The Diptera, commonly called true flies or two-winged flies, are a group of familiar insects that includes mosquitoes, black flies, _JL_ midges, fruit flies, and house flies. The Diptera are among the most diverse insect orders, with approximately 150,000 described species. These insects are diverse not only in species richness but also in their structural variety, ecological habits, and economic importance. The group is ubiquitous and cosmopolitan, having successfully colonized nearly every habitat and all continents, including Antarctica. Although brachyptery (wings reduced) or aptery (wings absent) are known in some Diptera (e.g., some Mycetophilidae, Tipulidae, Phoridae, and Hippoboscidae), adults usually are winged and active fliers. Depending on the group, adults can be nonfeeding or feeding, with the latter including diets of blood, nectar, and other liquefied organic materials.
Larval Diptera are legless and found in a variety of terrestrial and aquatic habitats. Most larvae are free-living and crawl or swim actively in water (e.g., Simuliidae, Culicidae, Chironomidae, Ptychopteridae, Blephariceridae), sediments (e.g., Tipulidae, Psychodidae, Ceratopogonidae, Tabanidae), wood (e.g., Tipulidae, Mycetophlidae), fruit (e.g., Drosophilidae, Tephritidae), or decaying organic material (e.g., Muscidae, Ephydridae, Sphaeroceridae, Sarcophagidae). Other larvae inhabit the tissues of living organisms (e.g., Oestridae, Tachinidae).
As expected for a ubiquitous group with diverse habits and habitats, the Diptera are of considerable economic importance. Pestiferous groups can have significant impacts in agriculture (e.g., Agromyzidae, Tephritidae), forestry (e.g., Cecidomyiidae), animal health (e.g., Oestridae), and human health (e.g., Culicidae, Simuliidae, Psychodidae). Other groups can be a general nuisance if present in high numbers (e.g., Muscidae, Ceratopogonidae) or because of allergic reactions to detached body hairs (e.g., Chironomidae). Despite these negative impacts, flies can play a valuable role as scavengers (e.g., Mycetophilidae, Muscidae, Calliphoridae), parasitoids and predators of other insects (e.g., Tachinidae, Empididae, Asilidae), pollinators (e.g., Syrphidae, Stratiomyidae, Bombyliidae), food for vertebrates (e.g., Chironomidae, Tipulidae), bioindicators of water quality (e.g., Chironomidae, Blephariceridae), and tools for scientific research (e.g., Drosophilidae).
MORPHOLOGY
Because of the structural variety in Diptera, especially among larvae, it is difficult to generalize about morphology. Despite this variety, flies share a number of features. Except for certain forms (e.g., cave-dwelling species), adult flies usually possess large compound eyes. In some species, eyes meet or almost meet dor-sally (holoptic); in other groups, eyes are widely separated (dichop-tic). Further modifications include eyes that are divided into distinct dorsal and ventral components, a feature found in many Simuliidae, Blephariceridae, and other groups. These modifications are among many that might be related to swarming behavior. The regions of a fly head include the vertex, a dorsomedial area above and posterior to the eyes; the frons, an area extending from the vertex to the antennal insertions; and the face, which extends from the antennal insertions to the clypeus, a region intimately associated with the mouthparts. All of these areas can bear a variety of setae, the number and position of which often are useful in identification.
Nearly all flies have well-developed antennae, with the flagel-lum being the most varied component. In nematocerous families, the antennae are usually composed of many segments and are filiform, plumose, or pectinate (Figs. 1 and 2), whereas brachycerous flies typically have the first flagellomere enlarged and the remaining flagellomeres stylate or aristate (Figs. 3-6). The mouthparts of adult
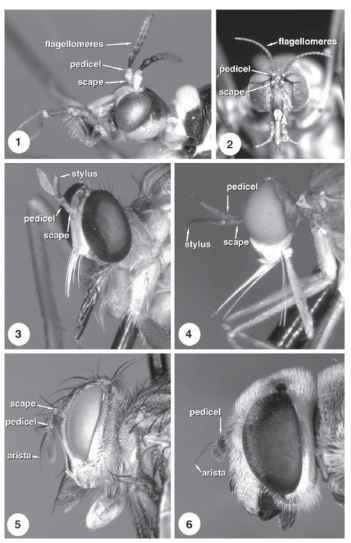
FIGURES 1-6 Adult head of (1) Tipulidae, (2) Blephariceridae, (3) Asilidae, (4) Empididae, (5) Tachinidae, (6) Syrphidae.
flies also vary between groups, ranging from vestigial forms (e.g., Deuterophlebiidae, Oestridae) to those that are well developed. The latter include two general types: (1) piercing and sucking, as seen in simuliids, culicids, and asilids, and (2) lapping and sucking, as seen in tipulids and most brachycerous groups. Typically, the proboscis comprises the unpaired labrum-epipharynx, labium, and hypopharynx and the paired mandibles and maxillae. In most groups, the base of each maxilla bears a distinct palpus and the apex of the labium is modified into a labellum, which consists of membranous lobes derived from the labial palpi.
Perhaps the most distinct feature of the adult fly is the single pair of wings (hence, the ordinal name, Diptera, meaning “two wings”). A related characteristic is the highly modified thorax, with a reduced prothorax and metathorax, and a greatly enlarged mesothorax. The latter includes several prominent dorsal and lateral sclerites and, internally, houses much of the wing musculature. Wing venation varies greatly throughout the Diptera and can be extremely important for identification. The metathoracic wings are modified into distinct club-shaped halteres, which are thought to play an important role as balancing organs. Interestingly, halteres are distinct in some groups that are otherwise wingless (e.g., Hippoboscidae). The legs of an adult fly are typical of most insects, each with a coxa, trochanter, femur, tibia, and, in nearly all groups, a tarsus comprising five tarsomeres. Beyond this basic arrangement, there is considerable diversity of leg structure in Diptera, with this diversity often providing useful taxonomic information.
The adult abdomen also shows considerable variety. In basic structure, the abdomen consists of 11 segments, the last two or three of which are highly modified for reproduction. Most abdominal segments consist of a dorsal and ventral sclerite, connected laterally by a pleural membrane of varying width. There is a general trend toward a shortening of the abdomen in Diptera (cf. Tipulidae and Muscidae). The terminalia of Diptera are complex, highly variable, and of considerable use in taxonomic and phylogenetic studies. Details of ter-minalic structure are beyond the scope of this article; however, the structural variety of Diptera terminalia and the controversy about interpreting their homologies can be found in some of the general references listed at the end.
The dipteran pupa also varies considerably in form. Some fly pupae look like a cross between the worm-like larva and the adult, whereas others are relatively featureless and seed-like in appearance. The former are typical of the Nematocera and are described as obtect, or having the appendages fused to the body (Figs. 7- 10 ). For instance, a crane fly (Tipulidae) pupa has identifiable head, thoracic, and abdominal segments, but the antennal sheaths, legs, and wing pads adhere to the pupal body (Fig. 9). Nematocerous pupae are frequently leathery to the touch. The exterior of the nematoceran pupa may be adorned with spines, gill-like respiratory devices, or locomo-tory paddles (Figs. 7-10). The Brachycera and Cyclorrhapha form the pupal stage in a different, more concealed manner. Families of the so-called higher Diptera form pupae that are described as coarctate, which literally means “compacted” or “contracted” (Figs. 11-15). These taxa (e.g., Syrphidae, Drosophilidae, Muscidae) form a pupar-ium that is composed of the hardened skin of the last larval instar (Fig. 14). This relatively tough, desiccation-resistant structure houses and protects the pupa; the adult also forms within the puparium. The enclosed adult must break through the puparial skin and it does so by extruding a balloon-like structure from the frons called the ptilinum. The ptilinum is used to break the cephalic cap, a lid-like structure positioned anteriorly on the puparium, thus liberating the teneral (or newly emerged) adult. Very few external features are noticeable on
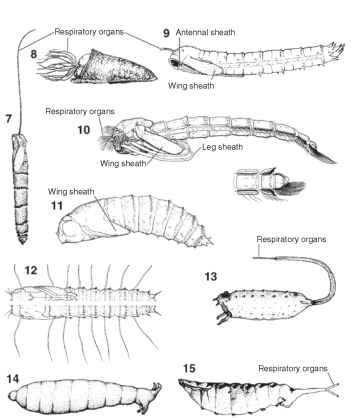
FIGURES 7-15 Pupa of (7) Ptychopteridae, (8) Simuliidae, (9) Tipulidae, (10) Chironomidae, showing anal division below, (11) Tabanidae, (12) Empididae, (13) Syrphidae, (14) Muscidae, (15) Ephydridae.
the puparium, although careful examination will reveal the spiracles through which atmospheric air is obtained by the pupa.
