with long scale fringes. The larvae are legless, obligate leafmin-ers, typically forming a serpentine track beginning just below the egg cemented to the leaf surface, gradually enlarging to a full-depth tube or irregular blotch (Fig. 17). At maturity the larva cuts a crescentic slit in the upper cuticle and drops to the ground to form a tough silken cocoon. Larval foods usually are mature leaves of woody plants, although a few larvae mine stems or cause petiole galls. Individual species are specialists, using more than 40 families of angiosperms, primarily Fagaceae and Rosaceae in the Holarctic; some species groups are specialists on one plant family, such as Anacardiaceae, Polygonaceae, or Fabaceae. There are nearly 800 described species, placed in 11 genera, occurring in all nonpolar regions. No accurate estimate is available, but the total named includes fewer than 10% of the species in tropical regions and probably less than half the North American species.
A related family, Opostegidae (100+ species), are slightly larger moths (FW 1.8-8.3 mm), with enormous eye caps, completely obscuring the eyes from a frontal view. The FW is relatively broad, white with sparse black markings, and the apices often are strongly bent upward. The larvae are leafminers in Rutaceae and cambium miners in stems and fruit of Betulaceae, Ranunculaceae, Polygonaceae, Saxifragaceae in the Holarctic, and Fagaceae in Chile.
Tischerioidea Tischeriidae adults are very small (FW 2.7-5 mm), with lanceolate wings of white, gray, or yellow. They are nocturnal with large eyes and when at rest they perch with the head appressed to the substrate and tail end lifted at a 45° angle. The larvae are slightly flattened leafminers with thoracic legs reduced to two vestigial segments or ambulatory calli, abdominal prolegs rudimentary with crotchets (Fig. 11). The linear or blotch mines are characterized by a heavily silk-lined nest within which the larva retreats when not feeding; the mines have been recorded from nine angiosperm families, most commonly Fagaceae, Rosaceae, and Asteraceae, more diverse on the last than is true of other lepidopterous miners. This group is primarily Holarctic, with about 80 described species in one genus, a few of which are in the Neotropical, Ethiopian, and Indo-Malayan regions, none in Australia and Oceania.
Incurvarioidea These are tiny to small moths having diverse biologies, but females all have a piercing ovipositor specialized for inserting the eggs into plant tissue, often the ovules or young seed. There are six families, Cecidosidae (seven species, gall inducers in South America and Africa), Crinopterigidae (one Mediterranean species, a larval case bearer with habits similar to some coleo-phorids), and four that are diverse and widespread: Heliozelidae, Incurvariidae, Adelidae, and Prodoxidae.
Heliozelidae Species of this family occur worldwide but because of their minute size and diurnal habits they are rarely seen and many more species are known from the characteristic abandoned larval mines than there are named. Adults (Fig. 38) are tiny to small (FW 1.7—7.0mm), typically with iridescent, metallic-appearing scaling. The eyes are small, characteristic of diurnal microlepidoptera. The larvae are flattened, usually legless leaf miners, having a thorax with paired ventral and dorsal movable calli; abdominal prolegs are absent. Early instars form a short, serpentine mine, and then enlarge it to a full-depth blotch (Fig. 18). The last instar constructs a portable case by cutting lenticular disks from the upper and lower epidermis and joining them with silk, giving rise to the common name ” shield-bearers”; the abandoned mines, with their distinctive ” shot holes ” are highly characteristic of heliozelids (Fig. 18). The larva crawls off and descends by a silken thread to attach to a lower leaf or bark, where pupation occurs in the portable case. Heliozelids are host specific, using at least 17 families of usually woody angiosperms, with preponderance in Myrtaceae in Australia and Cornaceae and Vitaceae in the Holarctic, the only Lepidoptera to specialize on the latter. There are more than 100 described species in about 12 genera, distributed in all major faunal realms except New Zealand but poorly known in tropical regions.
Incurvariidae Adults are small (FW 3.5—9mm), with rough head scaling, relatively small eyes, and proboscis short, half the palpi length; maxillary palpi are elongate and folded. They are somber moths with dark, monochromatic wings, sometimes iridescent brown, bronze, or bluish. The larvae are moderately flattened, with well-developed thoracic legs and reduced abdominal prolegs. Early instars form blotch mines; later the larvae cut through the upper and lower epidermis to remove oval sections, which they sew together to form a portable case. Larvae of a few genera remain in the mines throughout feeding, and then cut out a case in which they pupate. Oviposition is host specific; the ancestral, southern-continent genera use Myrta-ceae or Proteaceae, while Holarctic incurvariids use about 10 unrelated angiosperm families. There are about 100 described species in 11 genera, mostly Australian and Palearctic; they are poorly represented in Africa and the Western Hemisphere.
Adelidae (Fig. 39) are best known for their enormously long antennae, often 2.5 or 3 times the forewing length. Usually they are much longer in the male, which in many species possesses greatly enlarged eyes, but the eyes are small in some species, irrespective of antennal length. Holarctic and Neotropical species (Adelinae) are small (FW 4.5—9 mm), diurnal moths, often brightly colored, iridescent green, blue, or purplish, with white antennae, while the primarily African Nematopogoninae are crepuscular or nocturnal and dull colored. Both sexes have a well-developed proboscis and seek nectar from various flowers other than the larval food plant. Males of the large-eyed species form small, dancing groups, reacting to one another during mate seeking. Females insert the eggs into the base of the ovaries of unopened flowers. First instars of the few species studied in detail feed in the developing ovules; after molting they drop to the ground and construct flat, portable cases from silk and debris and feed on fallen leaves or the lower leaves of the host plant, which often are short-lived annuals. Pupation the following spring occurs in the figure 8-shaped case, with the long antennae free and coiled several times around the abdomen. Oviposition is restricted to one or a few closely related plants, which include members of at least 18 angiosperm families. Biologies of the Nematopogoninae of the Southern Hemisphere are poorly known. There are more than 300 species in five genera, occurring in all faunal regions except New Zealand.
Prodoxidae are famous for the close symbiotic relationship between species of Tegeticula and yucca plants (Agavaceae). Females possess enormous “tentacles,” appendages of the maxillary palpi, which are unique among all insects, used to gather pollen that is purposefully transferred to the stigmas while visiting other flowers for oviposition, thus ensuring cross-pollination. Other kinds of insects are not attracted to yucca flowers to collect pollen. Females are believed to leave a pheromone signal at the oviposition sites that deters later visiting females so that only a few larvae feed in any given seed pod and many unaffected seeds are produced. Adults (Figs. 40 and 41) are small (FW 4— 16 mm), generally dull colored, white or gray, although a few Greya and Prodoxus species have patterned or iridescent bronze-colored forewings. The maxillary and labial palpi are relatively prominent but usually shorter than the proboscis. The Agavaceae-feeding prodoxines apparently do not seek nectar, although individuals of Tegeticula maculata have been recorded living up to 9 days in the field. Early instars of the more ancestral genera (Lampronia, Greya) feed in young ovules of the host plant and then leave to spin overwintering shelters on the ground. In early spring they feed in flower beds or foliage shoots of the newly foliating host plants. These caterpillars and those of the pollen-carrying genera are stout, highly mobile, with well-developed thoracic legs, lacking abdominal prolegs, while those of the bogus-yucca moths (Prodoxus) are completely legless and apparently blind, living their entire life within the gallery and pupating there. Prepupal larvae of yucca moths are capable of maintaining the diapause for several years if optimal winter conditions are not experienced, up to 30 years followed by successful, seasonally synchronized development, in experimental trials. Holarctic Lamproniinae and species of the basal prodoxine genus Greya specialize on Rosaceae, Ericaceae, or Saxifragaceae, while the more derived prodoxines are Agavaceae specialists. Prodoxidae are predominately Holarctic, with Lamproniinae mainly Palearctic and Prodoxinae largely Nearctic, with a few species ranging into southern Mexico. About 75 species in 10 genera are known.
Ditrysia
The Ditrysia includes 98% or more of the described species, most of the superfamilies and families, almost all of the external plant-feeding caterpillars, and most of the special adaptations for prey avoidance. All members possess reproductive systems based on separate female copulatory and oviposition orifices with internal ducts for transfer of the sperm.
TINEOIDEA
The tineoids are generally recognized as the most ancestral living group of the Ditrysia. Most tineoids have erect, roughened head scaling and elongate, five-segmented maxillary palpi that are folded, usually longer than the labial palpi, which have lateral bristles, while the haustellum has short, unconnected galeae, used to lap up surface moisture from detritus or fungi. Females of most species possess elongate apophyses of segments A9 and 10 that anchor musculature, enabling the ovipositor to be telescoped outward to inject the eggs into crevices or other niches in the
habitat. Five families are regarded as comprising the superfamily, two of which are worldwide and more species rich, Tineidae and Psychidae. The others are smaller families of restricted distribution, Eriocottidae (70+ species) in the Mediterranean region and southern Africa to Australia and Taiwan, Acrolophidae (280 species) in the Neotropical and Nearctic regions, and Arrhenophanidae (30 species), Neotropical.
Tineidae ( Fig. 42) are slender, small to moderately large moths (FW length 2.5-25mm), usually shining brown, tan, or whitish, with FW patterns of black on pale or yellow on dark. Tineids are most easily recognized by the rough head vestiture and the short (or absent) proboscis. They lack bipectination of the male antennae, characteristic of other tineoid families. The larvae are slender with integument usually lacking color pattern, often living within silken tubes or portable cases (Fig. 43) . All instars have well-developed thoracic legs and abdominal prolegs with a single circle of crotchets. Larval foods—Tineids do not feed on flowering plants; they mostly are generalist detritivores or fungivores, and members of some subfamilies tend to be specialists on animal products such as fur or feathers (e.g., Tineinae). Some are capable of digesting wool, including several cosmopolitan species that feed on woolen clothes and other manmade products. Others are primarily fungus-feeders (e.g., Scardiinae, Nemapogoninae), especially in sporophores of wood-rot fungi (Polyporaceae) or wood permeated by the hyphae, sometimes quite specialized in host preference. Fungivory or detrivory presumably was the ground plan for the family and therefore for the Ditrysia. Larvipary, wherein eggs mature and first instars emerge within an enlarged oviduct in the female, is known in numerous Andean and Indo-Australian Tineinae. Many fewer eggs are produced than by tineids with conventional reproductive systems. There are more than 3000 described species worldwide, probably less than half the number known in collections, especially in tropical regions. These are assigned to more than 300 genera in 15 subfamilies.
Psychidae The common name “bagworms” applies to psy-chids because the larvae live in portable cases constructed from silk, plastered with debris or symmetrically arranged pieces of host plants (Fig. 23) . Adults are small and slender to rather large and heavy bodied (FW length 4-28mm). Males are fully winged, while females of some species may be fully winged, short-winged, wingless, or even larviform and never leave the larval case. Some species are female only (parthenogenetic) or bisexual only in some populations. The head vestiture is roughened, with long, slender scales directed forward, and the antennae often are strongly bipectinate in males, particularly in species with flightless females, but are filiform in both sexes of species having winged females. Nearly all psy-chids are gray or brown without color patterns. Psychid larvae are stout compared to tineids, with the head and thorax larger and more heavily sclerotized than the posteriorly tapered abdomen and variously pigmented. The thoracic legs are well developed and are used to pull the cases along on the host plant, while the abdominal prolegs are reduced. Bagworms feed on lichens, grasses, conifer foliage, or leaves of angiosperm trees and shrubs, sometimes as specialists but often as generalists. At maturity the larva attaches the case to a substrate and then inverts itself and pupates in the case with the head toward the distal (older) end, whence the moth emerges. There are nearly 1000 described species from all faunal regions, about 85% in the Old World. Psychids are generally better studied than most mic-rolepidopterans, owing to their fascinating behavior, biologies, and genetic complexity associated with the larviform females and parthenogenesis in five unrelated genera.
