Hemolymph
Uemolymph is the circulating fluid or “blood” of insects. It moves through the open circulatory system, directly bathing the organs and tissues. Insect hemolymph differs substantially from vertebrate blood, with the absence of erythrocytes and a high concentration of free amino acids being two of the common distinguishing features. The main component of hemolymph is water, which functions as a solvent for a variety of molecules. Water in hemolymph makes up to 20-50% of the total water in insect bodies, with larval stages generally having a larger relative hemolymph volume than adults. Hemolymph serves as a water storage pool for use by tissues during desiccation and as a storage depot for other types of chemicals. It also contains circulating cells called hemocytes. Hemolymph can function as a hydraulic fluid, for example, in the expansion of a newly molted butterfly’s wings. Hemolymph serves important roles in the immune system and in transport of hormones, nutrients, and metabolites.
INORGANIC COMPONENTS
The composition of inorganic ions in hemolymph varies widely among different insect groups. The pH of the hemolymph of most insects is in the range of 6.4-6.8. Apterygotes contain high levels of sodium and chloride, similar to mammalian blood. In hemolymph of exopterygotes, sodium and chloride are also high but magnesium makes up a large portion of the total inorganic cations. In endopterygotes, particularly Lepidoptera, Coleoptera, and Hymenoptera, concentrations of sodium and chloride tend to be much lower and are replaced with high levels of potassium, magnesium, and organic anions. This difference has been attributed to the coevolution of these insect groups with flowering plants and the consequent dietary importance of leaves (which contain high concentrations of magnesium and potassium). However, the concentration of inorganic ions is not a function of only the diet, because insects are able to regulate the ion composition of hemolymph to some degree.
LOW-MOLECULAR-WEIGHT ORGANIC COMPONENTS
Citric acid and other organic acids and organic phosphates (such as glycerol 1-phosphate and sorbitol 6-phosphate) account for much of the anion content in hemolymph from many insect species. The most abundant carbohydrate in hemolymph of most insects is the disaccharide trehalose. Transport of trehalose as an energy source for tissues is an important function of the hemolymph. Trehalose levels are hormonally regulated and can be increased through synthesis from glucose phosphate derived from glycogen stored in the fat body. Glucose may also be present in hemolymph, although generally at a lower concentration than trehalose. In some insects, diapause or exposure to low temperatures can stimulate synthesis of glycerol and sorbitol (from glycogen stored in fat body). The resulting high concentration of these compounds in hemolymph depresses the freezing point and protects the insects from damage that would occur if ice crystals were to form in hemolymph.
Hydrophobic lipoidal compounds present in hemolymph are carried by specific transport proteins. Diacylglycerol is the major transported form of lipid in most insects, but triacylglycerol, fatty acids, phospholipids, and cholesterol are also present. Pigments such as (3-carotene, riboflavin, and biliverdin, which give hemolymph of many insects a characteristic yellow or green color, are also carried by specific proteins.
Free amino acids are present at high concentration (up to 200 mM) in hemolymph and make a major contribution to hemolymph osmo-larity. All 20 of the amino acids found in proteins exist as free amino acids in hemolymph. Although the relative concentrations of the amino acids vary in different species, glutamine and proline are typically abundant. Proline is known to serve as an energy source for flight muscles in some species. Hemolymph may also contain some amino acids that are not found in proteins, such as ( -alanine and taurine. Tyrosine, which is metabolized for use in cuticle sclerotiza-tion, often occurs in hemolymph as a conjugate with glucose, phosphate, or ( -alanine, which increases its solubility. The phosphate and glucose substituents are removed from tyrosine by specific enzymes when tyrosine is needed for sclerotization. Catecholamines derived from tyrosine, which are used in cuticle sclerotization and pigmentation, are also present in hemolymph as conjugated forms.
PLASMA PROTEINS
Proteins are a major component of the hemolymph plasma. Typical protein concentrations in plasma range from 10 to 100 mgml—1. In most species, the concentration of proteins in plasma increases during each instar and decreases at each molt. The fat body is responsible for the synthesis of the majority of plasma proteins, but there is also a contribution of some specific proteins from epidermis and hemocytes. Plasma from each species contains a few very abundant proteins and more than a hundred other proteins at much lower concentrations. Although the identities and functions of the major proteins are understood, many of the minor hemolymph proteins have not yet been thoroughly investigated.
Storage Proteins
The most abundant proteins in larval hemolymph belong to a class known as storage proteins or hexamerins (because they are assembled from six ~80kDa polypeptide subunits). The storage proteins are synthesized by the fat body and reach extremely high concentrations in the last instar. At the end of this stage, most of the storage proteins are taken back into the fat body, through interaction with specific receptors, and stored in protein granules. During metamorphosis the storage proteins are broken down into free amino acids, which are used for synthesis of other proteins required in the adult stage. In some exopterygotes, hexamerins are again synthesized by the adult, although their function at this developmental stage is unclear. The hexamerins can be classified according to their amino acid compositions. Those rich in the aromatic amino acids (phenylalanine, tyrosine, and tryp-tophan) are called arylphorins, whereas another group of hexamer-ins are known as methionine-rich storage proteins. In addition, some other proteins that function as storage proteins but are not similar in sequence to the hexamerins have been identified in lepidopterans.
Transport Proteins
Several hemolymph proteins function to transport small molecules that have low solubility in water. Insect plasma contains two proteins that specifically bind iron; ferritin appears to sequester dietary iron, whereas transferrin acts as a shuttle to transport iron between tissues.