Diptera larvae can be distinguished from the larvae of most other insects by the lack of jointed thoracic legs. In other features, larval dipterans show tremendous structural variety. This variation is exemplified by cranial structure. Larvae of most nematocerous flies are eucephalic, i.e., characterized by a complete, fully exposed, and heavily sclerotized head capsule (Figs. 17-19 and 24). Larval tipulids are special among nematocerous flies, as the head capsule often is fully retracted into the thorax (Fig. 16) and the posterior cranial margin may possess small to extensive longitudinal incisions (Fig. 23). In contrast to the condition in nematoceran larvae, the cranial sclerites of brachyceran larvae are greatly reduced or absent. The hemicephalic head capsule of many orthorrhaphous Brachycera consists of slender arms and rods that are partly retracted into the thorax (Figs. 25 and 26). The culmination of cranial reduction is in the acephalic head of larval Cyclorrhapha, in which the external portions of the head are membranous, and much of the head is retracted into the thorax (Fig. 27). The internal portion, or cephalopharyngeal skeleton, is thought to comprise the remnants of internal cranial sclerites (tentorium) and various mouthparts. Although referred to as “acephalic,” the primary difference between the head of a cyclorrhaphan larva and that of a nematoceran larva is that most of the constituent segments are withdrawn into the thorax and thus externally hidden (Fig. 22). Cranial modifications are accompanied by general changes in the shape and rotation of mandibles and other mouthparts. The mandible of larval nematocerans typically consists of a stout, toothed structure that
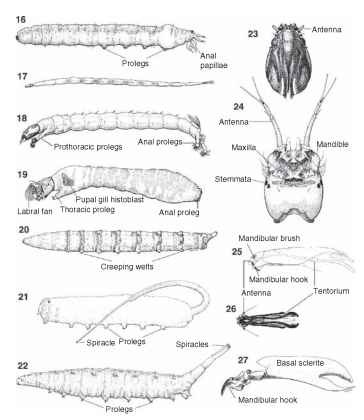
FIGURES 16-27 Larva of (16) Tipulidae, (17) Ceratopogonidae, (18) Chironomidae, (19) Simuliidae, (20) Tabanidae, (21) Syrphidae, (22) Ephydridae. Larval head capsule of (23) Tipulidae, (24) Chironomidae. Cranial sclerites and mouthparts of (25) Tabanidae, (26) Dolichopodidae. (27) Cephalopharyngeal skeleton of Sciomyzidae.
moves in a horizontal or oblique plane and operates as a biting and chewing organ. The brachyceran larval mandible usually is more claw-like, has fewer teeth along the inner surface, moves in a vertical plane, and operates as a piercing or slashing organ.
In most Diptera larvae, the thorax and abdomen are soft, flexible, and only occasionally provided with sclerotized plates. The thorax usually consists of three distinct segments and the abdomen usually has eight or nine segments (Figs. 17-19). Body form varies almost as much as does cranial diversity and ecological habits. In many nema-toceran groups (e.g., most Chironomidae, Tipulidae, and Simuliidae), the body is subcylindrical (Figs. 16, 18, and 19). Other groups are predominantly fusiform (e.g., Cecidomyiidae) or elongated and serpentine (e.g., Ceratopogonidae) (Fig. 17) . The latter body form is common in groups inhabiting soil and interstitial aquatic habitats. The larvae of some groups (e.g., Culicidae) are unusual in that the thoracic segments are indistinctly differentiated and form a single large segment that is wider than the rest of the body (Fig. 48). The typical body shape of a cyclorrhaphan larva is that of a maggot (i.e., pointed at the anterior end, with the thoracic segments approaching the maximum body diameter). The variation in body form is particularly impressive in families whose larvae feed on a variety of substrates (e.g., Syrphidae). Cyclorrhaphan larvae can be dorsov-entrally flattened, a feature often associated with the presence of segmental or branched body protuberances. The syrphid genus
Microdon has one of the most unusual larvae, being ventrally flattened, dorsally dome-shaped, and sluglike in overall appearance. Larvae with parasitoid and parasitic lifestyles (e.g., Pipunculidae, Oestridae) are often extremely stout or pear-shaped, their body form being closely adapted to that of the host.
Despite the absence of jointed thoracic legs, locomotion is highly diverse in fly larvae, reflecting the group’s diversity in habitat and habits. Locomotory appendages operate through a combination of turgor pressure and muscle action and include creeping welts, prolegs, and other specialized structures (e.g., suctorial disks). Creeping welts are transverse, swollen areas (ridges) that bear one to several modified setae or spines; creeping welts are characteristic of several groups, including many crane flies, dance flies, and deer and horse flies (Fig. 20). Among orthorrhaphous groups, ventral creeping welts are common in the larvae of Rhagionidae and Empididae. Cyclorrhaphan larvae typically use creeping welts as anchoring devices, with welts usually comprising bands of small spines on abdominal segments. The distribution and morphology of creeping welts vary considerably between families, species, instars, and segments. Prolegs usually are paired, round, elongate, fleshy, retractile processes that bear apical spines or crochets; prolegs come in a diversity of shapes, sizes, and positions and are typical of Chironomidae, Deuterophlebidae, Simuliidae, Rhagionidae, and various members of other groups (Figs. 16, 18, 19, 21, and 22). Other specialized structures used for locomotion or attachment include friction pads and suctorial disks. Several genera of Psychodidae possess friction pads, which are areas of modified cuticle on the ventral surface of the thorax or abdomen. Functionally similar structures may occur in certain Ephydridae, particularly in groups inhabiting waterfalls and thin films of flowing water. Suctorial disks are true suction devices on the ventral body surface of larval Blephariceridae and are an obvious adaptation to life in torrential streams.
Larval Diptera show a variety of respiratory adaptations, many a reflection of life in fluid or semifluid habitats. The basic respiratory system comprises an internal system of tracheae and the external spiracles. Respiration may be directly from the atmosphere, from plant tissues, or from oxygenated fluids. The presence of hemoglobin in the blood of some midges can assist the absorption of oxygen. Many aquatic larvae, particularly those from well-oxygenated streams, are apneustic (lack spiracles) and absorb oxygen directly through the skin. Some families (e.g., Psychodidae) possess spiracles on the prothorax and last abdominal segment, whereas others (e.g., Culicidae and most cyclorrhaphans) have spiracles on only the last segment. In several groups (e.g., many Ephydridae and Syrphidae), the spiracles are at the end of a retractile respiratory siphon (Figs. 21 and 22).
PHYLOGENY AND CLASSIFICATION
Traditionally, the Diptera have been divided into two or three suborders: Nematocera (“lower” Diptera) and Brachycera (“higher” Diptera), with the latter sometimes divided further into the Orthorrhapha and Cyclorrhapha. Although there is general agreement that the Diptera, Brachycera, Cyclorrhapha, and a few other subordinate groups are monophyletic, there is comparably general agreement that the Nematocera is a paraphyletic or grade-level grouping. No synapomorphies (shared, derived characters) unite the Nematocera, and the Brachycera are thought to have originated from some subgroup within the Nematocera. Despite this, it is useful to mention some of the primitive features shared by most nematocerans. The name itself (“Nematocera”) refers to the fact that adults of these flies typically have long, multisegmented antennae. Furthermore, adult nematocerans generally are slender, delicate, long-legged flies (e.g., Tipulidae and Culicidae); however, the group also includes some rather stout-bodied flies (e.g., Simuliidae and Ceratopogonidae). Larval nematocerans typically have a well-developed, sclerotized head capsule, and their mandibles usually rotate at a horizontal or oblique angle. Brachycera are characterized by the short, three-segmented antennae, the last segment of which is usually either stylate or aristate. Brachyceran larvae usually have a hemicephalic or acephalic head capsule, consisting mostly of slender, sclerotized rods that are partly or largely retracted into the thorax. Within the Brachycera, there are additional differences between orthorrhaphous and cyclorrhaphous groups. The former group, which includes Rhagionidae, Tabanidae, Stratiomyidae, and a few other families, is similar to nematocerous Diptera in that it is considered a paraphyletic group. Finally, within the Cyclorrhapha are two major subgroups, the presumed paraphyletic Aschiza (includes Phoridae and Syrphidae) and the monophyletic Schizophora (includes the majority of Brachycera, such as Tephritidae, Drosophilidae, Ephydridae, Agromyzidae, Muscidae, and Tachinidae).
ECOLOGY
Life History
As a holometabolous insect, or one that undergoes complete metamorphosis, the dipteran life cycle includes a series of distinct stages or instars. A typical life cycle consists of a brief egg stage (usually a few days or weeks, but sometimes much longer), three or four instars (typically three in Brachycera, four in nematocer-ous flies, and more in simuliids, tabanids, and a few others), a pupal stage of varying length, and an adult stage that lasts from less than 2 h (Deuterophlebiidae) to several weeks or even months (some female Culicidae). The eggs of aquatic flies are usually laid singly, in small clusters, or in loose or compact masses in or near the water and attached to rocks or vegetation. In Deuterophlebiidae and certain members of some other groups, the female crawls beneath the water to select oviposition sites, a behavior that ensures eggs are placed in a suitable larval habitat. The latter also is typical of many terrestrial flies, such as calliphorids, which will lay their eggs near the body openings (eyes, nose, mouth, anus) of carcasses. Some tephritid fruit flies use a rigid ovipositor to pierce plant tissue. Oviposition in parasitic flies can be complex and may involve placement of eggs in or on the host or in areas frequented by the host. Some parasitoids (e.g., some tachinid flies) produce eggs that are ingested by a feeding host. Larvae then hatch inside the host and penetrate the gut wall. Furthermore, some parasitic groups will oviposit on a blood-feeding arthropod (e.g., tick or another fly), with the heat of the next host stimulating hatching.