GRACILLARIOIDEA
This is the major clade of Lepidoptera adapted for larval mining in leaves (Figs. 19-21). Gracillarioids primarily mine woody trees, shrubs, and vines of angiosperms and conifers. The larvae are obligate leaf-, stem-, or fruit-miners in early instars; in many genera larvae leave the mines to feed exposed or in webs externally. The adults lack the tineoid lateral bristles of the labial palpi, have a smoothly scaled frons, and usually have a well-developed, elongate, coiled proboscis.
Gracillariidae Adults ( Fig. 44) are nocturnal, often are brightly colored, with the FW patterned in metallic orange, bronze, purple, or yellow. They are small, slender moths (FW 2-10mm) with a head with smooth scaling directed forward over the front and tufts of erect scales on the crown in Lithocolletinae; the antennae are 0.8 times to much longer than the FW and filiform. The HW is lanceolate with scale fringe broader than the wing. The larvae characteristically are hypermetamorphic with more than one form in successive instars; early instars are modified for mining, flattened with legs reduced or lacking, transforming in the third or later instar to cylindrical caterpillars, with chewing mouthparts and fully developed legs. They use silk to buckle the mine into a tentlike shelter or feed externally, often folding a leaf into a tightly closed shelter in which they graze. In some genera a variously modified, nonfeeding instar spins the cocoon. Pupation occurs outside the mine in most genera. Nearly all gracillariids are specialists on one or a few closely related plants, typically woody angiosperms, including more than 80 plant families. More than 2000 species have been described from all major faunal regions, assigned to about 75 genera, and certainly a much greater number remain to be defined, especially in tropical forests.
Phyllocnistidae Adults are tiny, slender moths (FW 2-3 mm) with long antennae, often with shining white or silvery FW, delicately banded with gray and rust distally; HW are lanceolate with a much broader fringe. The larvae are flat with legs reduced to stubs, mouth-parts highly modified for sap feeding; they create extremely long, meandering or regularly zigzag, subcutaneous mines (Fig. 21), often in new, still-soft leaves, causing them to curl conspicuously. More than 20 angiosperm families have been recorded as larval hosts, but many others in Central America are used, judging from the ubiquitous mines, probably all made by undescribed species. Phyllocnistid mines are described from mid-Cretaceous (97 mya) Magnoliidae, the earliest known ditrysian leaf mining. Fewer than 100 species are described, a small fragment of the fauna; mines are found on more kinds of plants at one lowland forest locality in Costa Rica than there are named New World species.
Bucculatricidae Adults are tiny to small moths (FW 2.5-7 mm), most easily recognized by their elongate frons and large, erect tuft of scales on the vertex. The appendages are short, antennae 0.6-0.9 times the FW length. The wings are lanceolate, FW often with tufts of upraised scales. Larvae are hypermetamorphic; they are legless leaf miners in the first two instars and later have well-developed legs, feeding externally as exposed grazers. Bucculatricid species are host-plant specialists, with more than 20 angiosperm plant families recorded, Asteraceae and Fagaceae dominant in the Holarctic; many species use Cupressaceae. There are about 250 described species, distributed on all continents except New Zealand, most numerous in the Holarctic.
YPONOMEUTOIDEA
This superfamily includes a heterogeneous conglomeration of dissimilar microlepidopterans that are grouped by default, that is, the nonapoditrysian Ditrysia that have nonmotile pupae and lack the scaled proboscis typical of Gelechioidea.
Yponomeutidae Adults are slender moths with elongate FW, ranging from tiny (FW 3.2—6.8mm), metallic golden, purple, or gray and white nocturnal Argyresthia to larger (FW 9—15 mm) and brightly colored, diurnal moths in Atteva (Fig. 45) and the white ermine moths in Yponomeuta. Larvae of typical yponomeutines live communally in extensive webs on trees and shrubs, sometimes causing economic damage to fruit trees; those of some Zelleria damage growing tips of conifers. Larvae of Argyresthia are miners in angiosperm buds or conifer foliage. Most species are specific to one or a few plants; Argyresthiinae feed on at least 13 gymnosperm and dicot families, with more than 40% of recorded species on conifers, including 25% on Cupressaceae, a greater degree of adaptation to conifers than by other Lepidoptera. There are about 600 described species, occurring in all biotic regions, with both the phylogeny and the taxonomy in tropical and south temperate faunas yet to be resolved.
Plutellidae and Ypsolophidae traditionally were treated as one family. The adults (Fig. 46) are small moths (FW 6—13 mm) with distinctive labial palpi, with the second segment broadly scaled and the third slender, smooth scaled and upcurved from the second preapi-cally. FW are narrow to lanceolate, with a flared terminal fringe. Large pleural lobes enclose the male genitalia in ypsolophids but are small and narrow in plutellids. These are typically nocturnal moths with yellow, brown, or gray FW, often with linear markings. The diamondback moth (Plutella xylostella) , a ubiquitous pest of cabbage, cauliflower, and other plants of the mustard family, is the best-known plutellid. The larvae are slender, tapered toward both ends, often with elongate abdominal prolegs, and pale green with unpat-terned integument, and they live in slight webs as external feeders. Pupation occurs in large-meshed, open cocoons (Plutella group) or dense, envelope-like cocoons (Ypsolopha group). Most Plutella feed on Brassicaceae, the only moth lineage adapted to do so, while members of this family group as a whole use 50+ families of angiosperms, rarely monocots, and a few gymnosperms, including Ephedraceae and Cupressaceae. Nearly 300 described species are assigned to one or the other of these families, but the systematics relationships and descriptive inventory are incompletely known, especially in Southern Hemisphere faunas.
Glyphipterigidae are small (FW usually 3.2—10mm), diurnal moths with a smooth-scaled head and porrect or decumbent, slightly upcurved labial palpi, often metallic gray; the FW have metallic markings and parallel, white chevron marks from costa and dorsal margin. The last abdominal tergum is greatly enlarged, forming a hood over the genitalia. The larvae are borers in seed, flowering stems, terminal buds, or leaves, primarily in sedges and rushes (Cyperaceae, Juncaceae), less commonly in grasses, and rarely in dicots, including Crassulaceae. Nearly 400 species are described in 20+ genera. The family is cosmopolitan and well represented in temperate, Palearctic, Nearctic, and Australian regions, including New Zealand.
Heliodinidae Adults are tiny, diurnal moths (FW mostly 3.2— 5 mm; rarely to 8 mm), resplendent in shining metallic body and wing scaling, often red or orange with raised silver or lead-colored spots. Adults of many species hold the hind legs aloft when perched, which has been regarded as characteristic of heliodinids, but not all of them do so. Larvae are unpigmented, grub-like, and host specific as leafminers or stem or seed borers, or a few feed externally in flowers and fruit. This is the only lepidopteran family to specialize on Caryophyllales (90% of the known hosts), especially Nyctaginaceae.
About 75 species are described worldwide, but the majority occur in the southwestern United States and Mexico.
GELECHIOIDEA
This is the largest superfamily of micro-moths by far, and because vast numbers of species remain unde-scribed, Gelechioidea may surpass Noctuoidea as the most diverse group of Lepidoptera. For example, even in North America only about one-third of the species known in collections are named, and a total of 4400 species is projected, 1000 more than Noctuoidea. In tropical regions the inventory is imponderably incomplete—more than 1100 species of Gelechioidea have been counted at one rainforest reserve in Costa Rica, comprising 20% of all Lepidoptera believed to occur there, the majority unnamed. In Australia, gele-chioids are estimated to make up about 40% of the Lepidoptera species.
Gelechioids all have overlapping scales on the dorsal surface of the haustellum, up to half its length, and most have a smooth-scaled head, four-segmented maxillary palpi, and upward curved labial palpi with the third segment long and acute (Figs. 4 and 55 ). The pupa is nonmotile (obtect), remaining in the cocoon until emergence of the moth. There is general agreement on the phylogenetic unity of this superfamily, but there have been wide differences of opinion on the number and relationships of the included families in recent analyses. About 25 groups have been treated as families, of which only the larger, worldwide ones are mentioned here.
Elachistid Assemblage This group of taxa is defined by having modified abdominal articulation in the pupa, lateral condyles, on A5—6 and A6—7, that prevent lateral movement (Fig. 16B), although those of Elachistidae s. str. are polymorphic and questionably homologous. The first three groups have been treated as families or subfamilies of Oecophoridae or Elachistidae. They are broad-winged moths with strongly curved labial palpi, often exceeding the top of the head (e.g., Fig. 4).
Stenomatidae Adults (Fig. 47) are small- to moderate-sized (FW 5—25 mm), with rectangular to nearly oval FW. The valvae of male genitalia have setae with prominent, multilobed apices. The male antennae usually have long cilia. Larvae of only a small proportion of described species are known; they are relatively stout caterpillars, often with heavily pigmented integument. They are external feeders in concealed shelters on diverse angiosperms (16+ families), predominately Myrtaceae in the Southern Hemisphere and Fagales in the Nearctic. More than 30 genera and 1200 species are named, 90% from the Neotropical region, where many are not yet described. This group and the Ethmiidae tend to be mutually exclusive on a broad geographical scale. Stenomatids are species rich in the southeastern United States and lowland wet forests of Central America and northern South America, while ethmiids are speciose in arid parts of western North America and thorn forest regions of Mexico, Central America, and the Antilles.
Ethmiidae Adults (Fig. 48 ) are small moths (FW mostly 4— 16 mm, rarely 24 mm), having elongate, narrow FW, often dark with a sinuate pale band along the dorsal edge that renders a bird-dropping appearance when the moth rests, or white with black spotting, superficially resembling Yponomeuta, and some tropical species are colorful. Most are nocturnal, but some high montane species are diurnal, as are a group of species in the southwestern Nearctic that fly in early spring, adapted to use annual plants. The family is characterized by a strongly recurved basal part of the phallus, secondary SV setae of the larva, and two separately derived pupal-anchoring mechanisms, either development of “anal legs,” ventral, setiferous, forward-directed extensions of the ninth segment (Fig. 16B) or grasping of the exuvial head capsule or cocoon silk between abdominal segments A6 and 7. Diapause occurs in the pupal stage, and development can be delayed several years, an adaptation to unpredictable, arid habitats. About 80% of the species for which the often colorful larvae (Fig. 49) are known feed on Boraginales (Boraginaceae, Ehretiaceae, Hydrophyl-laceae). About 300 species are named worldwide, with the greatest richness in areas of seasonal drought, especially microphyllous thorn forests of the northern Neotropical Region. This group is better studied than most micromoths, excepting the African fauna, and most species in collections are described.
Depressariidae Adults are small (FW 7-16 mm) and resemble the two preceding groups, with FW usually rectangular and labial palpi slender and strongly curved upward, lacking the multilobed setae of the valvae, the strongly recurved phallobase, and special pupal-anchoring mechanisms that characterize stenomatids and eth-miids. The larvae mostly are leaf tiers but some bore into stems or seed, using at least 17 families of dicots, with strong specialization on Apiaceae and Asteraceae in the Holarctic. More than 600 species are described, assigned to 80+ genera, occurring in all major faunal realms and best represented in north temperate and tropical regions.
Elachistidae Adults are tiny (FW 2.5-6.5 mm) with narrow wings; the HW fringe is much wider, and FW are usually white or black with white markings. The labial palpi are slender, strongly curved upward. The larvae are flattened, with head prognathous and recessed into the first body segment and legs short, adapted for mining. Elachista typically mine monocots (Poaceae, Cyperaceae, Jun-caceae), while other species are miners in a few dicot families. There are about 250 described species worldwide, but they are mainly Hol-arctic.
Xyloryctid Assemblage The remaining families of Gele-chioidea lack modified lateral articulation on the abdominal segments of the pupa but otherwise are not defined as a lineage by a derived feature.
