The most extensive studies of insect feeding behavior and its regulation have focused on two insects with completely different _JL_ feeding habits: the adult black blow fly (Phormia regina), which is a fluid feeder, and the final nymphal stage of the migratory locust (Locusta migratoria), a grass-feeding insect. Although their feeding habits are different, there are many common features in their feeding behavior patterns and the mechanisms that control their feeding behavior. It is likely, though unproven, that similar principles are involved in other insects, and this article focuses on these two insects, making reference to other species and other feeding habits where data are available. V G. Dethier initiated and guided the earlier work on the blow fly, summarized later by Stoffolano. The studies on the locust were initiated by R. F. Chapman and developed by E. A. Bernays and S. J. Simpson.
WHY INSECTS HAVE DISCRETE MEALS
Although casual observation may give the impression that insects feed nonstop, many insects eat in discrete meals separated by periods of nonfeeding (Figs. 1A and 1B). This is most extreme in species that feed on vertebrate blood: larval Rhodnius prolixus take a single meal in each developmental stage, and adult female mosquitoes usually have a single blood meal associated with each vitellogenic cycle.
![Patterns of meals taken by a caterpillar and a locust. Periods of feeding are shown by black bars. Notice that short pauses sometimes occur within meals, but the intervals between meals are markedly longer. (A) A final stage larva of M. sexta feeding on tobacco leaves. [Reproduced, with permission, from Reynolds, S. E., Yeomans, M. R., and Timmins, W. A. (1986). The feeding behaviour of caterpillars on tobacco and artificial diet. Physiol. Entomol. 11, 39-51.] (B) A final stage nymph of L. migratoria feeding on wheat. Patterns of meals taken by a caterpillar and a locust. Periods of feeding are shown by black bars. Notice that short pauses sometimes occur within meals, but the intervals between meals are markedly longer. (A) A final stage larva of M. sexta feeding on tobacco leaves. [Reproduced, with permission, from Reynolds, S. E., Yeomans, M. R., and Timmins, W. A. (1986). The feeding behaviour of caterpillars on tobacco and artificial diet. Physiol. Entomol. 11, 39-51.] (B) A final stage nymph of L. migratoria feeding on wheat.](http://lh3.ggpht.com/_X6JnoL0U4BY/S8G15rzrX_I/AAAAAAAAX9g/vz86N9qV3Hw/tmp4A37_thumb_thumb.jpg?imgmax=800)
FIGURE 1 Patterns of meals taken by a caterpillar and a locust. Periods of feeding are shown by black bars. Notice that short pauses sometimes occur within meals, but the intervals between meals are markedly longer. (A) A final stage larva of M. sexta feeding on tobacco leaves. [Reproduced, with permission, from Reynolds, S. E., Yeomans, M. R., and Timmins, W. A. (1986). The feeding behaviour of caterpillars on tobacco and artificial diet. Physiol. Entomol. 11, 39-51.] (B) A final stage nymph of L. migratoria feeding on wheat.
Nectar-feeding insects and phytophagous insects also feed in discrete meals, but the degree to which this is true in other insects has not been investigated. For predatory insects, a single prey item is commonly not sufficient to produce satiation, and it is likely that a “meal” would involve several prey, just as a “meal” for a nectar-feeding insect involves foraging from a number of flowers because no single flower contains sufficient nectar to produce satiation.
The underlying causes of this behavior are probably both physiological and ecological. Energy is expended in acquiring food and initiating digestion so that, when food is first ingested, there is a net loss of energy. Subsequently, as food is digested and absorbed, there is a gain in resources and energy, but as the process continues the rate of gain declines and the net gain plateaus as digestion and absorption are completed (Fig. 2). Consequently, there is an optimal period for which
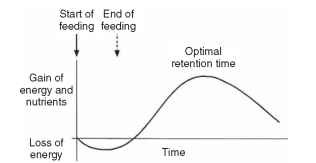
FIGURE 2 Nutrient and energy returns associated with eating a discrete meal. At first, the insect expends energy in obtaining its food. As the food is digested, nutrients are absorbed increasingly rapidly but as the nutrients are removed from the food the nutrient return decreases. To optimize the rate of nutrient return, most insects have discrete meals, with intervals between meals that approximate to peak rates of return.
an insect should retain food in its gut before replacing it with newer, undigested food. Thus, it is advantageous for the insect to eat a discrete amount of food (a meal) and process it before taking more food.
