The term “insectivorous” was used by Charles Darwin to characterize a group of plants that seemed to trap and feed on insects. Since Darwin’s time, observations have revealed that these plants capture and interact with a greater variety of animals, which can include spiders, lizards, sow bugs, tadpoles, and frogs, and judging from some reports, even small mammals such as rats and rabbits. Hence, because of this varied diet, many workers now prefer to describe such plants as carnivorous, rather than solely insectivorous. Yet the interactions between carnivorous plants and animals go beyond the presence of certain creatures as items on a plant’s menu. While it is true that the most spectacular and usually the most obvious activities of carnivorous plants seem to be in the often elaborate mechanisms for capture and digestion of prey, many other (often more subtle) associations occur between these plants and animals. Researchers are just at the beginnings of learning about these other fascinating interactions.
THE CARNIVOROUS HABIT
Plant carnivory is a rarity, occurring in only about 550-600 out of approximately 250,000 plant species. True carnivory has probably evolved a number of times independently. The carnivorous habit is not obligate, and carnivorous plants can grow without an insect meal, depending instead on photosynthesis and minerals supplied from the soil. In general, carnivorous plants grow in sunny areas, and in mineral-deficient, sometimes sandy soils. Often these soils have standing or gently moving water, with any dissolved minerals from the soil being easily carried away by the flowing water. The carnivorous plant habitat is typically low in nitrogen and phosphorus and, as suggested by some reports, in potassium as well. In this sort of habitat, plants that have alternative strategies for obtaining essential minerals are at a competitive advantage. The capture of insects and other animals thus provides carnivorous plants with a supplemental source of essential nutrients.
The carnivorous habit depends on an ability to trap prey. In the vast majority of carnivorous plants, the trap represents a modification of the entire leaf or of structures borne on the leaf. Given this rather straightforward requirement of a trap, it should be easy enough to characterize a plant as carnivorous, or not. However, the picture is not so simple: many plants can trap insects yet are not considered to be carnivorous. What truly distinguishes a plant as carnivorous is not only a trapping ability but also a mechanism to digest prey and to absorb the prey’s nutrients.
It is the specifics of this digestion that have caused some controversy. Some workers consider a plant to be carnivorous only if it has an inherent ability to digest prey—that is, if the plant itself produces enzymes to break down the insect. Other plants, sometimes considered to be semi-carnivorous, are able to trap prey but depend on the assistance of other organisms, usually, but not always, microbes, to digest the captured insects. However, for this short article, a plant is considered to be carnivorous if it traps and has a means, of its own making or not, for digesting prey.
The trap of a carnivorous plant is a true marvel. Traps can be grouped by whether they are “passive,” with no or slowly moving “parts,” often relying on gravity to aid in capture of the insect, or “active,” exhibiting some sort of usually rapid movement.
PASSIVE TRAPS
Perhaps the most familiar examples of passive traps are the sundews (Drosera) and the pitcher plants (Sarracenia and Darlingtonia in temperate climes and Nepenthes in the tropics). The sundews capture their prey by producing from stalked glands an adhesive, or glue (the drop of “dew”), which captures and holds fast the insect. As the prey struggles, it is covered with the sticky mucilage and, as a consequence, suffocates. The stalked glands then bend in toward the prey; in some species, the entire leaf enfolds the prey. A second type of gland on the leaf secretes digestive enzymes and acids, initiating the breakdown and subsequent absorption of nutrients. Darwin was so enthralled with the sundews that about two-thirds of his topic Insectivorous Plants is devoted to this group. He notes his surprise at “finding how large a number of insects were caught by the leaves of the common sundew,” and speculates that, “as this plant is extremely common in some districts, the number of insects thus annually slaughtered must be prodigious.”
A fascinating variation on the carnivorous plant passive trap theme is shown by plants that comprise the genus Roridula. These plants, considered by some workers not to be truly carnivorous, are native to South Africa and may be near extinction. Individuals in this group have leaves covered with stalked mucilage-secreting glands, which as in the sundews capture and hold fast insects. This is where the carnivorous story would end, were it not for another player, a species of assassin bug, which forms a mutualistic association with Roridula. Large numbers of these predaceous bugs may inhabit Roridula and are able to traverse the leaves, without themselves being ensnared by the glue. When other insects are captured by the plant, the assassin bugs move to the trapped prey, suck out

FIGURE 1 Habit view of the California pitcher plant (Darlingtonia californica) growing in a bog in northern California. The leaf is divided into a hood region and tube region. Digestion occurs in a well of water at the base of the tube.
their liquid contents, and, some time afterward, defecate a nutritious substance that is absorbed by the leaf and nourishes the plant.