Xyloryctidae Adults are small to moderately large (FW 1033 mm), nocturnal moths, often brightly colored, shining white or yellow patterned with black or brown, and relatively heavy bodied, having spiniform setae on the posterior part of abdominal terga 2- 6. The labial palpi usually are long, strongly curved, and slender. Larvae are robust caterpillars with a bordered submental plate and secondary SV setae on abdominal segments A3-6. They form silken tubes or shelters in lichens, on bark, or among foliage. Some species feed in bark or tunnel in bark or stems and drag leaves back to the gallery at night. Larval foods are lichens, living angiosperm plants (20+ families), about half the known species on Myrtaceae and Proteaceae, or dead eucalypt leaves. More than 500 described species are assigned to 60+ genera, in Africa, the Indo-Australian region, and Polynesia, with the greatest numbers in Australia.
Scythrididae are tiny to small (FW 3-12 mm) stiletto-shaped moths with narrow wings that wrap around the body, rendering a tapered appearance from thorax to wing tips (Fig. 50). Most are somber colored, gray or brown, with darker or white FW markings, the nocturnal species tending to be white or pale gray with larger eyes, while diurnal scythrids are mostly dark brown with small eyes and visit flowers for nectar. The male genitalia display an astonishing array of forms, from a relatively unmodified gelechioid plan in most nocturnal species to an extremely reduced and modified by fusion, often asymmetrical form that defies interpretation of homologies of the structures, mostly in diurnal forms. A remarkable species on coastal sand dunes in California, Areniscythris brachypteris, is flightless in both sexes, has greatly enlarged hind legs that enable it to leap 20 times or more its body length, and buries itself at night. The larvae are slender, with a small head, tapered toward both ends, usually without integumental markings but with sclerotized rings around setae SD1 on the abdomen. The head capsule has a submental pit in most genera, at least in early instars, which with the setal rings indicates relationship to Blastobasidae. Most species live in frail webs and feed on growing tips of herbaceous plants; some are leafminers in early instars. At least 20 families of angiosperms are hosts, mostly dicots, with Cistaceae and Asteraceae prevalent in the Palearctic; larvae of a few species eat lichens, mosses, grasses, or cacti. There are 700+ described species in 26 genera, with vast numbers awaiting study and naming (e.g., 90% of the North American fauna). Worldwide but most numerous in arid and seasonal drought areas such as the southwestern Nearctic, these moths are rare or lacking in wet tropical habitats.
Oecophoridae (s. str.) As now defined this is a worldwide assemblage of dissimilar moths, with a tremendous radiation of forms in Australia. Adults (Figs. 51 and 52) are small (FW 4-23 mm) with narrow to broad wings, mostly dull colored in Holarctic genera but wildly variable and colorful in Australia, where the FW are patterned in yellow, rose, rust, and browns. The abdominal terga are usually without spiniform setal bands. The larvae are cylindrical, with head often darkly sclerotized, sometimes with reduced numbers of stem-mata and integument usually not pigmented; the thoracic legs and abdominal prolegs are well developed. Most feed on dead plant material, leaf litter, and other vegetative refuse, and the rich fauna in Australia depends mainly on Eucalyptus (Myrtaceae), with about 60% feeding on fallen leaves and 25% on living foliage. There are more than 3000 described species in 500+ genera worldwide; this is the dominant group in Australia, with 2200+ named species in 340 genera and a projected 35-40% of the Lepidoptera fauna. Several species are cosmopolitan household moths whose larvae feed in stored meal, potted plant humus, etc.
Coleophoridae are typically tiny to small (FW 3.5-13mm), very slender moths with lanceolate wings (Fig. 53) ; the HW fringe is much wider than the wing. Usually these moths have rather long, nearly straight labial palpi that project forward, often slightly drooping. Paired patches of spiniform setae on the abdominal terga define their relationship with the Momphinae, which are tiny, stout moths with thick, diverging, upward-turned palpi. Mostly dull colored, yellowish, white, gray, or brownish, the FW often have linear, pale, or dark streaks. The larvae are slender, with very reduced abdominal prolegs in Coleophorinae, which are leafminers in the first instar and then live in a portable case constructed of silk covered with sand grains or pieces of plant material (Fig. 24); they feed by mining outward from the affixed case, forming characteristic, round mines with a central hole, as they move from spot to spot. Larvae of Momphinae are more stout, are grub-like, and feed within growing tips, stems, or galls they cause. Coleophorinae feed on more than 30 plant families, including conifers (rare); monocots, especially Juncaceae; and diverse dicots. Momphinae specialize on Onagraceae (70% of host records), the only Lepidopteran group to do so. More than 1100 species are described, with an estimated 500 unnamed species known in the Nearctic; they exist worldwide but are mainly Holarctic and are absent from Neotropical rainforests.
Blastobasidae Adults (Fig. 54) are small (FW 4-15 mm), nocturnal moths with narrow wings and a short abdomen that bears a conspicuous, transverse row of stout, rust-colored or black, spiniform setae on each segment dorsally. The labial palpi are usually short, strongly curved upward, and appressed to the head. Blastobasids are uniformly dull colored, usually gray, having FW with whitish or black steaks, sometimes tan or yellowish. The larvae are slender, cylindrical, often with heavily pigmented integument, a labium with a submental pit, and SD1 setae usually with sclerotized rings. Larval foods—the larvae are mainly scavengers, living in a wide variety of situations such as abandoned nests of insects, galleries of stem and root borers, and detritus associated with aphid and scale insect colonies, occasionally eating living insects, and a few species feed on living plant material. Owing to their consistently drab appearance and uniform genitalia structures, this family has been neglected in systematics studies. Worldwide, but more diverse in the Nearctic and Neotropical regions, there are 500+ described species and possibly 5—10 times that many awaiting study.
Cosmopterigidae This is a diverse group not defined by any uniquely derived characteristic. The adults are tiny to small (FW 3—13 mm), slender with lanceolate or somewhat broad wings, lacking a gnathos, and having a strongly hooked aedeagus. The larvae are morphologically most similar to Gelechiidae. Habits are diverse, most of these insects are internal feeders, leafminers or bud, stem, bark, or root borers, sometimes causing gall formation by the host plant. Hence they tend to be stout with short legs, without secondary setae and little integumental pigmentation. More than 25 families of angiosperms are hosts. Typical cosmopterigines are leafminers, Cos-mopterix often in monocots; others are seed feeders, and many are scavengers, in and under old bark, dead stems, etc., and in ferns and palms, especially on oceanic islands. Larvae of Euclemensia are pre-daceous on scale insects. Worldwide, there are 1650 described species in 100+ genera. The genus Hyposmocoma in Hawaii is the most famous example, with an estimated 450 mostly unnamed species that occupy diverse larval niches, including living plants, in dead wood or stems, on lichens, some feeding from a portable case, and in freshwater and littoral habitats, analogous to the Galapagos finches, but with a vastly more species-rich insular radiation.
Gelechiidae One of the major families of micromoths, especially in temperate latitudes, the adults are most easily recognized by the hind wing shape, with the terminal margin indented below the acute apex. Adults (Fig. 55) are tiny to small (FW 3—12 mm, a few tropical species to 18 mm). The great majority are nocturnal, somber colored, brown, gray, or black, but some are colorfully patterned. The larvae usually form concealed shelters in new growing tips of trees and shrubs, but many are leafminers at least in early instars, or stem and root borers, and a few live in plant galls they cause (Fig. 27). Some feed in seeds or dead plant materials, while a broad diversity of living gymnosperms and angiosperms (80+ families) are used. A few feed on ferns or mosses, especially on oceanic islands. There are more than 4500 named species placed in 500+ genera and unknown numbers of undescribed species (e.g., estimated 60% of the North American species). These moths are most diverse in temperate zone areas, including deserts and other seasonally arid habitats. Several are important agricultural insects, including the pink bollworm (Pectinophora gossypiella), a threat to cotton growers worldwide; Angoumois grain moth (Sitotroga cerealella), which feeds in stored grains; potato tuber moth (Phthorimaea operculella); and many conifer needle miners.
APODITRYSIA
All the more derived Lepidoptera are grouped in the Apoditrysia by possession of shortened apodemes on the second abdominal sternum that have enlarged bases, contrasted with the more ancestral state, continuations of longitudinal costae (venulae) of the sternal plate. Several superfamilies comprising the non-obtectomeran Apoditrysia retain the ancestral movable and spined abdominal segments in the pupa, which moves forward to protrude from the shelter, facilitating the moth’s eclosion. The more derived superfamilies of this lineage, the Obtectomera (the pyraloids and macromoths), have nonmotile pupae.
Choreutoidea The family Choreutidae is a small group of phe-notypically similar moths that have a scaled proboscis, an independent development from the Gelechiidae and Pyraloidea, and deposit upright eggs. The adults (Fig. 56) are small (FW 2—9 mm), diurnal, with broad wings, which they twitch in a characteristic fashion as they strut jerkily about on host-plant leaves. The male antennae usually have long ventral setae. Choreutids are mostly dark colored, with black or brown wings marked by metallic gray, white, or silver-white; some tropical species are orange, with harlequin patterns. The larvae are slender with elongate abdominal prolegs, living externally in slight webs, from which they graze on leaf surfaces. Larval foods include diverse dicot angiosperms (17+ families), concentrating on Moraceae in tropical regions, Fabaceae, Urticaceae, and Asteraceae in the Holarctic. About 400 species are described worldwide, with many undescribed tropical species.
Sesioidea This superfamily consists of three families, Bra-chodidae, Sesiidae, and Castniidae, the adults of which are markedly dissimilar in appearance and behavior; their proposed relationship is based on subtle features: the eye is more strongly pigmented anteriorly, they have large patagia, and the larvae have an unusual crotchet arrangement, two transverse, uniordinal rows.
Sesiidae These moths are wasplike (Fig. 57), with FW basally narrow and relatively short HW, usually lacking scales except along the veins and distal margin. The wings are tightly coupled, with the posterior margin of the FW bent down, engaging with the upcurved costal margin of the HW, and both have rows of stiff scales that interlock, in addition to the normal frenulum and retinaculum. Sesiids are small to moderately large and heavy bodied (FW length 5—28 mm), diurnal or crepuscular, and almost all species resemble wasps or bees, often startlingly so. This involves not only clear wings and a colorful, banded abdomen, but the legs are modified with tufts, even to the extent of having yellow-tipped scales resembling pollen carried by bees. Alcathoe are black with bright orange wings, and the males have a long, slender, scaled process from the tip of the abdomen, which in flight resembles the trailing leg posture of tarantula hawks (Pom-pilidae, Pepsis). Sesiids often visit flowers with quick, darting flights. The larvae (Fig. 25) are borers in stems, bark, and roots; they are stout, with heavily sclerotized head and mandibles and unpigmented integument. Larval foods include 40+ families of flowering plants, including conifers but not monocots. There are more than 1100 described species in 120 genera, speciose in both temperate and tropical regions. Sesiidae may be the most completely known of any mic-rolepidoptera. Many species are agricultural pests, borers in stems of berry and squash vines, fruit trees, and conifer trunks.
Castniidae are primarily tropical, large (FW 24—190 mm), strong-flying, diurnal moths that have broad, often colorful wings, and the antennae are swollen distally, so they resemble butterflies (Fig. 58). The antennal tip is abruptly narrowed and bears a tuft of long hairs, features shared with Sesiidae. Some species are crepuscular and occasionally come to lights. Larvae are stout, cylindrical, with short legs and a head retractable into the thorax. They feed in plant stems or form tunnels in soil and feed on subterranean plant parts. The larval life requires 4.5 months to 2 years. All confirmed feeding records are for monocots, and several species are pests of sugarcane, banana, or oil palm. There are about 180 species, placed in 33 genera. The distribution suggests a Gondwanan origin, with the subfamily Taschininae in Southeast Asia, the sister group of Castniinae in Central and South America and Australia. Castniids occur in tropical, subtropical, and warm temperate regions and are lacking from most of the Holarctic, southern South America, Tasmania, and New Zealand.