All instars occur in the same habitat in most taxa. Exceptions include flies that demonstrate hypermetamorphosis, which is characterized by an active, slender first instar (planidium) and grublike, endoparasitic later instars. Acroceridae, Nemestrinidae, and Bombyliidae are among the better known groups with hypermeta-morphic representatives. In general, the duration of the first larval stage is shortest, whereas that of the last instar is much greater, often several weeks or even months.
Habitat
The diversity of Diptera habitats is partly a reflection of the different ecological roles of larvae and adults, with larvae generally adapted for feeding and growth and adults for reproduction and dispersal. Whereas fly larvae occur in both terrestrial and aquatic habitats, virtually all adults are terrestrial and capable of flight. Wingless and, therefore, flightless groups include certain tipulids, marine chirono-mids, and phorids, as well as ectoparasitic adults of Hippoboscidae and Nycterobiidae. Adult flies are arguably one of the most aerial of organisms. Swarms of flies, which usually consist primarily of males, are a common sight in many areas. These aggregations, often for the purpose of enhancing male visibility to prospective female mates, may be seen along roadsides, over certain trees or bushes, above sunlit pools along streams, at the summits of hills, in sunny gaps of forest canopies, or at any number of other swarm markers. Swarming is probably a primitive feature of Diptera, which might explain the prevalence of this behavior in nematocerous groups. These Diptera and other flies share a number of structural features that might be adapted for swarming, including enlarged compound eyes and wings with well-developed anal lobes. These features and others are thought to assist flies in both maneuvering in flight and perceiving conspecific individuals in swarms. Swarming and related behaviors are especially developed in Bibionidae and Empididae. Males of the latter group are known for their predaceous habits and the elaborate behaviors and “nuptial gifts” for prospective female mates. Other groups (e.g., Bombyliidae, Syrphidae) are among the most agile flying insects, being particularly adept at hovering.
Diptera larvae have colonized a variety of terrestrial and aquatic habitats, including water (e.g., Simuliidae, Culicidae, Chironomidae), soil and damp sediments (e.g., Tipulidae, Ceratopogonidae, Tabanidae), rotting wood (e.g., Tipulidae, Mycetophilidae), fruit (e.g., Tephritidae, Drosophilidae), decaying organic material (e.g., Muscidae, Sarcophagidae), and the tissues of living organisms (e.g., Sciomyzidae, Oestridae, Tachinidae). Despite this diversity of habitats, most larvae are in a broad sense aquatic. Even “terrestrial” groups from decomposing vegetation, carcasses, leaf litter, rotting wood, or soil often live in a rather aqueous environment. This requirement for a damp environment partly reflects that the larval cuticle is usually thin, soft, and susceptible to drying. Truly aquatic larvae occur in coastal marine, saline, and estuarine waters, shallow and deep lakes, ponds, cold and hot springs, plant cavities (phytotelmata), artificial containers, slow to torrential streams, groundwater zones, and even natural seeps of crude petroleum! Aquatic habits are most prevalent in larvae of nematocerous flies, including all or most Culicidae, Simuliidae, and Chironomidae. Among brachycerous flies, aquatic habits are most common in Ephydridae, Sciomyzidae, and Tabanidae. In some groups, such as muscoid flies, only a few species are aquatic.
Trophic Relationships
Their trophic diversity and numerical abundance make the Diptera an important component in many ecosystems, both as primary consumers and as a food resource for other organisms. Trophic diversity is reflected in the wide range of larval feeding habits, which encompass nearly every category. In some groups (e.g., asilids, most empidids), larvae and adults belong to the same trophic category; in other groups (e.g., simuliids, tachinids) these life stages usually adopt different feeding strategies; in still others, feeding can be restricted to only the larvae or adults (e.g., chironomids, hip-poboscids, and nycteribiids). The latter comprise primarily the so-called Pupipara, in which the females are hematophagous and do not lay eggs and instead give birth to fully formed larvae (i.e., viviparous development). In addition to the above-mentioned variety of feeding habits, some groups may feed on multiple food resources during the same life stage (e.g., larvae that can be both saprophagous and pre-daceous and adults that are both nectarivorous and hematophagous). Larval sciomyzids may feed on dead or living mollusks, and some ephydrid larvae may consume algal, bacterial, or detrital resources during the same instar.
Saprophagous habits are among the most prevalent in Diptera, especially in brachycerous groups. Many fly larvae feed on decaying organic material or organic detritus, in which the resident bacteria and other microorganisms are the primary source of nutrition. Among the more common sources of these materials are animal carcasses, which are frequently colonized by calliphorids, muscids, phorids, sphaerocerids, and others. The sequence of colonization is often quite predictable, which contributes to the use of Diptera in forensic studies. Decaying fruit and vegetable material also is colonized by many groups, including especially ulidiid, sphaerocerid, and muscid flies. Decomposing plant fragments can be an important food resource in aquatic habitats, where it is consumed by the larvae of tipulids, ephydrids, ulidiids, and other groups. These groups and others (e.g., Psychodidae, Syrphidae, Stratiomyidae) also contain many species that feed on decaying, fine organic matter and associated microorganisms. Most Culicidae and Simuliidae consume fine particulate organic matter of varying size and quality, but use modified mouth-brushes or labral fans to extract particles from water. In most other saprophagous groups, including aquatic species, a sieve-like pharyngeal filter is used to concentrate microorganisms and other organic particles, whereas those feeding on carrion have well-developed mouthhooks for shredding and macerating raw meat.
Phytophagous groups, which consume live plants (including algae and fungi), are well represented by the larvae of bibionids, cecid-omyiids, mycetophilids, tipulids, phorids, tephritids, and agromyzids. Many of these flies can be serious agricultural pests. Aquatic habitats contain numerous flies that consume the thin films of algae and organic matter that occur on rocks and other substrata. Among the more obvious of these aquatic grazers are blepharicerids and certain species of psychodids, simuliids, and ephydrids.
Most predaceous Diptera attack other invertebrates as their primary food. Many families (e.g., Chironomidae, Culicidae, Tipulidae, and Ephydridae) contain a few predaceous species, whereas other groups (e.g., Ceratopogonidae and nearly all noncyclorrhaphan Brachycera) feed primarily or exclusively on invertebrates. Vertebrate prey (frogs and salamanders) can be part of the diet of larval Tabanidae. Whereas predaceous larvae typically kill multiple hosts, parasitic and parasi-toid larvae generally attack only one host. Parasitoids typically will kill that host, often after a long association with it. Twenty-two families of Diptera include parasitoid members, with tachinid flies perhaps the best known of these. Dipterans are parasitoids of other invertebrates, mostly other arthropods. Because other insects (some pests) often are attacked, parasitoids often are useful for biological control. The Diptera also includes several true parasites, which attack but do not kill the host, such as oestrids and various other groups that often exhibit distinct and complex migrations in vertebrate hosts.
ECONOMIC IMPORTANCE
Injurious Families
Several families of Diptera are of major economic importance and involved in the transmission of more disease pathogens to humans and other animals than any other group of arthropods. Biting flies cause annoyance that impacts tourism, recreation, land development, and industrial and agricultural production, whereas their effects on livestock can cause reduced milk, egg, and meat production.
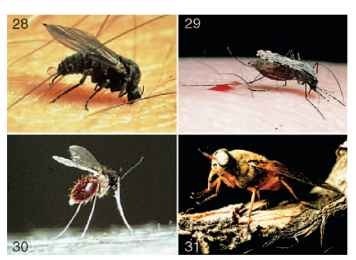
FIGURES 28-31 (28) Female black fly adult (Simuliidae) taking a blood meal. (29) Female mosquito adult (Culicidae: Anopheles) taking a blood meal. (Photographs by R. W. Merritt.) (30) Female sand fly adult (Psychodidae) taking a blood meal. (Photograph by B. Chaniotis.) (31) Female horse fly adult (Tabanidae).
The adults have mouthparts that have very effective piercing stylets, enabling these flies to “bite” and suck blood. Some major families with this characteristic include members of Simuliidae (Fig. 28 ), Culicidae (Fig. 29) , Psychodidae (Fig. 30) , Ceratopogonidae, Tabanidae (Fig. 31), and the bloodsucking Muscidae (Figs. 32 and 33). The bites from these groups can often cause severe allergic reactions, resulting in intense itching, rashes, and local swelling or, in some instances, hospi-talization as a consequence of toxemia or anaphylactic shock.