The risk of predation has almost certainly had a major role in shaping feeding behavior. Predation risks are much higher during feeding presumably because of the movements made by the insect that can be detected visually or mechanically by potential predators. For example, genista caterpillars, Uresiphita reversalis, feed for only about 3% of the day, yet 80% of predation by anthocorid bugs occurs during this period. Similarly, although tobacco hornworm caterpillars (Manduca sexta) on tobacco plants in a greenhouse fed for only about 7% of the time, 20% of predation occurred during this period.
Grasshoppers typically move away from a feeding site following a meal, sometimes backing down into the mass of vegetation and remaining unmoving and hidden until the time to feed again approaches. The caterpillars of U. reversalis move into silken shelters between meals. Most blood-sucking insects leave the host as soon as they are replete, usually moving to shaded places where they are inconspicuous, and it is probably true that most insects move away from the immediate area of feeding where food-related cues might reveal their presence to predators.
As a consequence of feeding in discrete, relatively short meals, the time spent feeding by most insects is only a small proportion of the available time; for most of the remainder they remain inactive and presumably minimize predation risks. Blood-sucking insects, which commonly ingest more than their own weight of food in a single meal, feed for less than 1% of the time; nectar-feeding butterflies and flies (feeding on unlimited supplies of nectar in the laboratory) feed for up to 14% of the time and this is also true for grasshoppers, both in the laboratory and in the field. All these insects have part of the gut modified for temporary food storage. Final-stage caterpillars of the tobacco hornworm spend about 35% of the time feeding in the field. In grasshoppers, the reduction in activity after feeding is controlled, at least partly, by a hormone released from the corpora cardiaca at the end of a meal. Hormonal release is induced by distension of the crop at this time.
Phloem-feeding homopterans appear to differ from most other insects. Planthoppers and aphids do not have discrete meals and ingest food more or less continuously. The phloem provides a continuous supply of sugars and free amino acids, requiring little or no digestion, so the availability of nutrients for absorption remains virtually unchanged over time. Under these circumstances the physiological necessity of eating discrete meals is eliminated. In these insects, the act of feeding is not associated with obvious body movements because once the feeding tube is plugged into a phloem sieve tube, the insect remains in one place for hours. This probably applies to xylem-feeding insects, which also need to process very large amounts of fluid because of the low concentrations of nutrients in xylem. Filter-feeding aquatic insects, such as some mosquito larvae, also probably feed continuously.
THE START OF FEEDING
As the time from the previous meal (the intermeal interval) gets longer, the likelihood that the insect will respond to food stimuli increases. A locust starts to move again and so the likelihood of encountering food is increased. Other factors, not related to the food, may also further increase the probability of feeding. In a locust, a sudden increase in light intensity or the act of defecation may have such effects. Conversely, an encounter with a highly unpalatable food source may delay the start of feeding and careless movements by an observer may have a similar effect. Simpson demonstrated that, in the migratory locust, there was in addition a tendency for meals to begin with some pattern of regularity which, in his observations, had a period varying from 12 to 16.5 min in different individuals. This does not mean that feeding or some other activity occurred every 15 min, but when it did so it was usually at some multiple of 15 min from a set time, which he determined to be lights-on in his experiments (Fig. 3). There is now evidence for similar rhythms in the caterpillars of an arctiid moth, Grammia geneura, and the sphingid M. sexta. The evidence for the latter is based on field observations, and the rhythm had a period of 3-4 min. The discovery of these rhythms was dependent on detailed, long-term observations on individual insects. Such sets of observations are rare, and the extent to which similar rhythms occur in other insects is not known because observations are lacking.
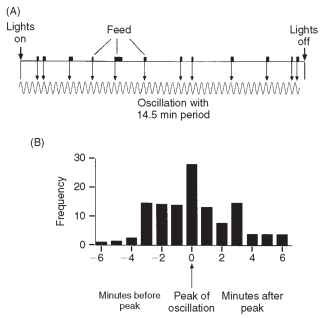
FIGURE 3 Oscillation underlying the feeding behavior of the migratory locust, L. migratoria. (A) Feeding record of an individual during a 12-h light phase. Notice that each meal begins close to the peak of a 14.5-min oscillation. (B) Times at which feeding started relative to the peak of the oscillation for eight insects on one 12-h day.