Cossoidea
Cossidae Goat moths are small to large, hawk moth-like, robust moths (Fig. 59) (FW 4-70 mm, rarely to 125mm), having short, usually bipectinate antennae in the male; the proboscis is not functional, reduced to a small triangular lobe; labial palpi are three-segmented, short, and upturned. The wing venation is more primitive than in related superfamilies, with the median vein complete and branched within the cell. Cossids typically are nocturnal, drab, mostly gray with black striae; a few have brown or orange patches. The eggs usually are laid in groups in crevices or under bark and may be produced in vast numbers, 18,000 counted for one species in Australia. First instars disperse and bore into branches or trunks of living shrubs or trees, sometimes living gregariously; later instars are stout, cylindrical, with heavily sclerotized head and mandibles and unpigmented integument. Larvae require 1-4 years to mature. Recorded hosts include at least 17 families of angiosperms, including 1 monocot, with woody legumes (Fabaceae) accounting for 25% of the records. More than 20% of the species are polyphagous. Cossids occur worldwide, with greatest numbers in tropical regions (40% Neotropical), including more than 670 described species placed in 83 genera.
Tortricoidea
Tortricidae This is a large and relatively homogeneous family, with three subfamilies, Chlidanotinae, Tortricinae, and Olethreutinae, each of which, along with several subordinate taxa, at times have been treated as families. Females possess modified papillae anales, which have been rotated 90% from the ancestral lateral position to form flat, expanded pads facing ventrally, usually with the outline of shoe soles. Adults (Figs. 60-62) are small to moderately large (FW mostly 3-25 mm, to 28 mm in the Asian Ceracini), generally with rectangular FW and broader, plicate HW. The antennae are about 0.6 times the FW length, filiform, with sensory setae in males typically short, but long in some groups; the labial palpi are porrect or bent upward but not curved as in Gelechioidea. Most species are nocturnal, with the FW cryptically colored in gray, brown, rust, or tan, but some species have colorful markings. A few are spectacularly polymorphic, notably species of Acleris (Tortricini) —2 species in England have more than 100 named color forms. Because many tortricids are economically important as agricultural and forest pests, there is a vast literature on the biology, ecology, host-plant selection, oviposition behavior, phe-romone chemistry, etc.—for example, more than 6000 references on the spruce budworms (Choristoneura fumiferana species complex) in North America. In all but the most ancestral tribes the eggs are flat, scalelike, and deposited singly in Chlidanotinae and Olethreutinae and the more ancestral tribes of Tortricinae, but derived Tortricinae deposit small to large, symmetrically shingled masses (100-150 eggs) (Fig. 9). Females of the Neotropical tribe Atteriini have thick mats of specialized (corethrogyne) scales of two types on sterna A6 and A7, which they deposit on and as upright fences around the egg masses. Two Australian Archipini and Epitymbiini fence the egg mass with scales from costal tufts on the HW. The larvae are cylindrical without secondary setae, with setal pattern and crotchet arrangements similar to those ofCossidae, usually with little or no integumental pigmentation other than the setal pinacula. Larvae of most Chlidanotinae and Olethreutinae feed as borers in stems (Fig. 26), roots, buds, or seeds, and most are specialists in host-plant selection. Larvae of a few species are miners in leaves (Fig. 22) or conifer needles or cause plant galls (Fig. 28). By contrast, nearly all Tortricinae are external feeders, often polyphagous, that form leaf rolls or other shelters in foliage, but species of Cochylini bore into buds and stems. Pupation usually occurs in the larval shelter or gallery, although some drop to the ground to pupate, especially those that diapause over winter as pupae or pre-pupal larvae in the Holarctic. External feeders of all three subfamilies possess an “anal fork,” used to flip frass away from the larval shelter. An enormous array of plants serve as hosts. A few small tribes (few genera) are specialists, for example, Bactrini on monocots. About 8500 described species are placed in 720+ genera, and incalculable numbers are unnamed in tropical regions—for example, 70-80% of recently studied species of Neotropical tortricines have been previously unnamed. Rich faunas occur in all biogeographic regions. In addition to the spruce budworms, important economic Tortricinae include the light brown apple moth (Epiphyas postvittana) in Australia and fruittree leafroller (Archips argyrospilus) in North America, while Olethreutinae include the codling moth (Cydia pomonella), pea moth (C. nigricana), larch and spruce budworms (Zieraphera), several seed and cone borers (Cydia), and pine tip borers (Rhyacio-nia) in the Holarctic.
Zygaenoidea This is a conglomeration of families of very different appearing moths and larvae. All share two features, larval head retractile into thorax and second abdominal spiracle of the pupa covered by wings. The mouthparts are vestigial in all the families treated here except Zygaenidae. The larvae are stout with the ventral surface slug-like, having short prolegs or suckers, often resembling caterpillars of lycaenid butterflies. Feeding habits vary, including predators of Homoptera or ants. Defensive secretions containing cyanogluco-sides are produced by some zygaenoids. Some families have been intensively studied because of their interesting larval habits, reproductive behavior, or chemical ecology. Taxonomists define 12 families, most of them small, with fewer than 50 species and limited to one geographical region.
Epipyropidae Adults are small (FW 4-10 mm), FW triangular, HW round, blackish or gray, rarely with white or orange HW. The antennae are bipectinate in both sexes, more broadly in the male. The moths are crepuscular or nocturnal but rarely come to lights. Females produce large numbers of eggs, up to 3000 in one African species, that are deposited on foliage of a plant frequented by the planthopper hosts. The larvae are hypermetamorphic, with first instar triungulin-like, slender, tapering posteriorly with long thoracic legs and ventral ambulatory setae. The first instar seeks the host, attaches by means of silk, and transforms to a grub-like larva, which may feed on secretions produced by the homopteran, and the host remains active. The larva is covered by wax secreted by glands in its integument. Pupation occurs away from the host in a dense cocoon impregnated with wax. Epipyropids feed on body fluids and secretions of fulgoroid leafhoppers of several families. There are about 40 described species assigned to nine genera, occurring in pantropical and warm temperate regions.
The Australian family Cyclotornidae (12 species) are similar to the Epipyropidae with an even more bizarre life cycle. Females deposit large numbers of eggs (1400 counted for one female) on vegetation infested with cicadellid leaf hoppers or Psyllidae (Homoptera). The active first instars follow Iridomyrmex ant trails leading to the homopterans. After feeding, the moth larva leaves and molts into a brightly colored, flat, broadened, scalelike larva, which curls its abdomen upward to expose the anus where a secretion is produced that is much sought after by the ants. The cyclotornid is seized by an ant and carried back to the nest, where it feeds on ant larvae and pupae.
Limacodidae Adults ( Fig. 65) are small- to medium-sized (FW 6—35 mm), mostly nocturnal moths with a stout body, relatively short, broad wings, and densely scaled head and body. The antennae are bipectinate in male, often to about half the length of the body. Mostly brightly colored, in yellow, tan, browns, these moths assume a characteristic resting posture, with the body held at an acute angle to the substrate. Larvae are hypermetamorphic, with first instar, which often is nonfeeding, oval, flat, and bearing rows of large spines that are not homologous with basic setal patterns of other Lepidoptera. Later instars (Fig. 66) are lycaenid-like in form, lack abdominal prolegs, and often have ventral suckers on segments A1—7 that adhere to the foliage, aided by a fluid secreted over the cuticle, and they move slug-like by peristaltic waves passing along the sole-like venter. Different species exhibit a great variety of body form, smooth, sometimes with gelatinous warts, with protuberances, spines, and hairs, some of which are stinging (nettle caterpillars), or densely hairy. Later instars often are green, but in stinging species they are brightly colored. The fecal pellets are characteristically cup-shaped. The cocoon, which usually is hard, incorporating calcium oxalate that is secreted by the prepupal larva, has a circular, dehiscent lid. A variety of dicot trees and shrubs serve as larval hosts, with many species polyphagous, and some feed on monocots, including coconut, banana, rice, sugarcane, and other economically important plants. There are more than 1000 described species, occurring in all zoogeographical regions, with greatest numbers in the tropics.
The sister family to the Limacodidae, the Dalceridae (40 species), is limited to the Neotropical region. Adults (Fig. 67) are small moths (FW 6.5—24mm), white, yellow, or orange, with broad, rounded wings and hairy bodies, similar to epipyropids and limacodids in having vestigial proboscis and small labial palpi; the antennae are bipec-tinate, more broadly in males. The larva (Fig. 68) has a brush-like spinneret, dorsum and sides with gelatinous humps secreted from glandular setae, venter with translucent cuticle, thoracic legs short, prolegs not developed, and crotchets absent in early instars, appearing in the final two.
Megalopygidae Adults (Fig. 69) are also similar to Limacodi-dae but generally larger (FW 11—28 mm) and relatively heavy bodied, with FW markings often brightly colored and elaborately patterned and scales hairlike, cleft, or tripartite. The larvae (Fig. 70) are superficially similar to some limacodids, but with three rows of spined protuberances (scoli) bearing variable development of typically urticating setae, sometimes beneath tufts of long, silky hairs like a fur coat (puss caterpillars), with the unusual complement of seven pairs of prolegs, those of A2 and A7 lacking crotchets. Pupation occurs in a tough, tapered cocoon blended into a branch. There are about 260 species, primarily Neotropical, with several genera ranging into the United States. The small subfamilies Somabrachyinae, in the Mediterranean region and Africa, and the Neotropical Aidinae are sometimes separated as families.
Zygaenidae comprise a worldwide group of dissimilar moths exhibiting a large number of remarkable specializations. Nearly all are diurnal and many are slow-flying and brightly colored (apose-matic) (Fig. 71) and are avoided by birds and other predators because both adults and larvae biosynthesize cyanoglucosides. They are able to release hydrocyanic acid by enzymatic breakdown of the cyanoglucosides. This has enabled many to take part in complex mimicry relationships. Moreover, adults and larvae are unusually resistant to cyanide, and naive collectors are startled to see a moth survive for half an hour in a potent vial that kills other moths in seconds. Adults are small to large (FW 5—50+ mm), with head and labial palpi smooth scaled, and they commonly visit flowers for nectar. The antennae are often thick and either clubbed in both sexes or bipectinate in males and narrowly bipectinate or filiform in females. Glands located between the eyes and the base of the proboscis produce a whitish or yellow liquid or foam when the moth is disturbed. They are often metallic colored, particularly vivid in European Zygaena, in bright red and metallic blues. The eggs are deposited in rows, clusters, or overlapping patches and sometimes covered with scales from a special abdominal hair tuft, which in some Australian genera are urticating. The larvae are stout and broad, with the head usually retractile under the extended prothorax; the body is roughened and covered with dense secondary setae. Larval foods include numerous plant families, although the species are mostly host specific. European zygaenines specialize on Celastraceae, cyanogenic Fabaceae, and noncyanogenic Apiaceae; some Australian Procridinae rely on Dilleniaceae, Myrtaceae, or Vitaceae, and the last is used by some Nearctic species. About 1000 species are described, and the family is relatively well studied owing to the colorful forms, diurnal habits, and mimicry associated with the chemical ecology.
Alucitoidea
Alucitidae The many-plume moths are so called because the wings are deeply cleft, so each wing has six fringed, plume-like segments (Fig. 63). The family was classified with the true plume moths (Pterophoridae) from which the alucitids differ by having discrete bands of spines on some or all of abdominal terga 2—7 and a relatively unspecialized pupa formed in a cocoon. Adults are small (FW 3—13 mm), slender moths that are unmistakable by their wing structure, but in two tropical genera with the largest members of the family, the wings are divided only a short distance. The antennae are filiform, proboscis is well developed, and labial palpi are usually fairly long, porrect or upcurved. Most are gray or brown, delicately banded with tan or white. They are nocturnal and collapse the wing plumes when at rest, holding them out from the body so as to resemble narrow-winged pyralids, but when active they strut about with the wings fully expanded, like miniature peacocks. The larvae are borers in flower buds, shoots, or in galls; the body is stout with short legs with short setae on inconspicuous bases. Pupation occurs in the larval shelter or in leaf litter in a cocoon. Larval hosts include at least eight dicot families, especially Caprifoliaceae in the Holarctic and Bignoniaceae and Rubiaceae in Australia and tropical regions; one species is a coffee pest in Africa. There are about 130 species described and likely there are many more in tropical regions.