FIGURES 32-35 (32) Female stable fly adult (Muscidae: Stomoxys calcitrans ) taking a blood meal. (Photograph by E. Hansens.) (33) Horn flies (Muscidae: Haematobia irritans) resting and feeding on the back of a bull. (Photograph by R. W. Merritt.) (34) Adult male midge (Chironomidae). (Photograph by R. F. Harwood.) (35) Adult blow flies (Calliphoridae: Phaenicia sericata) on a pig.
Some of the major human and other animal diseases resulting from the transmission of causative organisms by Diptera include human onchocerciasis (river blindness) by Simuliidae; leishmaniasis (sand fly fever) by phlebotomine sand flies belonging to the family Psychodidae; several protozoan and viral diseases of domestic and wild animals, poultry, and waterfowl by Simuliidae and Ceratopogonidae; malaria, yellow fever, filariasis, dengue, dog heartworm, the encephalitides, and related viral diseases by Culicidae; and tularemia and animal trypanosomiases by Tabanidae. Several other species belong to the bloodsucking muscoid flies and include the tsetse fly of Africa, responsible for transmitting the pathogen causing human sleeping sickness, and the stable fly (Muscidae) (Fig. 32 ) , whose vicious bites can annoy humans in recreational areas, and bother domestic animals such as horses, cattle, and sheep. The horn fly (Muscidae) (Fig. 33) is a well-established biting cattle pest throughout many tropical and temperate areas of the northern hemisphere, whereas its close muscoid relative, the buffalo fly, is particularly important to cattle and dairy industries of Australia.
In addition to the biting habits and disease agent transmission of the above groups, flies can cause annoyance and interference with human comfort. Members of the genus Hippelates in the family Chloropidae are referred to as “eye gnats” because they frequently are attracted to the eyes of the victim, feed on secretions, and may assist in the entrance for pathogenic organisms. A muscoid fly having similar habits, known as the “face fly,” has been associated with the transmission of “pink eye” to cattle. Several other species of muscoid flies (e.g., house fly, bush fly, latrine fly) generally breed in excrement and at times can be economically important pests of humans and/or domestic animals. Two families of Diptera that can cause annoyance and constitute a nuisance by their sheer numbers emerging from ponds and lakes are the Chironomidae (nonbiting midges) (Fig. 34) and the Chaoboridae (chaoborid gnats). These are commonly mistaken for mosquitoes (Culicidae), but do not bite. When one encounters swarms of these midges or gnats, it is difficult to keep them out of one’s eyes or avoid inhaling them.
The dipteran families Calliphoridae (blow flies) (Fig. 35) and Sarcophagidae (flesh flies) (Fig. 36) are the major producers of myia-sis, that is, the infestation of organs and tissues of humans or other animals by fly maggots. The larvae of these groups feed on necrotic tissue and may accidently be ingested or invade wounds of humans and domestic animals, causing severe discomfort and subsequent secondary infections. The primary and secondary screwworm flies (Calliphoridae) (Fig. 37) are attracted to the wounds and sores of animals, and the former was one of the most serious pests of livestock in the United States until it was eradicated through the sterile male release program. In recent times, the identification and aging of the larvae of some species of blow (Calliphoridae) and flesh flies (Sarcophagidae) have proved useful in establishing the time of death or “post mortem interval (PMI)” in forensic investigations.
One other family, the Oestridae (cattle, sheep, horse, human, and rodent bot flies), is involved in enteric myiasis of animals and sometimes humans. Damage caused by horse bots (Gasterophilus spp.) (Fig. 38) varies from violent reactions by horses due to the flies ovipositing, to irritation by larvae when burrowing into the oral tissue and subsequent interference with digestion. The larvae of cattle grubs (Hypoderma spp.) migrate through the host’s body and eventually reach the upper back where they cut a small opening in the hide and remain there for some time. Economic losses in cattle result from reduction in milk production, weight loss, and damage to hides. Another species of bot fly, the human bot or torsalo (Dermatobia hominis), is common in parts of Mexico and Central and South America. It parasitizes a wide range of

FIGURES 36-39 (36) Adult flesh fly (Sarcophagidae). (Photograph by R. W. Merritt.) (37) Secondary screwworms (Calliphoridae: Cochliomyia macelleria) on a pig. (Photograph by M. J. Higgins.) (38) Horse bot fly larvae (Oestridae: Gasterophilus intestinalis) attached to the stomach of a horse. (Photograph by R. W Merritt.) (39) Human bot fly larvae (Oestridae: Dermatobia hominis) under the hide of an ox in Costa Rica.
hosts, including humans, but is a more serious pest of cattle and oxen in these areas (Fig. 39).
Several families of Diptera are economically important to agriculture. The Cecidomyiidae or gall gnats “sting” the plant and make it grow a “gall home” for them (Fig. 40), within which they find not only shelter but also adequate and abundant food. Examples are the goldenrod ball gall and the pine cone gall. Some very destructive species in this family, such as the Hessian fly (Fig. 41), chrysanthemum gall midge, and wheat, pear, and cloverseed midge, feed on cultivated crops and do not always form galls. The Tephritidae, or fruit flies, contain some species whose larvae bore into the stems of plants; some produce galls, others are leaf miners, and most important of all are those that bore into the flesh of fruits and vegetables. The latter include some of the most important of all economic insects, specifically the apple maggot, cherry fruit flies (Fig. 42), walnut husk fly, and Mexican, Mediterranean, oriental, olive, and melon fruit flies. The Anthomyiidae, or root maggot flies, have larvae that feed on decaying vegetable matter from which a number have adopted the habit of attacking the roots of vegetables. These include the cabbage maggot, onion maggot (Fig. 43), seed corn maggot, and spinach leaf-miner. Larvae of the family Agromyzidae are known as leafminers and feed between the leaf surfaces, leaving light-colored, narrow, winding mines or large blotches that decrease photosynthesis and make produce unsalable. The leaves are weakened and the mines promote disease and decay.
The Diptera contain several families that can be considered beneficial to humans and their environment. First, and most important, is the role of all Diptera in food chains in nature. Groups such as Culicidae, Chironomidae, and Simuliidae occur in large numbers as larvae and adults and provide a major prey base for many other invertebrates as well as vertebrates such as fish, birds, bats, and amphibians. In turn, several families contain predators and parasitoids as larvae and adults, including the Asilidae, Empididae, Dolichopodidae, Syrphidae, and Tachinidae.

FIGURES 40-43 (40) Cecidomyiid gall on grape leaves. (Photograph by R. Isaacs.) (41) Hessian fly (Cecidomyiidae: Mayetiola destructor). (42) Cherry fruit fly adults (Tephritidae: Rhagoletis cingulata) on cherry. (Photographs by Department of Entomology, Michigan State University.) (43) Onion maggot adult (Anthomyiidae: Delia antiqua).
Many families are important decomposers and recyclers of decaying organic matter of different types. Examples include the Psychodidae, Tipulidae, Stratiomyidae, Mycetophilidae, Sciaridae, Sepsidae, Coleopidae, Muscidae, Calliphoridae, Sarcophagidae, Phoridae, Syrphidae, and Sphaeroceridae. Some Diptera are important pollinators of flowers and include some species of Syrphidae, Bombyliidae, and even adult male Culicidae who visit flowers to imbibe nectar.
Some families of aquatic Diptera have been important in water quality and bioassessment studies to classify the degree of pollution in a water body. For example, larvae belonging to the midge genus Chironomus in the family Chironomidae have been referred to as blood worms because of the hemoglobin in their blood. These and another group known as the “rat-tailed maggots” (Syrphidae: Eristalis) are often used as indicators of polluted water or water low in oxygen. The presence of Simulidae in a stream generally indicates clean well-aerated water. The Culicidae and Chironomidae have members that are associated with both polluted and clean water habitats. Finally, some Diptera have been the subject of study for scientists throughout the world. For example, chironomid midges are used in acute and chronic laboratory toxicity studies to compare toxicants and the factors affecting toxicity and to ultimately predict the environmental effects of the toxicant. The small fruit fly, Drosophila (Drosophilidae) (Fig. 44), has been the organism of choice in most genetic studies for years and has contributed significantly to studies ranging from neurobiology to evolutionary theory. Overall, the Diptera represent an order containing a variety of species that are economically very beneficial and equally injurious to humans.
BIOLOGY OF SELECTED FAMILIES
Suborder Nematocera
TIPULIDAE
Crane flies (Fig. 45) are a diverse group of > 15,000 species that inhabit a variety of freshwater and terrestrial habitats. Larvae of some groups are important shredders (e.g., Tipula, Pedicia) of leaves that enter streams, and others are predators (e.g., Hexatoma, Dicranota) in aquatic habitats. The moist transition zone between aquatic and terrestrial areas supports a distinct assemblage of taxa (e.g., Erioptera, Ormosia). Terrestrial habitats are home to species that feed on rotting logs (e.g., Epiphragma) or decaying organic material (e.g., Tipula), with the latter sometimes pestiferous consumers of sod. A few species can tolerate high salinity and inhabit the rocky intertidal zones of marine habitats (e.g., Dicranomyia). The adults generally do not feed, although they are frequently mistaken for “giant mosquitoes.” A few taxa possess a long proboscis that presumably allows nectar feeding. Large and gangly, adult crane flies are easily taken by vertebrate predators such as birds.