The second major group of plants that have passive traps are the pitcher plants. In this group, the leaf becomes variously modified, often into a tube, and develops at the base of the tube a well that must fill with water for the pitcher to function as a trap. The temperate species of pitcher plants (Darlingtonia in the western United States and Sarracenia in eastern North America) ( Figs. 1 and 2) are usually terrestrial. In these plants, the leaf lures flying insects by producing nectar, in some plants laced with volatile insect attractants (e.g., enol diacetal monoterpene and/or a series of methyl esters) that sometimes covers the colorful appendages. Crawling insects follow nectar trails running along the outside of the leaf. The nectar trails lead to the mouth of the tube, where the surface is smooth and slippery and from which the insect can easily lose its foothold, thus falling into the watery well. Escape from the well is almost impossible, since the inside wall of the tube leaf is lined with downward-pointing hairs. One would think that flying insects could fly out if they started to fall. To counter this possibility, pitcher plants such as Darlingtonia have developed a hooded leaf, transparent and sealed at its top. When an insect tries to leave the leaf, it flies toward light coming through the transparent upper portion of the hood. Since, however, the exit is sealed, eventually the insect becomes so exhausted that it falls into the well. There is some suggestion that pitcher plants may produce a “drug” to confuse the flying insect, and that fluids in the well may contain substances (in Sarracenia flava, a toxic alkaloid, coniine, [the poison in hemlock]) that is suggested to quiet and stun the fallen insect. In addition to nectar serving as an attractant, the
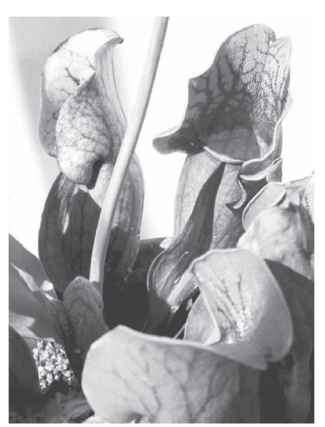
FIGURE 2 Eastern pitcher plant (Sarracenia purpurea) from Ohio. In this species a hood is absent.
possible development of ultraviolet signaling, as employed by flowers to attract pollinators, may also serve to lure insects to the trap. Many temperate pitcher plants secrete hydrolytic enzymes into the liquid in the well, thereby digesting the insect, whereas other pitcher plants (e.g., Darlingtonia) produce none of their own digestive enzymes but instead rely on bacteria to decay the insect. In either model, the digestive enzymes can be quite powerful, with only the hardest parts of insects, such as legs or shells, remaining undigested.
The tropical pitcher plants belong to the genus Nepenthes in which the pitcher develops at the end of a leaflike petiole. Given, the complexity and variety of pitchers in Nepenthes , it is often difficult, hard to believe that what one is indeed looking at is a leaf. Like their temperate cousins, Nepenthes spp. produce nectar to lure prey, which subsequently become intoxicated, lose their foothold, and fall into the trap. Nepenthes spp. generally produce climbing stems, thus elevating the pitchers, and perhaps thereby making them more accessible to potential prey.
Species of Nepenthes, and likely all carnivorous plants, do not seem to be designed to trap one particular species. An inventory of the traps shows that their diets are ever-changing and can be quite varied. For example, from 10 Nepenthes pitchers over a season, Erber found arthropods of 150 identified species, belonging principally to the orders Diptera, Hymenoptera, Collembola, and Acarina, and the families Formicidae and Aphididae. Similar tallies

FIGURE 3 Habit view of the Venus flytrap (Dionaea sp.). In this genus the blade is divided into two halves, which are attached along one side. On the inner surfaces of the blade a lure is produced, and here too are located the trigger hairs.
in Sarracinea reveal victims of 115 families belonging to 14 orders of insects, including several species of Mollusca. This strategy no doubt ensures some prey, if, for example, a particular insect species is not present one year, or becomes extinct, and also allows the plant to trap a variety of insects over a very long season. Additionally, such variety may be important in supplying a diversity of nutrients.
ACTIVE TRAPS
Plants with rapid movement usually first come into mind when carnivorous plants are mentioned. These so-called active traps imprison their prey by a quick movement of all or part of the leaf. Into this group are placed several genera: one well known, the Venus flytrap (Dionaea) (Fig. 3) and the others less familiar (e.g., the bladderworts, Utricularia ) (Fig. 4). It is believed that the rapid movement comes about when the prey makes contact with a triggering mechanism, resulting in the generation of a small electric current and the activation or closure of the trap. As with the passive traps, the lure for the insect is usually some sweet nectar. In the case of the Venus flytrap leaf in which the two halves of the blade are joined along one side, as in an open topic, nectar is produced on the inner surface of the leaf (Fig. 3). As an insect wanders along this inner surface collecting nectar, it may contact the trigger hairs, of which there are about two to four on the inner surface of each half-leaf. For the trap to close, either an individual trigger hair “must be touched twice, or two different trigger hairs must be touched sequentially, within a time period that is neither too short (<0.75 s) nor too long (>20 s).” When the hair or hairs are touched within the right time interval, the trap literally snaps shut, though at first, not completely. Initially, small openings remain between the two halves, presumably to allow smaller insects to escape from the trap. When unsuitable prey gain their release, the trap reopens and awaits the main course. But if the insect is unable to escape

FIGURE 4 Scanning electron micrograph view of the trap of Utricularia neglecta. The large hairs (“antennae”) may act as guides luring the prey to the trap mouth (arrow).
through the small openings and continues to struggle, the trap closes more fully. Subsequently, enzymes are secreted by special gland cells, and the insect is digested over a period of one to two weeks, and its nutrients are absorbed by the leaf. Leaves of the Venus flytrap apparently can be redeployed several times in capturing insects.