Pterophoroidea
Pterophoridae The plume moths are recognizable by their deeply cleft wings in all but the most ancestral genera. They lack proboscis scaling and abdominal tympana, the hind tibia is 2 or more times the length of the femur, and abdominal terga 2 and 3 are elongated. Adults (Fig. 64) are small (FW 4—18mm), with long and slender bodies, legs, and wings; FW is cleft for about 0.25—0.33 its length and the HW deeply twice-cleft in most species. When at rest, the plumes are overlaid and rolled under the leading edge of the FW, resembling sticks held out from the body. They usually are nocturnal and dull colored, tan, brown, or gray with paler and darker markings; although a few are colorful members of tropical mimicry complexes. The larvae (Fig. 29) typically are elongate and cylindrical with long

FIGURES 65-92 Adults and larvae of zygaenoid, pyraloid, and macro moths. Zygaenoidea: (65) Parasa indeterminata (Limacodidae) (New Jersey); (66) Larva of Isa textula (Limacodidae) (Maryland); (67) Dalcerides ingenita (Dalceridae) (Arizona); (68) larva of D. ingenita (Dalceridae) (Arizona); (69) Trosia revocans (Megalopgyidae) (Amazonas); (70) larva of Monoleuca semifascia (Megalopygidae) (New Jersey); (71) Zygaena ephialtes (Zygaenidae), pair in copulo (France). Pyraloidea: (72) Petrophila confusalis (Crambidae, Nymphulinae) (California); (73) Pyraustinae species (Crambidae) (Costa Rica); Crambus species (Crambidae) nectaring at composite flower, whereas the larval host is a grass (Arizona). Geometroidea: (75) Urania fulgens (Uraniidae) (Ecuador); (76) Dichorda illustraria (Geometridae) (California); (77) Neoterpes edwardsata (Geometridae) (California); (78) flightless female of Tescalsia giulianiata (Geometridae), a winter moth (California). Bombycoidea: (79) Phyllodesma species (Lasiocampidae) (California); (80) Bombyx mori (Bombycidae), pair in copulo of the commercial silk moth; (81) Eacles species (Saturniidae, Citheroniinae) (Costa Rica); (82) Hemileuca eglanterina (Saturniidae, Hemileucinae), a diurnal species, female ovipositing (California); (83) Argema maenas (Saturniidae, Saturniinae) (Malaysia); (84) Smerinthus cerisyi (Sphingidae) in predator avoidance posture (Utah); (85) Hemaris senta (Sphingidae), a diurnal bumble bee mimic (California). Noctuoidea: (86) Clostera apicalis (Notodontidae) (California); (87) Phryganidia californica (Notodontidae, Dioptinae); (88) larva of P. californica, the California oak moth; (89) Lymantria dispar (Lymantriidae), mating pair of the notorious gypsy moth (Russia); (90) Orgyia vetusta (Lymantriidae), wingless female tussock moth (California); (91) larvae of O. vetusta; (92) Horama panthelon (Arctiidae) (Texas). (Photographs by: E. Buckner, 71; R. Carde, 89; C. Covell, 75, 83, R. Coville, 72, 73, 76, 77, 79, 80, 81, 86, 87, 88, 90; J. Hafernik, 74; C. Hanson, 67; L. Penland, 65, 68; J. Powell, 78, 84, 91; D. Rubinoff, 82, 85, 92; J. Ruffin, 66, 70; K. Sandved, 69.)
prolegs. Most are external feeders on foliage and usually have dense setae that may be forked, clubbed, or glandular, secreting a sticky fluid. Some species are borers in stems and have short setae, even a strongly sclerotized anal shield with two stout hornlike processes resembling the urogomphi of coleopteran wood borers. The pupae are strongly spined or setose and either are formed in the galleries or are affixed to host-plant stems or debris by the anal cremaster to a silk pad, fully exposed, and they can bend and curl over by their movable abdomen. More than 20 dicot families are recorded as larval hosts, principally Asteraceae, usually herbs, but not monocots. The larvae of Buckleria in Europe are remarkable for feeding on sundews (Dros-eraceae). About 1000 species are described worldwide, with many unnamed tropical species.
APODITRYSIA: OBTECTOMERA
Pyraloidea This is one of the largest superfamilies of Lepi-doptera, with more than 17,500 species described and probably at least as many more from tropical regions awaiting study. The fundamental features that define the pyraloids are a basally scaled proboscis, well-developed maxillary palpi, and tympana consisting of paired chambers on the venter of abdominal segment A2. In recent decades specialists have agreed that differences in the morphology of the tympana and other adult and larval characters warrant treating the former Pyralidae as two families, the Crambidae and Pyralidae. While it is difficult to recognize crambids and pyralids as distinct groups on the basis of superficial appearance owing to the enormous variability within each, subfamilies of each family are distinctive. Pyraloids occur worldwide, other than Antarctica, and range from high alpine to low desert and tropical habitats but are most prevalent at low and middle elevations in the tropics. They are highly successful at dispersal and colonization and are especially well represented on oceanic islands.
Pyralidae These are small to relatively large moths (FW 575 mm, mostly under 30 mm) that have the tympanal organs almost completely closed, with their conjunctiva and tympanum in the same plane; they have vein R5 of the FW stalked or fused with R3 + R4 and lateral “arms” at the base of the uncus in the male genitalia; the larvae almost always have a sclerotized ring around the base of seta SD1 on abdominal segment A8 and often around SD1 of the metathorax. The larvae usually are stout and cylindrical, with relatively short legs and setae; the body typically is unpigmented, although some species are well patterned, even brightly colored. Almost all are concealed feeders, most often borers in seed, fruit, or stems or live in tunnels they construct in the soil beneath plants. Many others construct shelters among tied leaves, often of quite tough silk. A variety of flowering plants are hosts, as well as wood-rot fungi (Xylariaceae), dry vegetable matter including seeds, and the papery structure of social Hymenoptera nests (Galleria, Achroia, Aphomia, Galleriinae). Many are household and granary pests that have been transported worldwide by human activities (Corcyra, Galleriinae; Pyralis, Pyralinae; Plodia, Ephestia, Anagasta, Ectomyelois, Phycitinae). A few species are predaceous on scale insects (Laetilia, Phycitinae) or live in ant nests (some Chrysauginae); three genera of Chrysauginae feed in the dung of sloths, and the adults live in their fur. Several species of Phy-citinae have been used for biological control of cactus. The majority of species feed on flowering plants, including conifers (Dioryctria, Phy-citinae); and monocots, including pests of coconut and other palms (Tirathaba, Galleriinae) and corn (Epipaschiinae), and a wide range of forest trees, ornamental shrubs, and crops are damaged, especially in tropical regions. There are more than 6000 described species,about two-thirds of which are Phycitinae, and the tropical faunas are not thoroughly studied.
Crambidae Crambids are more diverse and variable in morphology and biology than pyralids, but all share ” open ” tympanal organs (i.e., with a wide anterior aperture, and the conjectiva and tympanum meet at a distinct angle); vein R5 of the FW usually is not stalked with R3 + 4, male genitalia are without basal uncus “arms,” and A8 of the larva lacks sclerotized rings around seta SD1. About 14 subfamilies are defined, and usually members of each can be recognized on superficial bases. Adults (Figs. 72-74) vary from small and slender to large and stout bodied (FW 3.5-47 mm), with FW narrow and HW plicate and folded under it at rest (e.g., Crambinae, Fig. 74), to broadly triangular with HW similarly colored and held flat (e.g., Pyraustinae, Fig. 73). The labial palpus is prominent, obliquely ascending or porrect (typically very long in Crambinae, snout moths), maxillary palpi are typically small, often with broadened scaling that connects a profile of frons with the labial palpus. Proboscis is usually well developed. Legs are long, and those of males often have structural modifications and/or androconial scale tufts. Larval food habits and morphology vary among subfamilies: Crambinae (lawn moths) live either as ground dwellers feeding primarily on grasses or as stem borers in various monocots; Schoenobiinae are borers in marsh grasses; Cybalomiinae and Evergestiinae specialize on Brassicaceae and Cap-paridaceae; Midliinae are borers in Araceae; Musotiminae larvae feed on ferns, a rare niche for Lepidoptera; many Odontiinae are leafmin-ers or flower, bud, and stem borers, on diverse dicots; and Pyraustinae, which make up 65% of the known species of Crambidae, are mostly webworms but some borers, in an enormous variety of monocots and dicots, pest species of many crops, including corn, bananas, palms, pasture grasses, cucurbits, tomatoes and other solanaceous fruits, coffee and other tropical trees, garden mints, and conifers. Scopariinae are specialists on mosses and lycopods, tunneling in the roots and stems, and on ferns, or on seed-bearing vascular plants. Nymphulinae larvae are aquatic, living either in ponds on vascular plants, often in cases, or in rapid streams, usually under webs on rocks and feeding on algae. Larvae either breathe through open spiracles, living in air-filled cases or stems, or absorb dissolved oxygen through tracheal gills. Pupae are formed in cases in chambers within the plants or in gas-permeable cocoons and breathe through the spiracles. In the Palearc-tic Acentria (Schoenobiinae), females are wingless, enter the water, and are parthenogenetic until a bisexual generation of winged adults late in the season. More than 11,500 species are described, nearly 90% of which are members of Nymphulinae, Crambinae, or Pyraustinae.
Geometroidea This group includes five families, Drepanidae, Epicopeiidae, Sematuridae, Uraniidae, and Geometridae, although they have been separated as two or three superfamilies by some authors, based primarily on the structure of the larval mandibles and tympanal organs. Geometroids typically are broad-winged, with slender bodies, small to large moths (FW 5-78 mm); they have abdominal tympana of structures different from those of pyraloids and lack scaling of the proboscis. Larvae of Uraniidae have well-developed abdominal prolegs, while most Drepanidae and Geometridae have some of the prolegs vestigial or absent.
Drepanidae Adults have internal abdominal tympana unlike any other Lepidoptera, associated with the dorsal-ventral sclerites that connect tergum 1 with sternum 2, opening dorsally. Adults are medium- to large-sized (FW 8-31 mm), broad winged and geometrid-like in typical Drepaninae, often with the FW apex produced or curved; Thyatirinae are stout bodied and noctuoid-like. The antennae usually are short, lamellate or bipectinate to the tip for most of the length, sometimes filiform. The larvae have few secondary setae or rarely numerous but very short setae, sometimes with an eversible vesicle just above the prothoracic coxa in Drepaninae, often with notodontid-like protuberances in Thyatirinae; anal prolegs are usually vestigial but those of A3—A6 are well developed, and the anal shield is conspicuously elongated. At least 20 families of diverse dicots and one monocot (Zingiberaceae) are hosts; some Holarctic species are gen-eralists. More than 650 species are described in 120+ genera, mostly in the Holarctic and Oriental regions; Drepaninae are absent in the Neotropics. A few species are pests of coffee.