PSYCHODIDAE
Sand flies (Fig. 30), drain flies, and moth flies are typical representatives of this family and contain 2900 species. Adult sand flies (Phlebotomus) are tropical hematophagous (blood-feeding) flies that can transmit leishmaniasis, a disease caused by parasitic protozoa spread by sand fly bites. However, most psy-chodids do not bite and are harmless to humans and livestock. Drain flies (e.g., Psychoda, Telmatoscopus) and moth flies (e.g., Maruina, Pericoma) resemble tiny moths (about 2-4 mm in length) with hairy, pointed wings. The former have larvae that develop on the rich organic material that builds up in domestic pipes and drains and can be abundant in households and public restrooms. Moth flies have aquatic to semiaquatic larvae that breathe atmospheric oxygen by maintaining contact with the atmosphere using hydrofuge hairs on their posterior spiracles. Eutrophic lakes, marshes, and wastewater treatment plants may produce large numbers of adults. As detriti-vores, the larvae of moth flies probably are significant nutrient recy-clers in lentic ecosystems.
BLEPHARICERIDAE
The net-winged midges (320 species) have peculiar larvae (Fig. 46) that use ventral suckers (suctorial disks) to maintain their positions on rocky substrates in torrential streams.

FIGURES 44-47 (44) Adult female small fruit fly (Drosophilidae: Drosophila). (Photograph by R. D. Akre.) (45) Adult crane fly (Tipulidae). (Photograph by Department of Entomology, Michigan State University.) (46) Ventral view of larva of Blephariceridae showing suctorial disks. (47) Adult net-winged midge (Blephariceridae).
A hydraulic, piston-like apparatus gives the larvae the ability to generate suction that allows their suckers to work—even waterfall habitats are occupied by blepharicerid larvae. The head capsule, thorax, and first abdominal segment are fused into a “cephalic division,” which helps keep the anterior part of the body close to the substrata while the larva is feeding. The larval mouthparts are specialized for scraping thin algal films off of rocks within fast-flowing environments. Diatoms and other unicellular algae are most often consumed, but fungi and bacteria may also be included in the larval diet. Pupae are also firmly attached to rocks within the flow with permanent adhesive pads. The emerging adult splits the pupal case by applying downward pressure against the substratum, and the adult (Fig. 47) usually reaches the stream surface in an air bubble. Because the wings develop to full size within the pupal case and merely unfold during emergence, adults can fly immediately upon reaching the water surface. Adult females with well-developed mandibles usually are insect predators, whereas males and nonmandibulate females appear to be primarily nectarivorous. Adults are known as net-winged midges because of the finely divided venation of the wings.
CULICIDAE
Mosquitoes (3600 species) ( Fig. 29 ) are well-recognized for their roles in disease agent transmission and as pests to humans, livestock, birds, and a variety of other vertebrate hosts. However, adults may emerge in high numbers and provide food for avian, bat, and certain predatory invertebrate populations. Mosquitoes may colonize new aquatic habitats quickly and can survive in confined container habitats. In terms of mosquito control, the ecological importance of the larvae, pupae, and adults is rarely considered. The larvae are mostly filter feeders, but some scrape organic material and algae from solid substrates in standing water habitats. The clearance rate of particles from standing water is impressive and may alter the characteristics, such as turbidity, of the water the insects inhabit. Larval populations are a major component of the neuston, or water-surface inhabitants, and maintain contact with the atmosphere with their spiracles. Larvae (Fig. 48) are known as “wrigglers” because of their frantic swimming action that allows them to dive when threatened; lessening of light intensity by a mere shadow will initiate the wriggling action in some Culex, making

FIGURES 48-51 (48) Larva of mosquito (Culicidae: Aedes aegypti). (49) Pupa of mosquito (Culicidae: Anopheles quadrimacu-latus). (50) Adult Ceratopogonidae. (51) Adult march fly (Bibionidae).
them difficult to collect. Some taxa, such as Culex, Culiseta, and Aedes, have their spiracles positioned apically on respiratory siphons; others, such as Anopheles, lack this breathing-tube apparatus. Mansonia and a few other genera possess siphons that are specialized for piercing the roots of wetland plants such as cattails to obtain oxygen and therefore do not need to come to the water surface to breathe. The mosquito pupa (Fig. 49) is free-swimming with respiratory trumpets that allow individuals to obtain atmospheric oxygen; pupae are known as “tumblers” because of their tumbling action that propels them below the surface when disturbed. Emergence occurs quickly at the water surface as the pupal skin breaks to liberate the adult. Although females feed on sugar sources and may take a blood meal for the purpose of egg production, males feed only on nectar and lack bloodsucking proclivities.
CERATOPOGONIDAE
This family is known as biting midges, punkies, and no-see-ums and contains more than 5600 species. The adults (Fig. 50) are minute bloodsuckers that swarm around mammalian hosts, including humans (e.g., Culicoides, Lasiohelea). Certain taxa also feed on other invertebrates as ectoparasites, including crane flies, dragonflies, and mantids. The tiny black to gray adults frequently have darkly patterned wings and relatively long antennae. Larvae are encountered in a variety of standing water habitats, including saturated mud and sand, tree holes (Dasyhelea), rain pools, marshes, lakes, and even hot spring algal mats (Bezzia) . The genus Leptoconops can be pestiferous and biting adults are encountered at ocean-side beaches. The larval feeding habits of biting midges consist mostly of scavenging and predatory behavior.
SIMULIIDAE
Although the general public is often aware of the pest nature of mosquitoes, knowledge of blood feeding by black flies (nearly 2100 species) is often restricted to anglers and those who recreate within or near aquatic systems. Like mosquitoes, the larvae of black flies play an important role as filter feeders; however, black flies are restricted to flowing water systems. Larval simuliids spin silken threads on the surface of riffle rocks, vegetation, and other substrata, and maintain a hold on the threads with hooks positioned on the posterior abdominal segment. They are able to move downstream on these threads without being swept away by the current. The mouthparts of most taxa (e.g., Simulium, Prosimulium) are modified into head fans, which allow the larvae to capture organic material, including particles as small as bacteria. Rocky substrates below dam spillways where organic-rich water flows may support tens of thousands of larvae per square meter. The larvae of a few groups lack head fans and scrape or collect food materials from benthic substrates (e.g., Gymnopais, Twinnia). Black-fly larvae are apneustic (i.e., lack spiracles) and therefore require moving water for cutaneous respiration. Pupae are firmly attached to substrata exposed to current, where thoracic pupal respiratory organs (gill-like structures) dangle in a downstream direction, supplementing spiracular respiration (Fig. 8). Most of the pupa is enclosed within a sheath-like cocoon. As in mosquitoes, male black flies have weak mouthparts and may feed on nectar, whereas the females of most species have short probosci and cutting mouthparts for obtaining a blood meal from their vertebrate hosts. The adults are small and grayish black, lack distinct patterns on the wings, and have short antennae (Fig. 28). Some species can swarm in large numbers and are capable of causing shock in domestic animals (e.g., cattle) due to blood loss.
BIBIONIDAE
March flies (750 species) are named for their early spring appearance in temperate habitats. The stout, dark-colored adults (Fig. 51) feed on flowers; in contrast, the worm-like larvae are general detritivores and can be common in organic soils and compost heaps. The genus Bibio overwinters as larvae prior to forming pupae after being exposed to cold temperatures. One pre-daceous species, Plecia nearctica, was introduced to the southeastern United States to control mosquitoes. Although its impact on mosquitoes is questionable, its impact on human residents is very real. The adults appear for brief periods (about 2 weeks) in such large numbers as to smear automobile windshields and clog radiators. The smashed bodies may even damage a car’s paint if not washed off quickly. The adults are known as “l ove bugs” because males and females are frequently seen flying in copula.
SCIARIDAE
These are known as dark-winged fungus gnats (2200 species) because of their small, gnat-like size and smoky grayish-black wings. Sciara is the genus most frequently encountered by people, as the pale, slender larvae develop in a variety of materials, including potting soil used in greenhouses and household planters. In nature, larvae consume the fungus-rich detritus formed under the bark of rotting trees, as well as within the logs themselves. Organic-rich compost heaps and mushrooms are also inhabited by sciarid imma-tures. Although a few taxa are pests (e.g., Pnyxia attacks mushrooms), most species of this common family are harmless.
CECIDOMYIIDAE
Gall midges and gall gnats are minute flies that are abundant, species-rich (6000 species), and cosmopolitan. More than 1000 species occur in North America, and many undescribed species await taxonomic attention. Most species form distinctive galls within which the maggots develop (Fig. 40). Indeed, it is frequently easier to determine what species is attacking a plant based on gall morphology rather than adult or larval morphology. Many people are quite familiar with the plants that are affected, such as the damage from maple leaf spot (Rhabdophaga), or the attack of the Hessian fly (Mayetiola destructor), which can be a serious pest of wheat. However, the Cecidomyiidae as a family shows impressive breadth in the plant species it attacks. A few species (e.g., Miastor) exhibit paedogenesis, whereby the larvae reproduce. The ” mother larva” produces a number of larvae within her body, which eventually consume the mother and then escape.