The effects of these varying factors on feeding can be accounted for by an, as yet hypothetical, excitatory state in the central nervous system, first postulated for the blow fly and subsequently elaborated for the migratory locust (Fig. 4) . Only when the central excitatory state exceeds a certain threshold can feeding occur, but feeding is not an automatic consequence of reaching the threshold; it is a probabilistic event. At the end of a meal, the central excitatory state is assumed to be depressed below threshold. As time since the previous meal increases, so does the level of the central excitatory state so that it approaches and ultimately exceeds threshold. Rhythmic changes in the central excitatory state are presumed to account for the basic rhythmicity of feeding, and other events, such as defecation, may temporarily elevate it, while others (disturbance) may depress it.
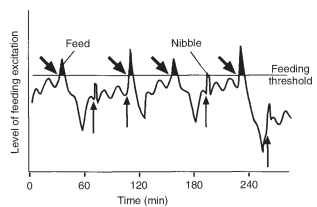
FIGURE 4 Model of the control of feeding in a locust eating wheat. Similar principles are believed to apply to other insects. The irregular line shows the level of feeding excitation (the central excitatory state). When this exceeds a threshold, the insect feeds. Notice that after a meal, the excitatory state declines sharply. Subsequently it rises slowly and the level oscillates with a period of about 15 min. Defecation (upwardly pointing arrows) causes a sudden rise in excitation. If this causes excitation to exceed the threshold, the insect may feed. Biting the food (oblique arrows) releases juices from the food and phagostimulants cause a sharp rise in the central excitatory state.
THE SIZE OF A MEAL
The size of a meal, assuming the food supply to be unlimited, depends on the net phagostimulatory effects of the food and the nutrient requirements of the insect. Net phagostimulatory effect is the balance between nutrient components of the food that stimulate taste receptors leading to feeding and any other factors, such as the presence of toxic compounds or undue hardness, that tend to inhibit feeding. For many insects, sugars are major phagostimulants and higher concentrations in the food result in larger meals. Amino acids may also influence the phagostimulatory input. Plant secondary compounds, such as alkaloids, are often feeding deterrents even for insects, such as the blow fly, whose food does not normally contain them. Inorganic salts at higher concentrations are also deterrent although at low concentrations they may stimulate feeding. Phagostimulatory compounds do not just switch on feeding that then continues until the insect is replete; their continued input is necessary for feeding to continue and the behavior of some insects appears to reflect this. Chemosensory receptors usually adapt within a few seconds if continually stimulated, but the palpation behavior of grasshoppers and caterpillars appears to permit a continual flow of information by bringing the receptors into contact with the food for frequent very brief periods. The sensilla on the palp tips of a locust make about 10 contacts per second and each contact may be only 1020 ms. As a result they remain largely unadapted.
Although continual stimulation is important to maintain feeding, meal size seems to be determined by the level of phagostimulation when the insect first bites into its food and releases the internal fluids containing a mixture of stimulating chemicals. This was demonstrated by an experiment in which the mouthpart chemoreceptors of locusts were stimulated with a highly phagostimulatory solution that they were not allowed to ingest. These insects subsequently ate larger meals than others stimulated with water alone, despite the fact that during the meal the receptors of both sets of insects were equally stimulated. Events before feeding started determined how much was eaten. Comparable experiments have shown that distasteful compounds can reduce meal size. Such experiments are interpreted as reflecting changes in the central excitatory state. A high concentration of phagostimulant is believed to elevate the central excitatory state well above threshold and feeding continues, provided some level of input is maintained, until the excitatory state declines to threshold. Thus, the higher the initial level, the longer it takes the excitatory state to reach threshold and the larger is the meal. It is supposed that a high level of deterrent compounds would inhibit feeding by depressing the level of the central excitatory state below threshold.
The elevated level of the central excitatory state is also believed to account for the “dances” of flies and the palpation behavior of locusts and grasshoppers following loss of contact with food early in a meal. When a fly loses contact with a drop of sugar, it moves in an irregular path with frequent turns as if “searching” for the food. The more concentrated the solution, the more frequent the turns (Fig. 5A).
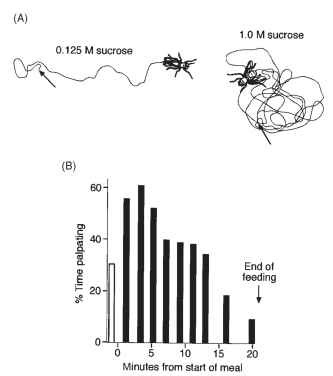
FIGURE 5 Searching for food after loss of contact during a meal. (A) Blow flies dance when they lose contact with a drop of sugar. After experiencing more concentrated sugar solutions the high rate of turning is much more sustained. (B) The migratory locust palpates when it loses contact with a blade of grass. Each bar represents the percentage of time palpating after losing contact with the food at a different stage of the meal. Soon after the start of a meal it palpates for most of the time, but toward the end of a meal it is less persistent. The open bar represents the time palpating just before starting to feed.