Uraniidae This family is defined by the sexual dimorphism of the tympanal organs, which are on the lateral, posterior part of tergum A2 in males and on the lateral part of sternum A2 in females. Adults (Fig. 75) are small to large (FW 7—78 mm), broad winged, usually with a relatively slender body; wings are often resplendent in brilliant, iridescent colors; HW veins are sometimes produced into one or several swallowtail butterfly-like tails. Most are nocturnal, but three tropical genera are day flying, including Urania, some species of which are famous for their massive migratory flights, involving thousands of the spectacularly colored moths. Epipleminae are smaller, nocturnal, and dull colored; they rest with the FW extended and rolled, HW appressed to the body. The antennae are filiform, lamellate, or pectinate, sometimes thickened preapically. The larvae are more or less bare, with few secondary setae, occasionally with spatulate setae, and prolegs are well developed. Larval foods are recorded in about a dozen dicot families, including specializations on Oleaceae, Ascle-piadaceae, and Euphorbiaceae (all known larvae of Uraniinae feed on euphorbs), unusual for Lepidoptera and likely sources of distasteful qualities, and many species appear to be aposematic. Worldwide, these moths are primarily pantropical; around 700 described species are assigned to 90 genera.
Sematuridae are tropical, similar superficially to Uraniinae, often brightly colored with HW tails, but adults lack abdominal tympana, and the antennae usually are distally thickened, like those of skipper butterflies.
Geometridae This is one of the three most speciose families of Lepidoptera. Adults (Figs. 76—78) are small to large (FW 5—55 mm), typically with broad wings and slender body and abdomen with basal tympanal organs in deep, ventrolateral cavities. The great majority of geometrids are nocturnal and rest by day with the wings outspread, with cryptic resemblance to tree bark, lichens, green or fallen, brown leaves, often ornate with lines simulating leaf veins or spots resembling necrotic or eaten areas of foliage. Some genera hold the wings upright, butterfly-style, and their undersides are cryptically colored. Some geometrids are day flying, including early spring species in the Holarctic or mimetic species in tropical regions. A few species that are winter moths or occur at high elevations have flightless females (Fig. 78), and these have the mouthparts and tympana reduced or vestigial. The larvae usually have prolegs of segments A3—A5 reduced or absent (Fig. 13), so the caterpillar walks by advancing in measured, looping steps, from which both the family name and the common name (inchworms) are derived. They are bare but with a variety of colors, protuberances, ornate body forms, and behavior; most are exposed feeders that depend upon cryptic resemblance to flowers, leaves, twigs, etc., to avoid predators (Fig. 30). Most species are general feeders on trees or shrubs. Owing to their worldwide species richness, an enormous variety of gymnosperms and angiosperms are eaten. At least 21,000 species and 1500 genera have been described, and no doubt many remain unnamed, especially in tropical regions.
Several species are defoliators of hardwoods or conifers (e.g., spring and fall cankerworms, Paleacrita vernata and Alsophila pometaria, and the hemlock looper, Lambdina fiscellaria, in North America), but geometrids are not a major pest group.
Bombycoidea These are macromoths that have no thoracic or abdominal tympana. The group is distinguished by deep clefts between the prescutum and the mesoscutum of the mesothorax. There are 12 families, including 4 worldwide groups summarized here that are sometimes treated as superfamilies: Lasiocampidae, Bombycidae, Saturniidae, and Sphingidae. Other bombycoids are Mimallonidae, a mostly Neotropical family with 200 species; Anthelidae, a small Indo-Australian group; Eupterotidae, worldwide with 300 species; Endromidae, Mirinidae (Palearctic), and Carthaeidae (Australian), each with 1 or 2 species; and 2 small Eurasian and African families related to Sphingidae, Lemoniidae and Brahmaeidae.
Lasiocampidae Adults ( Fig. 79) are small to large (FW 9— 80 mm) with moderately broad wings and stout bodies. The eyes usually have fine hairs between the facets; mouthparts are absent or vestigial; antennae are bipectinate to the tip in both sexes; labial palpi are porrect, the first segment with a scaleless patch bearing sensory setae, unique in Lepidoptera. The wings have interlocking tiny setae but no retinaculum and frenulum. Lasiocampids are nocturnal, but males of a few species are primarily diurnal, mostly somber-colored moths, browns and tan. The female often is much larger than the male, with a stout abdomen having a fully developed complement of eggs upon emergence, sometimes deposited in a single large mass. The larvae do not have the fore coxae fused as in other bombycoids. The body is covered with dense, often long, unbranched secondary setae and frequently is brightly colored. Larvae of some genera are gregarious (e.g., Holarctic Malacosoma, tent caterpillars, Fig. 33), and some have urticating hairs that produce a skin rash in humans. Pupation occurs in a dense, parchment-like cocoon. Larval foods are diverse, mainly trees and woody shrubs, Betulaceae, Fagaceae, and Salicaceae in the Holarctic; low-growing Asteraceae, Brassicaceae, and grasses in Africa; and predominately Myrtaceae in Australia but also mistletoes (Santalaceae). Some species are polyphagous. There are about 1500 named species, in 150 genera, occurring worldwide, mostly in the tropics; they are absent from New Zealand.
Bombycidae Including Apatelodidae of recent authors, this family is defined by a few subtle skeletal features and by short pupal galeae that fail to reach the foreleg apices. Adults are small- to medium-sized (FW 9—28mm), nocturnal with broad wings, which usually are held outward from the body when at rest. The mouth-parts are vestigial. The antennae are bipectinate nearly to the tip in both sexes, and the thorax and legs are clothed in long, hairlike scales. Larvae are similar to lasiocampids, but the secondary setae are either short and minute or long in Apatelodinae. The fore coxae are separate (Apatelodinae) or fused. Segment A8 has a middorsal scolus except in Apatelodinae. Larval foods are largely Bignoniacease, Symplocacace-ae, Moraceae, and Theaceae—some species are reported to defoliate commercial tea. There are about 350 species referred to 40 genera, worldwide except in Europe, best represented in the Oriental and Neotropical regions. The silk moth (Bombyx mori, Fig. 80) is the most well-known species; it has been domesticated for centuries and is not known in the wild, its adults no longer capable of flight.
Saturniidae The emperor or giant silk moths include many of the world’s most spectacular moths. They are medium- to very-large-sized (FW 14—130+ mm), broad winged with highly variable color patterns (Figs. 81-83), often with eyespots with concentric rings (hence the family name), which presumably act as a defense mechanism against predators of the sluggish moths, many of which must warm themselves by pumping the abdomen before they can fly. Most genera are nocturnal, some mating only during early morning hours, while species of a few genera are diurnal (e.g., Hemileuca in North America, buck moths, Fig. 82). The proboscis and maxillary palpi are rudimentary or absent. The male antennae are bi- or quadripectinate, those of the female filiform to quadripectinate. The male FW usually is produced apically, while the hind margin of the HW sometimes has short to very long tails. The larvae are stout with a prominent, smooth head and body protuberances that often are branched; secondary setae are numerous but small, mostly on the ventral half of body and prolegs. The scoli of some genera bear poisonous spines that cause nettle-like stings in humans, and females of some species coat the eggs with urticating hairs that cause dermatitis. Abdominal segment A8 usually has a middorsal horn or scolus. Pupation occurs in a strong, sometimes double-walled silken cocoon that may be covered with plant fragments or in the soil without a cocoon. Many saturniids are polyphagous, and an enormous array of plant hosts are recorded—90 genera in 48 plant families recorded for one species of Attacus—but some are specialized feeders. There are about 1500 described species in 165 genera, occurring worldwide except at the highest latitudes, most abundant in moderate to high-elevation habitats, richest in the Neotropical region, particularly the South American Andes.
Sphingidae Sphinx or hawk moths are among the largest, most easily recognized, and best known Lepidoptera. Adults (Figs. 84 and 85) are medium- to very-large-sized (FW 16-90 mm), having a stout body with the abdomen typically tapering posteriorly. The FW is narrow and HW relatively short, its hind margin produced, angulate at the tip of veins 1A + 2A, emarginate beyond. The antennae are distinctive, usually lamellate ventrally or bi- to quadripectinate, tapering toward the apex, which is upturned or hooked; males with two rows of long sensil-lae that meet dorsally; shorter and filiform in females. The proboscis usually is well developed, sometimes much longer than the body, and used to imbibe nectar while hovering in flight, hummingbird-style, and in some habitats sphingids have significant roles in pollination. Most are nocturnal, extremely strong fliers, among the fastest insects, and several are well-known long-distance migrants. Some genera are diurnal, a few resembling bumble bees, with mostly transparent wings (e.g., Hemaris, Fig. 85); such species have fully scaled wings upon eclosion, but after drying, the scales are shed, all but along the margin and veins. Wing coupling is usually by frenulum-retinaculum, a long bristle in males, multiple setae in females. The larvae have a prominent, triangular or globose head; the body is covered densely with minute secondary setae and usually no other setae or protuberances except a middorsal horn or button on A8. The lateral markings are distinctive, each abdominal segment with an oblique stripe ascending posteriorly, those of A7 reaching the base of horn on A8. Color is often polymorphic; they usually feed completely exposed and rely upon cryptic coloration for protection. A distinctive characteristic is their resting pose, with the thorax raised and head turned down, resembling the pose of an Egyptian sphinx. Pupation usually occurs without a cocoon, in soil or ground litter, but rarely in a silken cocoon. Sphingids feed on a very broad range of gymnosperms and angiosperms, often specialists on plants with chemical defenses that repel most insects, including Apocynace-ae, Cleaceae, Solanaceae, Rubiaceae, and Violaceae. There are more than 1200 described species in about 200 genera, distributed worldwide, best represented in the tropics. It is probably better inventoried and cataloged than any other moth family.
Noctuoidea This is the largest superfamily by far, with more than 7200 genera proposed for nearly 60,000 species, about 40% of all described Lepidoptera. As such, there is a tremendous variety of form, size, color, morphology, and behavior in both larvae and adults. Four major, cosmopolitan families are recognized, Notodontidae, Lymantriidae, Arctiidae, and Noctuidae, from which several regional lineages have been split as families by some authors. Monophyly of Noctuoidea is unequivocally based on complex metathoracic tym-panal organs and associated abdominal structures. The tympana are assumed to have evolved in response to bat echolocation, and many species have been observed to engage in bat avoidance behavior upon receipt of their sounds. Tympana may also receive mating signals, especially in Arctiidae.
Notodontidae With Thaumetopoeidae and Dioptidae included as subfamilies, this is a large and diverse family. Adults (Figs. 86 and 87) are medium- to large-sized (FW 16-50 + mm), typically with relatively long FW and stout body that extends 2 or more times the width of the HW. The head often has scale tufts or crests; antennae are usually bipectinate to the tip in the male, filiform or sometimes bipectinate in the female. Proboscis is usually well developed and coiled; labial palpi are often quite short. The abdomen is densely covered with long, slender scales and sometimes dorsal scale tufts at the base. The tips of the tibial spurs are serrated. These are mostly dull-colored, tan, brown, or gray moths, but many tropical dioptines are diurnal and brightly colored, involved in mimicry complexes. The larval body is stout, nearly bare, sometimes with long secondary setae, often possessing one or more protuberances, a modified body form (Fig. 31), a median knob or horn on A9, or anal prolegs modified into slender, single or double caudal processes (stenopods). All larvae except Dioptinae have two MD setae above the spiracle on abdominal segments, whereas other noctuoids have only one. Late instars have a smooth mandibular cutting edge, derived from the serrate ancestral state in other noctuoids. When disturbed, some species emit formic acid or ketones from a cervical gland (adenosoma). Many have various cryptic colors correlated with modified body forms, but others are brightly colored and aposematic, especially Dioptinae (Fig. 88). Larval foods include a wide diversity of dicot angiosperms, mainly woody shrubs and trees, and a few feed on grasses. Many specialize on plants containing toxic substances, including Anacardiaceae, Apocynaceae, Aristolochiaceae, Fabaceae, Passifloraceae, and Violaceae. There are more than 2800 described species, distributed worldwide except in New Zealand and the Pacific Islands and particularly rich in the Neo-tropics. A few are occasional defoliators of orchard or forest trees.