Suborder Brachycera
TABANIDAE
The horse flies (e.g., Tabanus, Hybomitra) (Fig. 91) and deer flies (Chrysops, Silvius) (Fig. 52) contain nearly 4400 species and are a familiar insect group to people who frequent rural outdoor areas. The adults are rapid fliers; one species was estimated to fly over 150 km per hour! Eggs are normally laid in masses, frequently on vegetation overhanging water or saturated soils. The cryptic larvae are restricted to aquatic and semiaquatic habitats where most species are predators of other invertebrates. The life cycle generally takes about 1 year to complete, whereas some of the larger horse flies require up to 3 years. Although most species inhabit stagnant habitats, some are found at the margins of streams. Females are blood feeders and may inflict a painful bite. Rather than puncturing a host’s skin and sucking blood like mosquitoes, tabanids create a laceration on the host’s skin and quickly lap up the pooling blood. The attack on livestock can be so severe as to reduce milk yields in dairy cattle. Like many other families of biting flies, females use visual cues to locate hosts and also sense plumes of carbon dioxide produced during vertebrate respiration. Horse flies tend to be large (about 10-25 mm) with nearly colorless or smoky wings, whereas deer flies are smaller (around 8 mm) and have yellow or black bodies that support darkly patterned wings. Human disease transmission by Tabanidae (i.e., tularemia, anthrax) is possible, but not significant in North America.
However, transmission can be significant in other areas of the world (e.g., Africa).
RHAGIONIDAE
The snipe flies (700 species) superficially resemble some deer flies, but have a more slender body. Most common in woodlands, snipe flies are often dull yellow to brown (e.g., Rhagio), but the gold-backed snipe fly (Chrysopilus ornatus) of eastern North America has brilliant gold hairs adorning the thorax and abdomen. Most adults are nectar feeders, whereas a few taxa are predators of flying insects. Larval rhagionids tend to be predators of small invertebrates within masses of rotting wood, organic-rich soil, or compost. One genus (Symphoromyia) of western North America has blood-feeding adults that will bite humans in woodland areas.
MYDIDAE
Mydas flies (460 species) include the largest adult Diptera, with some tropical species over 50 mm in length. The adults are dark and have red to yellowish coloration on some abdominal segments. Little biological information is available on this family, although some larvae are predators in decaying logs in woodlands. Pupae occur a few centimeters below the soil, and are adorned with heavy spikes for digging to the surface just prior to adult emergence. Adults are also thought to be predators that specialize in capturing other flying insects, but a fair number of species have vestigial mouthparts. The females of the latter may simply live on the accumulated fatty tissue in the abdomen.
ASILIDAE
The robber flies (7400 species) occur in a vast number of terrestrial habitats; most adult activity occurs in areas that are sunny or at least partially sun lit. Adults (Fig. 53) may reach approximately 30 mm in length (e.g., Proctacanthus), whereas others are less than 10 mm in length (e.g., Holocephala). There is great morphological variation in this family among adults, but all species have a conspicuously sunken vertex. Adults are predators that are able to take larger prey such as dragonflies, but the selected prey size varies among species. The type of prey, whether stationary, crawling, or flying, is also species-specific among robber flies. The mouthparts contain a stout proboscis that the adult uses to exsanguinate prey species. Some species (e.g., Laphria) mimic bumble bees, which reduces predatory attempts on the adult. Most larvae live in soil or rotting wood where they hunt other insect larvae and nymphs; however, some species are ectopara-sitic on Diptera, Coleoptera, Hymenoptera, and Orthoptera imma-tures. Very few life history studies have been done on the Asilidae.
BOMBYLIIDAE
Bee flies (>5000 species) are stout, hairy-bodied flies and, as the name implies, are frequently mistaken for hymenopterans because of their beelike appearance (Fig. 54). Furthermore, the adult behavior often involves hovering at flowers, beelike, and extending a long proboscis to obtain nectar while in flight! Some taxa have bold patterns on the wings (e.g., Anthrax, Exoprosopa) or have the anterior margin of the wing darkened (e.g., Bombylius). Although a widespread family, most species occur in arid areas. The biology of the immature stages remains unknown for most species, but species for which the larval feeding habits are known appear to be parasitic on Diptera, Lepidoptera, Hymenoptera, Coleoptera, and Neuroptera larvae or pupae. Many larvae have relatively large, tong-shaped mandibles, presumably suited to aid the parasitic life history. A few bombyliid species consume grasshopper eggs.
DOLICHOPODIDAE
The long-legged flies (>7000 species) are small to minute flies that are often brilliant green, blue, or copper colored (Fig. 55 ) . Males may have genitalia that are nearly as long as the other abdominal segments combined (e.g., Dolichopus). Adults participate in complex courtship rituals, and males of some

FIGURES 52-55 (52) Adult horse fly (Tabanidae). (Photograph by R. W. Merritt.) (53) Adult robber fly (Asilidae). (54) Adult bee fly (Bombyliidae). (55) Adult long-legged fly (Dolichopodidae).
species have legs adorned with flattened hair-like scales used as flags to communicate with females during courtship. The family is impressively diverse in its habitat use, as adults can be abundant in freshwater marshes and lake edges, stream margins, woodlands, open fields, and coastal marine areas. Adults appear to be exclusively predaceous. Larvae have been taken from water, damp soil, grass stems, under bark, and other places. Most taxa are predaceous, but a few (e.g., Thrypticus) are phytophagous. One genus (Medetera) has predaceous larvae that feed on bark beetles.
Suborder Cyclorrhapha
PHORIDAE
This family, also known as humpbacked flies and scuttle flies (4000 species), is another group that exploits a wide range of habitats and exhibits diverse feeding habits. The humpbacked appearance and reduced venation make the adults easy to identify. Many species are consumers of decaying organic matter and can infest household garbage cans; the females are strongly attracted to the odor of decay. Other species are more unusual, specializing on the consumption of slug eggs (some Megaselia) or parasitic on spiders, millipedes, and at least nine insect orders (e.g., Apocephalus, Melaloncha). Some species are currently targeted as potential biocon-trol agents of fire ants, a serious pest in the southern United States. One species is known as the coffin fly (Conicera tibialis) because it was reported to maintain many generations on a single human body in the confines of a buried casket.
SYRPHIDAE
Like the bee flies, flower fly adults (nearly 6000 species) resemble Hymenoptera and can mimic bees, bumble bees, and hornets (Fig. 56). Syrphids have the ability to hover (thus, they are also known as hover flies), and individuals are frequently found flying near flower heads where they obtain nectar. Adults are common near wetlands and lakes (e.g., Eristalis, Allograpta) but are also abundant in terrestrial areas (e.g., Merodon, Syrphus) where appropriate flowering vegetation grows. In aquatic habitats, the larvae of rat-tailed maggots (Eristalis) are collector-gatherers and may use retractable siphons for respiration (Fig. 57). Emergent vegetation, or vegetation at the aquatic-terrestrial interface, may be infested with aphids and
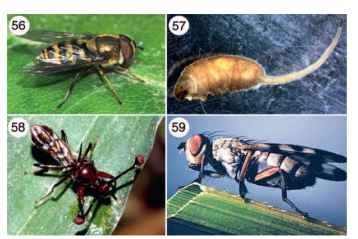
FIGURES 56-59 (56) Adult flower fly (Syrphidae: Eristalis sp.). (57) Rat-tailed maggot (Syrphidae). (58) Adult stalk-eyed fly (Diopsidae). (59) Adult picture-winged fly (Ulidiidae: Melieria similies).
other homopterous herbivores that certain flower fly larvae devour upon discovery. Some aphids enter plant stems compromised by boring larvae of other orders (e.g., Lepidoptera) to feed on decaying plant juices; it is not uncommon to find flower fly larvae that have also entered the damaged areas of plants to obtain prey. Predatory habits are also seen in terrestrial habitats, where larvae of certain species will inhabit dung, rotting logs, and decaying vegetation, as well as the exterior of plants. Overall, larval habitats are diverse, including dung, rotting cactus, peat, and hymenopteran nests. Some species have also been implicated in intestinal myasis.
DIOPSIDAE
The stalk-eyed flies (Fig. 58) are one of the most aptly named and morphologically unusual dipteran families (180 species). Each eye and its antenna are positioned at the end of individual stalks that protrude laterally from the head; the distance from eye to eye may be approximately equal to the entire body length! North America’s one species (Sphaerocephala brevicornis) has very short eye stalks and breeds in decaying organic matter. The adults of this species exhibit no particular courtship displays, whereas highly adorned males of Afrotropical species (Diopsis) battle with one another using their stalks as levers during aggressive “wrestling matches.” The larvae of Diopsis are herbivorous, and some species develop within the stems of rice plants.