Less well known as active trappers but possessing traps more complex than the Venus flytraps are the bladderworts, which grow in wet or periodically wet areas. Bladderworts, the largest genus of carnivorous plants, grow worldwide, on every continent. They develop diminutive, often microscopic traps that cover the leaves (Fig. 4). The size of the trap determines what creatures will enter: paramecia, rotifers, water fleas, worms, and mosquito larvae, for example. As in the Venus flytrap, contact with trigger hairs initiates the trapping mechanism, which involves the opening of a “door” leading to a chamber maintained under a vacuum, a sucking in of the prey, and a resealing of the trap; all this occurs within 10 to 15 thousandths of a second! With the secretion of enzymes, the prey is digested, usually within hours. There is some speculation that the trap can also lure prey.
OTHER INTERACTIONS BETWEEN CARNIVOROUS PLANTS AND INSECTS
Thus far, plant-insect interactions have been presented in the context of insects serving as prey. But the associations can be much more varied and complex, as seen in the case of the assassin bug and Roridula. Entomologists are now only beginning to appreciate the many other ways in which carnivorous plants and insects/animals interact. Some of the more fascinating examples are found in the pitcher plants, where other animals turn the traps to their own advantage. Spiders often can be found prowling about the mouth of pitchers, then lowering themselves on silken strands to retrieve prey from the pitcher well. In Nepenthes, spiders also use the pitcher for protection from predators. If a predator is detected, the spider will lower itself on a silk thread to the pool and, if necessary, will even hide under the water until danger passes.
Other insects spend part or most of their lives in the wells of pitchers. For example, the pitcher plant mosquito (Wyeomyia smithii) lays its eggs on the moist inner surface of the leaf, or more often, in the pool of liquid. The larvae hatch and feed on detritus from trapped insects, bacteria, and protozoans. As winter approaches, the larvae go into a dormant state and overwinter in the pitcher, exiting the pitcher in the spring as adult mosquitoes. In climates where water freezes, the larvae spend winter frozen in the ice of the pitcher. Interestingly, the liquid that digests the trapped insects seems to have no detrimental effects on the larvae. Fish fly larvae also live in pitchers and, like the mosquito larvae, are not injured by the pitcher’s digestive enzymes because, it is speculated, their bodies produce a protective substance.
One of the most fascinating examples of an association between plant and insect, benefiting the insect, is seen in species of Exyra moths (Noctuidae) that exploit the pitcher leaf to shelter their young. The cycle begins with the female moth entering an open leaf and laying its eggs on the inner wall of the pitcher leaf. When the larvae hatch, they move about on silken strands, feeding on the inner wall. As they grow, hence becoming more visible to predators, the larvae move to the top of the pitcher, severing vascular strands carrying water to the upper regions of the pitcher leaf, which causes the top of the pitcher leaf to dry, collapse, and fold over the opening. The developing larvae are now shielded from predators. Just before a larva prepares to pupate, it chews a hole in the wall of the leaf. Through this hole, the moth exits the leaf.
Yet another example of an insect exploiting its association with carnivorous plants is the solitary sarracenia wasp (Chlorion har-risi). This insect uses the pitcher as an incubator for its eggs.
In preparation for the laying of eggs, the wasp packs into the bottom of the pitcher tube a layer of grass, which is then overlayered with freshly killed grasshoppers or crickets. This process may be repeated several times, resulting in alternating layers of insects and plant materials. Eggs are then laid among the dead insects and the whole construction is covered by another layer of grass. The eggs can now develop protected, and when the young hatch, they have a food supply.
The last example represents what may come closest to a commensal, or symbiotic, association between plant and insect. Certain species of Nepenthes (e.g., N. bicalarate) have enlarged, hollow petioles in which ants take up residence. In return for this “home” (domatia), the ants, it is suggested, protect the plant from predators.
Carnivorous plants grown outdoors probably catch more than enough insects to maintain proper nutrition, whereas plants grown indoors may be fed insects by hand. Interestingly, carnivorous plants are generally not able to digest large non-insect food, such as bits of raw meat, suggesting that some characteristics of the insect may stimulate the plant’s digestive processes.
Finally, note should be made of the apparent conflict between the plant-pollinator and plant-prey systems. Some plants separate spatially, or temporally, the flowers from the traps. But in most cases traps and flowers are produced at the same time. Indeed, in some cases, as in the pitcher plants, the leaves mimic the flower characteristics (Fig. 1). In Pinguicula, plants in bloom increase the prey capture of small visitors, especially thrips and small beetles. Thus, at a cost of some pollination flowering can promote increased vegetative success through prey capture, which usually translates into increased reproductive success. It would seem, therefore that under conditions in which pollinators were not limiting, trapping a portion of the pollinators would not be detrimental.