If a locust loses contact with its food, it palpates vigorously and such behavior lasts longer if loss of contact occurs earlier in a meal. Toward the end of a meal, loss of contact with the food results in only a limited period of palpation (Fig. 5B). The so-called searching behavior of other insects, such as that described for coccinellid larvae when feeding on aphids and temporarily losing contact with the prey, probably has a similar basis.
THE END OF A MEAL
The end of a meal is ultimately determined by the degree of distension of the part of the gut in which the food is temporarily stored. In grass-feeding grasshoppers and nectar-feeding flies, this temporary store is the crop (part of the foregut). In R. prolixus, feeding on vertebrate blood, the food is stored in the anterior midgut. In each case, the degree of distension is monitored by some form of stretch receptor. In locusts, these receptors are multipolar cells on the wall of the foregut, and receptors on the most anterior part of the foregut, which is the last part to fill; these receptors are responsible for inhibiting further feeding. R. prolixus has chordotonal organs in the body wall. The input from these stretch receptors has an inhibitory effect on feeding, presumably by leading to a decline in the level of the central excitatory state to below threshold. If food quality and the nutritional and feeding status of the insect are constant, stretch receptor input determines that an insect ingests a similar amount of food at each meal.
CHANGES IN FEEDING BEHAVIOR
The pattern of feeding changes with the age of the insect, its previous experience, and its nutritional needs. Phytophagous insects in general tend to eat greater amounts in the middle of a developmental stage and more in the light than in the dark. The average meal size taken by the final-stage nymph of the migratory locust, for example, increases from about 50 mg on the day of molting to almost 100 mg 4 days later, whereas the average interval between meals declined from 82 to 71 min. At night, the insects take fewer meals even though the temperature may be constant.
Some phytophagous insects become less selective if they experience a long period without food and this has given rise to some confusion in the literature. For experimental purposes, it is often convenient to use insects that feed readily when presented with food. This is achieved by depriving them of food, often for 24-h periods. However, because such insects are less selective than insects with continual access to food, grasshoppers, for example, were generally considered to be unselective in their choice of foods. More critical observations, however, show that this is not accurate. With increasing periods of food deprivation, several grasshoppers have been shown to accept a wider range of food plants. It is probable that this acceptance of previously unacceptable plants reflects a need for water rather than for other specific nutrients, although this hypothesis has not been thoroughly investigated. It is, however, clear that a well-hydrated locust actively moves away from wet filter paper, whereas a dehydrated one attempts to eat it. Similarly, dehydrated flies drink water, whereas hydrated ones do not.
The tendency of grasshoppers and caterpillars, and probably other insects, that are deprived to sample food that would otherwise be rejected can play a major part in the subsequent acceptance of food. This becomes possible because taste receptors that initially signaled rejection because of some distasteful component of the food become habituated and are no longer stimulated by the distasteful compound. At the same time, detoxifying enzymes are probably mobilized within
the insect, providing it with the capacity to minimize any harmful effects that the compound might have.
The nutritional requirements of insects vary through life and this is reflected by changes in their feeding behavior. During larval development, the amount of food consumed is usually maximal in the middle of each developmental stage, falling to zero for a period before each molt. Changes also occur in adults in relation to somatic development and, in females of many species, in relation to egg development. This variation is illustrated for adult red locusts (Nomadacris septemfasciata) in Fig. 6. When the insect first becomes an adult the cuticle is soft and the flight muscles are poorly developed. During this teneral period, both sexes feed actively during the day (Fig. 6A).
Subsequently, the insects enter reproductive diapause and feeding is reduced to a single meal each day (Fig. 6B). During the reproductive period, females eat much more than males (Fig. 6D).
Among some adult flies and grasshoppers, there is good evidence that mature females change their feeding behavior to acquire protein for the synthesis of vitellogenin. This is most obvious in blood-sucking flies, such as mosquitoes and tabanids, females of which use nectar as a flight fuel, but vertebrate blood as their primary protein source. Males of these same species feed only on nectar. This is also true of blow flies. Mature female grasshoppers, given the opportunity, tend to select food with a higher protein level than do males or immature females. Thus, they tend to eat the seed heads of developing grain rather than foliage.