ULIDIIDAE
These flies (previously in the family Otitidae) are also known as picture-winged flies because their boldly patterned wings are used in courtship and species recognition (Fig. 59). Adults are commonly found walking along vegetation flashing their wings. Ulidiids are abundant in both aquatic and terrestrial habitats (700 species). In marshes and vegetated lake margins, picture-winged flies are herbivores (e.g., Eumetopiella), secondary invaders of damaged plants (e.g., Chaetopsis) , and general scavengers (e.g., Seioptera). Herbivory also occurs in terrestrial species (e.g., Tetanops, Tritoxa), but scavenging of decaying organic material appears to be more common (e.g., Delphinia, Euxestra, Notogramma). Some groups also attack fungi (e.g., Pseudotephritis).
PYRGOTIDAE
Although some species of certain fly families (e.g., Tipulidae) are attracted to collecting lights at night, pyrgotid flies are unusual in that they are exclusively nocturnal. These flies (350 species) are relatively large and usually have strongly patterned wings (Fig. 60 ) . Adults (e.g., Pyrgota, Sphecomyiella) seek scarab beetles, most notably June beetles, and apparently attack flying beetles by laying a single egg on the dorsum of the thorax or abdomen that is exposed when a beetle’s elytra and wings are spread. The larva hatches from the egg and burrows into the body, acting as a parasi-toid. The feeding larva eventually kills the host and consumes the remaining tissue. Larvae pupate within the hollowed host, and the adult exits the beetle exoskeleton to continue the life cycle.
TEPHRITIDAE
These true fruit flies (>4600 species) are essentially entirely terrestrial in their habitat selection, although the host plants exploited by the family sometimes grow at the margins of lakes and marshes. Adults (Fig. 42) oviposit on the flower heads of the plant family Compositae or on fleshy fruits. Like the Ulidiidae, the wings of most adults are distinctly patterned, and adults flash their wings during courtship; this behavior has earned the Tephritidae a second common name, “peacock flies.” Species tend to be fairly specific in their host plant preferences or at least attack a narrow spectrum of plant taxa. Fruit fly species are also specific in the area of a plant that they infest. Some species are frugivorous (e.g., Ceratitis, Rhagoletis), seed-head predators (e.g., Euaresta, Trupanea, Tephritis), gallmakers (e.g., Eurosta), or leafminers (e.g., Euleia). Frugivorous larvae damage the host fruit, causing it to rot quickly; seed predators usually select young, developing seeds. Galls may be formed on a variety of plant areas, including stems, leaves, and flower heads.
DRYOMYZIDAE
These are relatively uncommon flies (30 species), with the biology of only two of the eight North American species known. Dryomyza anilis is a scavenger and breeds in decaying mammalian carcasses; it can be reared on raw ground beef. A contrasting life history is found in Oedoparena glauca, which preys on barnacles in the intertidal zone of western North American shorelines. This character makes O. glauca one of the truly marine insects, as it is tied intimately to an ocean-inhabiting invertebrate. Adults lay their eggs into the barnacle’s operculum when dropping tide levels expose them. Larvae consume the soft tissue, and mature larvae frequently move to new barnacles to continue feeding. Pupariation occurs within the final host.
SEPSIDAE
The black scavenger flies (375 species) are fairly abundant in both aquatic (e.g., Enicomira, Themira) and terrestrial (e.g., Sepsis) environments, where the larvae are scavengers of decaying organic matter. Dung of a variety of mammalian animals, carcasses, rotting snails, and washed-up seaweed have been exploited. The adults of many species are easily recognized by their rounded heads and the presence of a black dot at the apex of each wing.
SCIOMYZIDAE
Called marsh flies and snail-killing flies, neither name encompasses all the habits of this well-studied family (600 species). Some species (e.g., Dictya, Limnia) are found in marshes, but some species of certain genera (e.g., Sciomyza, Pherbellia) are fully terrestrial. Many species are larval parasitoids or predators of snails, and some attack slugs (e.g., certain Tetanocera, Euthycera) or fingernail clams (Renocera); the larvae of one genus (Antichaeta) prey on the eggs of aquatic and semiaquatic snails. The adults range in color from yellowish brown to brownish black, have antennae that may be long or short, and vary in size from a few millimeters to nearly 1cm (Fig. 61). However, the trophic niche of exploiting freshwater or terrestrial Mollusca (i.e., snails, slugs, and clams) ties all Sciomyzidae together evolutionarily. Ovipositional habits vary from certain species that lay their eggs directly on the host (e.g., Sciomyza) to species that

FIGURES 60-63 (60) Adult of Pyrgotidae. (61) Adult marsh fly (Sciomyzidae: Limnia). (62) Adult shore fly (Ephydridae: Ochthera mantis ) . (63) House fly adudomesticalt (Muscidae: M. ).
lay eggs on plants, thus requiring larvae to search for their hosts (e.g., Tetanocera). Only one species (Sepedonella nana) from Africa seems to deviate from the trophic tie to mollusks, as laboratory-reared larvae have fed and survived on aquatic oligochaetes in the laboratory.
CHAMAEMYIIDAE
Aphid flies are predators of aphids, mealy bugs, and other homopterous herbivores (350 species). Adults lay their eggs on plant surfaces, in galls, or in the egg sacs of scale insects, and the maggots can reduce homopteran populations (e.g., Leucopis). One report from Mexico showed that the adults of an aphid fly fed on the secretions of vertebrate animals and that the larvae may have developed in bird nests (e.g., Paraleucopis). Some taxa are found within emergent and shoreline vegetation of aquatic habitats, whereas others are encountered in woodland or open fields.
PIOPHILIDAE
These flies (80 species) are most commonly represented by the cheese-skipper fly (Piophila casei), a cosmopolitan consumer of proteinaceous materials. Larvae frequently infest cheese and exhibit the rather peculiar escape strategy of grabbing the posterior body segment with their mouthhooks to form a U shape and then releasing their grip, which causes the larvae to propel, or skip, away from their original location. These behaviors give them the name cheese-skipper, even though the larvae are known to consume dung and the drying tissues of aging mammalian carcasses, the latter making the group potentially important forensically. While most species appear to be scavengers of decaying materials and carcasses (Protopiophila) or mushrooms (Amphipogon), the larvae of some species are parasitic on birds (Neottiophilum).
SPHAEROCERIDAE
The small dung flies represent a speci-ose family (nearly 1600 species) that have predominantly scavenging larval feeding habits. One genus (Leptocera) consistently appears to be scavengers; however, the spatial niches inhabited by the larvae are highly diverse. Larvae have been found in decaying vegetable matter, sewage, dung, dung beetle broods, stranded masses of seaweed, fungi, slime molds, carrion, and the organic matter accumulated within cups of bromeliads. Muddy, organic-rich margins of aquatic habitats, such as marshes and ponds, will support virtual clouds of adults. Some species are quite habitat-specific, such as those that inhabit bogs. The adults of common species have long, stiff bristles dorsally and are black to gray in color, and the arista is several times longer than the other segments of the antenna.
EPHYDRIDAE
These flies are also known as shore flies, and most taxa are associated with aquatic habitats. This is another taxo-nomically rich family of Diptera (nearly 2000 species) and one of the most diverse in feeding habits. Larvae are consumers of decaying organic matter (e.g., Discocerina), secondary stem borers of damaged plants (e.g., Typopsilopa), primary herbivores (e.g., Hydrellia), gener-alist feeders of algae (e.g., Scatella), specialist consumers of algae and cyanophytes (e.g., Hyadina), diatom specialists (e.g., Parydra), predators (e.g., Ochthera), and consumers of spider eggs (e.g., Trimerina). Virtually all aquatic habitats, from flowing water to stagnant environments, temporary to permanent, freshwater to hypersaline, and cold water to hot springs, are occupied by ephydrids. Terrestrial environments are less likely to support ephydrid populations, but these flies are found in moist woodlands and even in sod from suburban areas. Shore flies can be abundant in human-made habitats, including constructed wetlands and sewage treatment plants. It is difficult to make generalizations about the overall morphology of this family, except that the adults tend to be small; the smallest adults (e.g., Lemnaphila), only a couple of millimeters across, mine the thalli of duckweed plants. Adult body color ranges from silvery gray to jet black, and the wings are completely colorless to highly patterned with various shades of gray and brown (Fig. 62). The species, habitat, and feeding diversity led the dipterist Harold Oldroyd to state that the shore flies are currently “in the flower of their evolution.”