The most abundant transport protein in hemolymph is lipophorin, which transports lipids between tissues. Like lipoproteins in mammalian plasma, lipophorin is composed of proteins that complex with lip-ids in such a way that the lipids are protected from contact with the surrounding water. Lipophorin docks with specific receptors on the surface of tissues to either accept or unload diacylgycerol. Lipophorin contains two polypeptide subunits, apolipophorin-I and apolipo-phorin-II, which are produced by proteolytic cleavage of a larger protein precursor. In insects that use lipids as a fuel for flight muscles, diacylglycerol is released from the fat body into the hemolymph under control of a peptide hormone known as adipokinetic hormone. As lipophorin accepts large amounts of diacylglycerol, its volume increases and its density decreases as it is converted from high-density lipophorin to low-density lipophorin. Low-density lipophorin contains a third type of protein subunit, apolipophorin-III, which binds to the surface to stabilize the expanding lipid-water interface.
Juvenile hormone (JH), a sesquiterpenoid lipid, has low solubility in water and is transported through hemolymph bound to a specific carrier protein. JH binding proteins of ~30kDa have been well characterized from plasma of lepidopterans, whereas in other insect orders lipophorin or a specific hexamerin takes on the role of JH transport. In addition to keeping JH in solution, these proteins also protect the hormone from degradative enzymes that help to regulate JH concentration in plasma. JH binding proteins may also aid in delivery of the hormone to target tissues.
Egg Yolk Proteins
In adult female insects, certain proteins synthesized by the fat body and secreted into the hemolymph are delivered to the ovary, where they are taken up by developing oocytes. The most abundant of these is called vitellogenin. Once vitellogenin becomes a part of the egg yolk, it is called vitellin. Vitellogenins are typically large,
phosphorylated lipoglycoproteins that are expressed specifically in adult females. Lipophorin is also taken up from hemolymph into eggs and provides additional lipids for use by the developing embryo. Vitellogenin and lipophorin are related in their amino acid sequences, indicating that they have a common ancestral gene. Vitellogenin, lipo-phorin, and a few other plasma proteins are taken up into oocytes by receptor-mediated endocytosis.
Proteins and Peptides Involved in Immune Responses
A group of plasma proteins functions in defense against microbial infection. Hemolymph of many insects contains lysozyme, an enzyme that degrades bacterial cell walls. In addition, low-molecular-weight antimicrobial peptides are synthesized in response to bacterial or fungal infection. Many of these peptides act by disrupting the integrity of bacterial cell membranes. Phenoloxidase, an enzyme present in plasma of some species and stored in hemocytes of others, is synthesized as an inactive precursor, prophenoloxidase. In response to infection or injury, prophenoloxidase is activated and catalyzes the production of quinones that polymerize to form the pigment melanin, which helps to trap and kill invading organisms. The tendency of hemolymph to darken has been known for more than 100 years, but this melanization has only recently become understood at a molecular level. Plasma contains proteins that bind to carbohydrates on the surface of microorganisms. This causes activation of a cascade of proteases that results in the proteolytic activation of prophenoloxidase. To regulate this immune response, plasma contains several types of proteins that function as protease inhibitors.
HEMOCYTES
The circulating cells in hemolymph are called hemocytes. Insects lack erythrocytes, and hemocytes cannot be directly equated with vertebrate leukocytes. Some fraction of hemocytes remains sessile and attached to the surfaces of tissues, and in some species (e.g., mosquitoes) such cells may account for a majority of the hemocytes. Several different morphological types of hemocytes can be identified in each insect species. Some commonly observed hemocyte types are illustrated in Fig. 1. Prohemocytes are small, round cells that may be precursors from which some other cell types develop. Granular hemocytes contain conspicuous cytoplasmic granules that can be discharged as part of a defensive response to invading parasites. Plasmatocytes usually contain few granules and are characterized by their ability to change from round or spindle-shaped cells in suspension to extensively flattened, ameboid cells after attaching to a substrate. Spherule cells contain very large cytoplasmic granules, which may contain mucopolysaccharides. Oenocytoids are large cells that synthesize prophenoloxidase.
Plasmatocytes and granular hemocytes are usually the two most abundant hemocyte types, although their proportions can vary between species and within a species at different developmental stages. These two hemocyte types participate in immune responses, including (1) phagocytosis of small organisms such as bacteria; (2) nodule formation, in which multiple hemocytes aggregate to trap microorganisms; and (3) encapsulation, in which hemocytes attach to the surface of a larger parasite and form a multilayered hemocyte capsule, in which the parasite is killed. Nodules and capsules often become mela-nized through the action of phenoloxidase. Hemocytes, especially plasmatocytes, also aggregate in a type of coagulation response, sealing wounds to prevent hemolymph loss. Another function of hemocytes is
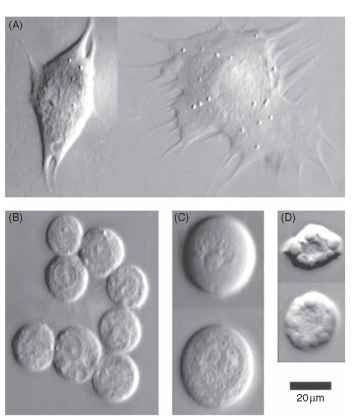
FIGURE 1 Examples of hemocyte types from a lepidopteran, Manduca sexta. (A) Plasmatocytes, the plasmatocyte shown on the left has just begun to spread, whereas the one on the right has spread extensively; (B) Granulocytes; (C) Oenocytoids; (D) Spherulocytes.
in synthesis of the extracellular matrix that covers tissues exposed to the hemolymph. Granular hemocytes appear to be the primary cell type involved in this aspect of hemocyte function.