Lymantriidae The tussock moths are so called because larvae of many have thick tufts of erect secondary setae on the dorsum, like those of a toothbrush (Fig. 91). Adults are small to moderately large (FW 7-45 mm), nocturnal, usually dull-colored moths, mostly brown or yellow; they are usually broad winged and densely hairy; the FW is triagnulate, HW rounded, hidden under the FW at rest, with the wings appressed to the substrate and the densely hairy forelegs extended in front of the head. Females of some genera are flightless, their wings vestigial (Fig. 90). Antennae of males and usually females are bipectinate to tip. The proboscis is vestigial or absent. Females have a large, abdominal tuft of deciduous scales used to cover the egg masses. Males of many genera possess a tymbal organ, a pair of finely corrugated pockets on A3. The larval integument often is brightly colored, with hair tufts from verrucae, but the body form is not strongly modified as in notodontids. Lymantriid larvae all possess a middorsal, eversible gland, often yellow or red, on segment A6 and usually another on A7. The larval hairs of Euproctis in Australia
and some other genera are hollow, barbed, and urticating and cause a severe skin rash in humans. The body hairs are woven into the cocoons and often retrieved by the emerging female and redeposited on the egg masses and then used by first instars, which feed in protected aggregations. Many lymantriids are polyphagous, frequently on arborescent shrubs or trees, and a broad array of plants is eaten. Appreciable generic radiations feed on flowers and fruit of low herbs and grasses, and many Asian and African species feed on algae, fungi, and detritus. There are more than 2500 species placed in 360 genera, distributed in all geographic regions, reaching their greatest development in the Old World tropics. Many species in several genera are forest defoliators in Europe, North America, and Indo-Australian tropics, the most notorious being the Palearctic gypsy moth (Lymantria dispar, Fig. 89), which was introduced into North America in the late 1800s.
Arctiidae The tiger moth family includes Ctenuchidae and Pericopidae, formerly treated as families, which now are interpreted as artificial groupings. There are three subfamilies, Lithosiinae, Syn-tominae, and Arctiinae, all characterized by a pair of dorsal, eversible, single or branched pheromone glands from the terminal abdominal segment in the females. In addition, many arctiines and lithosiines and some syntomines have metathoracic tymbal organs in both sexes. Sound is produced by contraction of muscles that deform ridges on the tymbals rapidly to produce bursts of ultrasonic clicks, stimulated by tactile cues or in response to hunting signals of bats. Many species of all three subfamilies have prothoracic glands from which a liquid is extruded containing acetylcholines and histamines and probably pyro-zines, the odor of which is believed to signal distasteful or toxic properties to predators. Adults (Figs. 92 and 93) are small to moderately large (FW 5—50 mm), usually brightly colored moths with a myriad of patterns, predominately orange, red, and black, often aposematic, advertising their toxic qualities and involved in mimicry complexes, or they resemble wasps, with mostly scaleless wings. Females of some species have a large tuft of abdominal scales used to mix with or cover egg masses. Most arctiids are nocturnal and come to lights, even many tropical species with elaborate Hymenoptera resemblances. Some genera are strictly diurnal, such as North American Ctenucha, Gnophaela, and Lycomorpha that accompany aggregations of lycid beetles they mimic. Larvae of many arctiids have dense secondary setae over the body (woolly bears, Fig. 94), but setae are sparse in some arctiids, especially Lithosiinae. The body form is typically cylindrical with a full complement of prolegs; most are rapid crawlers and can move great distances in search of food or pupation sites. Lithosiinae possess an enlarged basal molar area of the mandibles, used to macerate algae and lichens, which are the principal foods (rarely liverworts and mosses). Some Syntominae also feed on algae and lichens, many are scavengers or fungivores, and some feed on flowers, especially Asteraceae, or grasses. Arctiinae are polyphagous plant feeders or specialize on one of a variety of angiosperms, some genera on latex-producing plants (Apocynaceae, Euphorbiaceae, Moraceae) or those with toxins (Asteraceae, Boraginaceae). More than 6000 species are described in 750 + genera, occurring worldwide, particularly rich in tropical regions. Tropical lithosiines are poorly studied, and there are numerous undescribed species.
Noctuidae This is the largest family of Lepidoptera, with more than 35,000 species grouped into about 30 subfamilies, many of which have been regarded as families, including Pantheinae and Nolinae, by recent authors. The composition and classification of most of these subfamilies is debatable, with critical larval characteristics unknown for most genera. Members of this vast lineage do not share a distinguishing derived feature; they posses the wing venation and tympanal form of advanced noctuoids, retain a well-developed proboscis, and lack the eversible abdominal gland present in larval Lymantrii-dae and the eversible pheromone glands of arctiid females. Adults ( Figs. 95—97) are small to very large (FW 4—140 mm), including Thy-sania agrippina in Central America, with the world’s largest insect wing expanse, exceeding 10 in. The FW is triangular to narrow and the HW broad, usually folding fanlike under the FW when at rest. The body is relatively heavy, the thorax having powerful musculature in most noctuids, and many of these moths migrate long distances. The head has long scales, sometimes forming erect tufts or a conical projection in front; the proboscis is long and coiled, its tip armed with thorns for piercing fruit in some genera. Wing coupling is accomplished by frenulum—retinaculum, usually a single bristle in males, two or three finer bristles in females. The abdomen often has eversible coremata or tufts or pouches of specialized scales. Most noctuids are nocturnal and somber colored, with the FW cryptic against bark or leaf litter when the moths are at rest during the daytime. Some have brightly colored HW that are flashed when the insect is disturbed, presumably having a startle effect on would-be predators (e.g., Catocala, underwings, Fig. 96) . Other Catocalinae hold the wings out flat at rest, with the HW cryptically colored, matching the FW. Some noctuids are diurnal and brightly colored, like flowers they visit, while many high-latitude and montane species are diurnal and dark colored. The larvae typically are cylindrical robust caterpillars, bare with only primary setae (cutworms). Those of some Catocalinae have lateral fringes that appress to the substrate, eliminating shadows (Fig. 32). Acronictinae, Pantheinae, and Nolinae have various secondary setae, sometimes in rows or as tufts on verrucae similar to the arctiids. The prolegs are reduced in some subfamilies, with those of segments A3—5 nonfunctional (semiloopers), especially in sedentary species that feed on herbs. Noctuids feed on all kinds of plants, probably nearly every gymnosperm and angiosperm family. Many are polyphagous, foraging on low-growing plants at night, while others are specialists on one or a few plants, including those with toxic chemicals or latex, such as Anacardiaceae, Apocynaceae, Asclepiad-aceae, Euphorbiaceae, Moraceae, Urticaceae, and Vitaceae, as well as grasses and sedges, Liliaceae and Amaryllidaceae. Several groups feed on fallen leaves or in plant detritus (e.g., Hypenodinae) or on algae, lichens, and fungal-ridden plant matter. Several species are predaceous on scale insects or feed on detritus in spiderwebs or mammal nests. Many are important economically worldwide, especially polyphagous cutworms and armyworms (e.g., Agrotis, Autographa, Trichoplusia, Heliothis, Pseudaletia), eating soybean, sugarcane, cereal, legume, rice, and other field crops. There are more than 35,000 named species in 4200+ genera, worldwide, occurring from high elevations above timberline to low deserts and especially numerous in tropical regions.
Hedyloidea Hedylids are peculiar moths that superficially resemble geometrids, with which they were classified until recently proposed as the ancestral butterfly lineage. The hypothesis is based on 10 characteristics: mesothoracic aorta configuration, six features of the adult skeletal structure, an upright egg, larval anal comb, and pupal girdle. None, however, is unique or universal for all members of either Hedyloidea or Papilionoidea to affirm their mono-phyly. Probably these resemblances evolved independently, as they are not present consistently in ancestral members of the respective butterfly lineages. Adults (Fig. 98) are nocturnal, medium-sized (FW 16—32mm), with broad, semitranslucent wings, weakly scaled in patterns of gray or brownish and white. Wings coupled by a reti-naculum and frenulum with a single bristle in the male, weak bristles

FIGURES 93-116 Adults and larvae of Noctuoidea, Hedyloidea, and butterflies. Noctuoidea: (93) Apantesis (Grammia) virgo (Arctiidae) (E. U.S.); (94) larva of Lophocampa maculata (Arctiidae) on Salix (California); (95) Megalographa biloba (Noctuidae) (California); (96) Catocala species (Noctuidae) (California); (97) Xanthopastis timais (Noctuidae) (Florida). Hedyloidea: (98) Macrosoma species (Hedylidae) (Ecuador). Hesperioidea: (99) Phocides species (Hesperiidae, Pyrrhopyginae) (Ecuador); (100) Autochton cellus (Hesperiidae, Pyrginae) (Texas); (101) Poanes melane (Hesperiidae, Hesperiinae) (California); (102) larva of Hesperiidae (California). Papilionoidea: (103) Papilio rutulus (Papilionidae) (California); (104) larva of Papilio polyxenes (Papilionidae) (Costa Rica); (105) Anthocaris stella (Pieridae) (California); (106) Lycaena (Tharsalea) arota (Lycaenidae, Lycaeninae) (California); (107) Callophrys (Incisalia) eryphon (Lycaenidae, Theclinae) (California); (108) Plebeius acmon (Lycaenidae, Polyommatinae) (California); (109) larva of P. acmon on Eriogonum, tended by ants (California); (110) Apodemia mormo (Lycaenidae, Riodininae) nectaring at Eriogonum, the larval host (California); (111) Vanessa tamea-mea (Nymphalidae, Nymphalinae) (Hawaii); (112) Cercyonis oetus (Nymphalidae, Satyrinae) feeding on ripe fruit (Nevada); (113) Agraulis vanillae (Nymphalidae, Heliconiinae) (California), (114) larva of A. vanillae on Passiflora (California); (115) Ithomiini species (Nymphalidae, Heliconiinae) (Costa Rica); (116) Morpho peleides (Nymphalidae, Morphinae) (Costa Rica). (Photographs by: C. Covell, 98, 99; R. Coville,95, 96, 97; J. Hafernik, 100, 105, 106, 109; W. Hartgreaves, 111; W. Middlekauff, 102; P. Opler, 115; J. Powell, 94, 101, 103, 104, 107, 108, 110, 112, 113, 114, 116; unknown, 93.)
in the female, typical of most ditrysian moths. The resting posture is characteristic, with the thorax titled so the HW nearly touch the substrate, and the slender abdomen is raised above them. The head is small, eyes are large, proboscis is well developed, labial palpi are ascending. The antennae are usually filiform, bipectinate in a few species, lacking the apical club of butterflies. There are small, tympana-like structures at the base of the FW, similar to those of some Nymphalidae, and there are no abdominal tympana that would link hedylids with Geometridae. The forelegs of males are reduced and not used for walking, like nymphalid butterflies. The egg is pierid-like, upright, spindle-shaped, and ribbed. The larval head is bizarre with elongate, trifid, barbed horns, similar to some nymphalids; the body is smooth and slender, and the last abdominal segment is bifid, somewhat like some notodontid moths and satyrine butterflies. The pupa is exposed, anchored by a silken girdle, similar to Pieridae and some Papilionidae. Recorded larval foods are Euphorbiaceae, Malpighiaceae, Malvaceae, and Sterculiaceae. There are about 40 species, all in the genus Macrosoma, restricted to the Central and South American tropics, Cuba, and Trinidad.