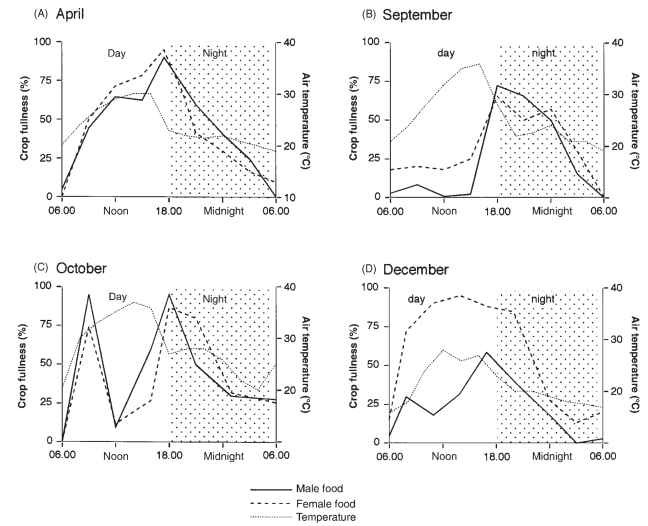
FIGURE 6 Variation in feeding behavior during adult life. The red locust, N. septemfasciata, in the field. (A) April. Soon after becoming adult both sexes feed for most of the day. This is a period of somatic growth when flight muscles and cuticle become fully developed. (B) September. Despite moderately high temperatures during the day, very little feeding occurs until late afternoon. The insects are in reproductive diapause. (C) October. Little feeding occurs in the middle of the day, perhaps because of the high temperature. The insects are beginning to become sexually mature. (D) December. Females eat much more than males during the period of egg development. All these samples were taken from the same generation and population of insects, which live for about 9 months as adults. Each graph shows the amount of food in the foreguts of a sample of insects taken at each time point over a 24-h period; 100% would indicate that all the locusts were full, 0% that they were all empty. When the temperature is 30°C or above, the foregut becomes more than half empty within an hour, so that crop fullness above 50% during the day indicates recent feeding. At 25°C and below, the food takes several hours to leave the foregut so that night time values largely reflect feeding before dark.
Under laboratory conditions, when fed on artificial diets, locusts and caterpillars are able to select from the foods with different amounts of proteins and carbohydrates to maintain an appropriate balance of the two classes of compound. Locusts can make the adjustment from one meal to the next, with an interval of less than an hour between meals. The extent to which insects can fine-tune their nutritional balance when feeding on natural food with much smaller deficiencies of protein or carbohydrate has yet to be demonstrated.
FEEDING BEHAVIOR UNDER NATURAL CONDITIONS
In the field, feeding behavior is determined to a large extent by environmental factors, although relatively few extensive studies have been carried out. Temperature has a major effect on feeding behavior, as it does on other insect activities, with little feeding occurring at low or at very high temperatures (Fig. 6A and 6C). The effects of temperature are most obvious in insects living under extreme conditions of low or high temperature. For Gynaephora groenlandica caterpillars living within the Arctic Circle, feeding is possible only when the insect has raised its body temperature by basking. As a result, most feeding occurs in a relatively narrow window of time around noon each day, when the sun is highest in the sky (Fig. 7).
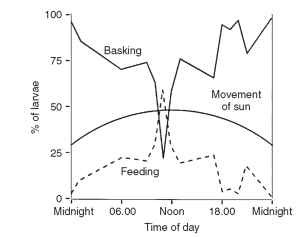
FIGURE 7 Feeding is limited by temperature. Most caterpillars of G. groenlandica, living within the Arctic Circle, feed during a 2-h window when the sun is at its zenith. For most of the time, the insects bask to raise their body temperatures, enabling them to feed efficiently; during feeding their temperature falls rapidly.
Darkness also tends to reduce feeding. For many visually foraging insects, finding food at night is impossible, although night-flying moths obtain nectar only during darkness and some blood-sucking insects, such as mosquitoes, feed most actively at night or in the crepuscular periods. These insects locate their host primarily by odor, although night-blooming flowers often also present conspicuous targets because of their size and whiteness.
Biotic factors may also have a profound effect. For example, a caterpillar of M. sexta that has defended itself from the attack of a tachi-nid fly does not feed for some time after it has successfully repelled the attacker.