Hesperioidea Hesperiidae includes six subfamilies, and the giant skippers, formerly accorded family status (Megathymidae), have been relegated to a subset of the subfamily Hesperiinae by recent authors. Adults (Figs. 99-101) are small to moderately large (FW 835 mm), stout bodied with powerful thoracic musculature; they are called skippers because of their quick, darting flights. The third axillary sclerite at the FW base is unusually wide and forms an irregular Y-shaped structure for muscle attachment, a defining character for Hesperiidae. The HW has an area of very small, specialized scales at the base of R + Sc, also not found in other Lepidoptera. Skippers typically perch with the wings outspread (Pyrginae, Fig. 100) or hold the HW out horizontally and the FW upright, slightly cocked open (Hesperiinae, Fig. 101), or they close both wings above the body like other butterflies, especially when taking nectar. The head is broader than the thorax, with the antennae widely separated and enlarged distally into a club with its tip (apiculus) attenuated and curved. The flagellum is strongly bent at the club in Pyrrhopyginae. The proboscis is well developed, and nearly all species feed at flowers, bird droppings, or other nutrient sources. Most skippers are rather drab, predominantly tan, brown, gray, or black, but many tropical species are colorful. The larval head is prominent (Fig. 102), frequently with protruding lobes, separated from the body by a constricted “neck” in all but the Megathymus group, a unique condition in Lepidoptera. In megathymids the head is narrower than the thorax, and the pupa moves in the larval tunnel, protruding at adult eclosion, a unique reversal among Obtectomera. The terminal body segment has an anal comb, analogous to that of some moths, used to flip frass from the larval shelter, sometimes remarkable distances. Trapetzinae in Australia feed on monocots, mainly Xanthorraceae, Poaceae, and Cyperaceae, as do Hesperiinae, mostly on grasses. Coeliadinae of the Old World tropics, Pyrrhopyginae in the New World, and Pyrginae specialize on dicots, with more than 50 plant families recorded, and a few feed on monocots. About 3500 species are described in 500+ genera, and many species complexes in tropical regions are not thoroughly studied. Hesperiidae are distributed worldwide except in New Zealand, with greatest richness in the Neotropical region.
Papilionoidea To most people butterflies are among the most conspicuous and recognizable insects. Their diurnal behavior, esthetic beauty, and limited species numbers render them favorite subjects for beginning naturalists and amateur collectors and teaching insect metamorphosis to primary school children. Moreover, butterflies have been of special significance to biologists, in studies of geographical distribution patterns, chemical defenses and mimicry, migration, genetics and population biology, and host-plant relationships. In recent decades they have become poster children in conservation efforts. Butterflies are vastly better studied than most moths, but they are negligible in insect biodiversity, making up fewer than 0.1% of all insect species and 9% of described Lepidoptera (likely less than 4%, were moths equally well cataloged). Several adult skeletal features define members of this superfamily as monophyletic, and the antennae have apical clubs, without an apiculus like that of Hesperiidae and not subapically broadened as in sphingid, castniid, and sesiid moths. Systematists recognize four families of butterflies: Papilionidae, Pieridae, Lycaenidae (including riodinids), and Nymphalidae (including libytheids, satyrids, and danaids). There are an estimated 14,000 species, a total that is greatly inflated relative to moths and other insects, because they are more thoroughly studied, with a propensity by the industrious specialists to accord species status to geographically disjunct populations that differ in color patterns and size but not morphologically or by molecular analysis.
Papilionidae The swallowtail family is one of the most easily recognizable of Lepidoptera, one whose phylogeny is well supported, based on several wing venation and skeletal features and the eversi-ble gland of the larval prothorax (osmeterium). Adults (Fig. 103) are medium- to very-large-sized (FW 14-105mm), including the largest butterflies (birdwings of the Indo-Australian region); wings are broad, with the FW triangulate and the HW rounded, and often with one or more veins extended into “tails.” Most are brightly colored, often aposematic, warning of their distasteful properties, and many swallowtails are models in mimicry complexes; some are polymorphic, with several forms each with different corresponding mimics, often nymphalids. Swallowtails are strong fliers and have been recorded dispersing several miles from a point of origin. Some tropical species engage in mass migrations. The larvae (Figs. 34 and 104) are plump, often ornate with filaments or protuberances. They appear bare but usually have numerous tiny secondary setae. They live exposed on foliage, inactive by day, and depend upon cryptic coloration for protection, often resembling bird droppings in early instars and then graduating to foliage or flower colors, as they grow, or they are apose-matic, brightly colored (Fig. 34). The osmeterium is hornlike, forked, usually bright pink or orange, and everted to emit a foul aroma intended to ward off predators. The caterpillars feed on a wide variety of dicot angiosperms, including several groups on Aristolochiaceae and others on Magnoliaceae, Apiaceae, Rutaceae, Lauraceae, and other plants not used by most Lepidoptera. There are about 600 species in 26 genera, with virtually all the world’s species described, distributed worldwide, with Parnassiinae in high latitudes and elevations of the Holarctic and Papilioninae mostly subtropical and tropical and with their greatest richness in the Old World tropics.
Pieridae The whites and sulfurs make up a well-established, monophyletic family, based on several characters: the presence of pterin pigments in the wing scales; the foretarsi with inner claw sub-equal in length to the outer, whereas the inner is much shorter in other butterflies; and wing venation and thoracic skeletal features. Adults (Fig. 105) are small- to medium-sized (FW 11-48mm), broad winged, mostly white, yellow, or orange, with some tropical species brightly colored, containing flavone pigments, mimicking other butterflies (e.g., South American Dismorphiinae). Most pierids display sexual dimorphism in color patterns, sometimes to the extreme, and many have marked seasonal variation. Remarkable mass migrations by some tropical pierids occur, often moving from seasonally dry to wet habitats. Larvae are slender caterpillars, relatively uniform in structure, without protuberances, and covered with short secondary setae, and each segment is divided superficially into six annulets. They are mainly green, including the head, or spotted with yellow and blue in species that feed in flowers. Some species possess an anal comb. Tropical Dismorphiinae feed on legumes, as do some Coliadi-nae, while most Pierinae specialize on Brassicaceae, Capparidaceae, Loranthaceae, or Santalaceae. The American genus Neophasia feeds on pines. Several species have achieved important pest status, particularly the cabbage white (Pieris rapae), which was introduced from Europe into North America in the 1880s, feeding on cabbage and other crucifer crops, and species of Colias, feeding on alfalfa. More than 1000 species in 75 genera have been described, including probably nearly all the world’s species. This group is cosmopolitan except in New Zealand and the Pacific Islands, with greatest development in the tropics. Species range to the extreme limits of Lepidoptera habitats, Colias to 83° N latitude and Baltia to 5000 m (16,350 ft) in the Himalayas and several genera to similar elevations in the Andes.
Lycaenidae The coppers (Fig. 106), hairstreaks (Fig. 107), blues (Fig. 108), and metalmarks (Fig. 110) together form a diverse butterfly family, with a remarkable array of larval biologies. Inclusion of the metalmarks (Riodininae) is debatable because they have several uniquely derived traits and because they have foreleg morphology and function that resemble those of Nymphalidae. However, exclusion of the riodinids leaves the remainder of the Lycaenidae an incomplete lineage (paraphyletic). Adults (Figs. 106—108, 110) are mostly small (FW 6—25 mm; Neotropical Eumaeus and African Liphyra reach 35 mm) and the upper surface of their wings is usually brightly colored, entirely or patterned, in blue, orange, or red, often brilliantly metallic, especially in the males, while the undersides, which are exposed when the butterfly is inactive and the wings are held together above the body, tend to be more cryptic, gray, brown, or green. The wings are relatively broad, the FW usually triangular and the HW rounded; most hairstreaks and a few blues have one or more slender filaments arising from the hind margin, often preceded by a colorful eyespot on the underside. During perching the wings are moved alternately, giving an impression of antennal movements, a behavior thought to deflect predator attack to the HW rather than to the head and thorax. Metalmarks exhibit a bewildering array of wing forms and color patterns, especially in tropical species, resembling diverse kinds of butterflies and moths. Lycaenid antennal bases are adjacent to and usually indenting the eyes. In Riodininae the antennae usually are long, more than half the FW length, and the forelegs are atrophied in males. The antennae are shorter and the male forelegs functional in other lycaenids. Lycaenid larvae are peculiar caterpillars, shaped like a sowbug, with the body segments broadened laterally and the small head retractable and hidden under the thorax; they are usually covered with short secondary setae, giving a velvety appearance (Fig. 109). Species that live in association with ants are bare, and Riodininae usually have long secondary setae. All lack eversible prothoracic glands characteristic of other butterfly caterpillars. Many Lycaenidae have evolved glands on the last abdominal segment that produce a sweet, honeydew-like fluid that is much sought after by ants, which display various behaviors. Some tend and “milk” the larvae on their food plants (thereby presumably warding off parasites and invertebrate predators) (Fig. 109), others transport the young caterpillars to their nests, where they are fed by the ants or eat the ant brood. A wide variety of flowering plants serve as hosts, including a few conifers and monocots. Most species are specialists, but some are polyphagous. Larvae of the African Poretiinae feed on algae and lichens. Those of
Miletinae (African and 1 species in North America, Feniseca) feed exclusively on Homoptera or their secretions or in ant nests. The association with ants has developed in many Riodininae and unrelated genera of other lycaenids. Feeding on legumes has led to minor pest status for a few species, including the bean lycaenid (Strymon meli-nus) in North America, a polyphagous species also called the cotton square-borer. There are more than 6000 described species in 640 + genera, and many tropical taxa are not thoroughly studied. Lycaenids occur worldwide, with endemic species even in New Zealand and the Pacific Islands, but the majority occur in the Neotropics and Africa.
Nymphalidae This is a large and diverse family that includes the typical Nymphalinae (brush-footed butterflies, admirals, check-erspots, Fig. 111) , Libytheinae (snout butterflies), Satyrinae (wood nymphs, ringlets, Fig. 112), Heliconiinae (long wings, fritillaries, Fig. 113 ) , Morphinae (morpho and owl butterflies, Fig. 116) , and Danainae (milkweed and glasswing butterflies, Fig. 115). All possess three longitudinal ridges (carinae) on the ventral surface of the antennae that are unique in Lepidoptera, and the forelegs of males are reduced or modified (less so in Libytheinae), usually lacking claws and nonfunctional for walking. Adults are small to very large (FW usually 10—50mm, ranging to 75 mm in tropical Morpho and Caligo), mostly broad winged except in Heliconinae, and usually brightly colored, often with orange, black, and white dominating, but mostly brown and tan in Satyrinae. Many tropical nymphalids are involved in mimicry complexes, either as models (Danainae, Heliconiinae) or as mimics of them or other distasteful butterflies and moths and/or they benefit in both roles. Glasswing butterflies (Ithomiini) live primarily in deep shade of tropical forests and have sparsely scaled areas or transparent wings, with subtle color patterns, while owl butterflies fly at dusk. They, morphos, and satyrines have conspicuous eyelike spots near the margins of the wing undersides, presumably confusing would-be predators or diverting their attacks from the body. The larvae are cylindrical caterpillars with full complement of abdominal prolegs (Fig. 114), but there are diverse modifications, e.g., densely spinose or with dorsal projections (verrucae) that are spinose (Nym-phalinae), smooth with filaments (Danainae), smooth with bifid caudal segment (Satyrinae), pubescent with hair tufts and usually bifid caudally (Morphinae). The pupa hangs head downward, attached by a cremaster, without a silken girdle. The larvae feed on a diverse array of flowering plants, with considerable specialization within subfamilies: Morphinae and Satyrinae almost exclusively on monocots, including Arecaceae, Bromeliadaceae, Helioconiacae, and Musaceae (a few species are pests on bananas) in the tropics, mostly Poaceae and Cyperaceae in the Holarctic, with 2 genera on Selaginellaceae; other nymphalids eat mostly dicot angiosperms, often specializing on plants with toxic chemicals (e.g., Heliconiinae on Flacourtiace-ae, Passifloraceae, Urticaceae, Violaceae) or latex-producing plants (Danainae on Apocynaceae, Asclepiadaceae, Moraceae). About 6500 described species are placed in 630+ genera, occurring worldwide, ranging from Arctic—Alpine Boloria in the Holarctic to extremely rich tropical faunas in most subfamilies, several of which are not represented in New Zealand.