DROSOPHILIDAE
Pomace flies, vinegar flies, and small fruit flies are another species-rich family (>3900 species). The latter name has led to some confusion as the Tephritidae are also known as fruit flies. The adults (Fig. 44) are small (generally only a few millimeters in length), but can disperse about 10 km in 1 day. Some dro-sophilids are frugivorous, but a vast array of food sources are used. For example, many feed on fungi (e.g., Amiota, Mycodrosophila, Stegana, Scaptomyza), living flowers (e.g., Apenthecia, Styloptera) or are predaceous on other invertebrates (e.g., Rhinoleucophenga, Cacoxenus, Acletoxenus). Indeed, the genus Drosophila, mostly known for the experimental studies of D. melanogaster, in the wild exhibits a vast trophic ecology and includes species that develop in rotting vegetation, rotting fruit, tree sap, fungi, living flowers, and plant stems and that prey on other invertebrates. Two Drosophila species are commensal with crabs: larvae live attached to the crab exoskeleton and consume semiliquid excretions from the crab or develop in the crab’s branchial chamber and consume its microflora.
CHLOROPIDAE
Flies in this family are called chloropid flies or frit flies (nearly 2900 species). Larvae are generally scavengers of decaying organic matter, secondary invaders of damaged plants, or primary herbivores. Adults are common and abundant among vegetation in terrestrial and aquatic situations. Species have been reared from dozens of different substrates around the world. Grasslands commonly support populations of certain genera (e.g., Meromyza, Parectecephala), and fungi are the sole food of others (e.g., Fiebrigella, Apotropina). However, a great diversity occurs in vegetated areas of aquatic habitats (e.g., Chlorops, Epichlorops, Eribolus, Diplotoxa) where most taxa are detritivores in decaying masses of vegetation, secondary stem borers, and primary herbivores of aquatic or semiaquatic plants. A few taxa are predatory on Homoptera (e.g., Thaumatomyia). Other, less common, larval food sources include dung (Cadrema), decaying wood, and bird nest debris (Gaurax), and one Australian genus (Batrachomyia) is subcutaneously parasitic on frogs and toads.
The adults tend to have rounded flagellomeres and range from dull colored to bright yellow or green, and many species have a distinctly shiny triangle positioned at the vertex of the head.
AGROMYZIDAE
These are known as the leaf-miner flies because of their highly herbivorous nature. Like the Chloropidae, agromyzids are well represented in both aquatic and terrestrial environments (>3000 species). Herbaceous and woody plants are both attacked, but larvae tend to feed on a single host plant, or a narrow spectrum as host plants as leaf miners, stem borers, or seed-head predators. Wetland taxa can form large populations in which both monocot and dicot flora are used as host plants (e.g., Agromyza, Cerodontha, Liriomyza, Phytomyza). One species (Melanagromyza dianthereae) is a specialist stem borer of water willow, a flowering plant found at the edge of streams. The females of this fly lay eggs on the exterior of the plant, and upon hatching the larvae burrow into the stem to initiate feeding. Leaf mines are frequently seen as dead or brown areas on a leaf surface, and mine morphology is sometimes distinctive enough to determine which agromyzid species is responsible for plant damage.
ANTHOMYZIDAE
These common flies are a group about which little is known of their biology (100 species). One genus (Anthomyza) has small yellowish adults that may feed on the culms of wetland sedges, but it is unclear if they are herbivorous or act as secondary stem borers after plants have been attacked by other herbivorous insects.
MUSCIDAE
This large family (5150 species) includes anthro-pophilic species such as the house fly (Musca domestica) (Fig. 63) and the stable fly (Stomoxys calcitrans) (Fig. 32). The house fly is well known for its “filthy habits,” and the stable fly bites both humans and livestock. The reproductive rate of the house fly is noteworthy, as one female can eventually give rise to 2 billion other female flies after several summer generations are produced (assuming all flies live, which is never the case). A short life cycle (12-14 days required for development from egg to adult in summer temperatures) is at least partially responsible for the success of this species and is necessary for developing in such ephemeral, human-made habitats such as dung heaps, garbage cans, and mammalian road kill. However, most muscid species are not directly associated with human populations. The larval feeding habits found among the Muscidae include herbivory (e.g., Atherigona, Dichaetomyia), scavenging (e.g., Graphomyia), and predatory behaviors (e.g., Coenosia, Lispe, Spilogona). A few taxa cause myiasis in birds (e.g., Muscina) or are avian blood feeders (e.g., Philornis). Some Muscidae form a cocoon prior to pupariation, which is uncommon among Diptera. Adult mus-cid flies may be predaceous on other insects, but most are generalized scavengers or feed on pollen.
OESTRIDAE
These are commonly known as bot or warble flies (150 species). The larvae of all species are endoparasites. Species that attack livestock burrow into the host skin to feed on living tissue and either form their pupae under the skin, forming warbles (Hypoderma), or drop off the host and pupariate in soil (Oestris). Four species of the horse bot fly (Gasterophilus) infest the alimentary tract of horses (Fig. 38), donkeys, and mules. One genus (Cuterebra) (Fig. 64) infests lagomorphs and rodents and is among the biggest bot flies (about 2.5 cm). The human bot fly (Dermatobia) lays eggs on mosquitoes and other biting flies. When a larva hatches, it hangs onto the bloodsucker’s leg until it lands on a human to take obtain a blood meal. The maggot then drops onto the host and burrows into the skin. Human bot flies are restricted to the Neotropical areas of the world and use a variety of mammalian hosts in addition to humans.

FIGURES 64-65 (64) Adult rodent bot fly (Oestridae: Cuterebra jellisoni) (65) Adult of Tachinidae.
North American vacationers and workers visiting the fly’s home range frequently return home with a painful welt, under which lays a feeding maggot that respires through a small hole in the person’s skin. The experience is painful, and most infected travelers have the larva removed surgically prior to pupariation or adult emergence.
HIPPOBOSCIDAE
Louse flies and bat flies (nearly 800 species) are specialized ectoparasites of birds, mammals (including bats) and even a few large reptiles. Like many blood-feeding calyp-trate flies, both males and females take a blood meal. Adults of many groups lack wings, which probably reduces the host’s chances of removing the fly. Females bear mature living young (i.e., they are pupiparous), which is uncommon among Diptera. The reproductive biology of these flies is unusual in that the eggs hatch one at a time in the female oviduct, and the larva receives nutrients produced by glands within the female abdomen. Female bat flies deposit the larva on the walls of bat roosts, and females of some species will sit on top of the larva and briefly press it to the wall to ensure good adhesion. Pupariation occurs quickly after larviposition. The adult emerges, and then seeks a host to continue the bloodsucking habit. Therefore, larval feeding does not occur outside of the adult female fly.
CALLIPHORIDAE
The blow flies are thought to have been given their name from Homer’s classic topic, The Iliad, in which he wrote about the “blows of flies” infesting the wounds of injured and dead soldiers. Most of the >1500 species of this cosmopolitan family are attracted to rotting flesh and can sense the chemical scent of decay within minutes of death. In nature, the adults accelerate the decomposition of all types of vertebrate carcasses, and most blow flies (e.g., Calliphora, Cochliomyia, Lucilia, Phaenicia) are specialist scavengers. Because of the ability to find dead bodies rapidly, forensic entomologists use the stage of larval development (i.e., age of a larva) found on corpses of people who died from suspicious causes as a way to determine the time between death and corpse discovery (i.e., the PMI). It is well documented that by using this method, the time of death often can be estimated with a fair amount of accuracy. Other taxa of blow fly, however, exhibit other feeding habits, such as parasitism of land snails (e.g., Helicobosca), earthworms (e.g., Pollenia), and amphibians (e.g., Bufolucilia). The adults are also known as blue bottle and green bottle flies because some taxa have metallic brightly colored bodies (Figs. 35 and 37 ).
SARCOPHAGIDAE
Flesh flies have been given a name that often contrasts their biology. Only a few of the more than 2600 species invade or consume carrion (e.g., Sarcophaga) or living tissue (e.g., Wohlfahrtia). Dung is a more common habitat in most groups (e.g., Ravinia). Many taxa are parasitic on other invertebrates (snails, earthworms, insects, and others), whereas some specialize in consuming the decaying bodies of insects found in the bottoms of pitcher plants of wetland habitats (e.g., Fletcherimyia). Females are viviparous, young hatch within the female’s abdomen, and she deposits them as first instars on the desired substrate. This may give flesh flies an advantage over potential competitors for food because mortality of eggs by predation or parasitism is avoided, and larvae can feed immediately rather than waiting to hatch for some days prior to feeding. The adults of most genera are easily recognizable by the gray thorax possessing longitudinal black stripes (Fig. 36).
RHINOPHORIDAE
This is an unusual fly family in that nearly all of the 150 species for which biological details are known are specialist endoparasitoids of terrestrial isopods (also known as sow bugs, pill bugs, and potato bugs). This family represents the only dip-terans that attack isopods. The larval life is tenuous, because larvae hatch from eggs laid in moist soil and must wait for a passing isopod. Perhaps the proleg-like apparatus present on the first instar is an adaptation to securing itself to a host. Both species of rhinophorids found in North America were probably introduced from Europe.
TACHINIDAE
These flies (>9600 species) (Fig. 65) are important parasites of a variety of other insects and are used in biological control programs against pestiferous Lepidoptera. Eggs are deposited on hosts or in areas where hosts are common. Some species retain their eggs so that they will hatch almost immediately after being laid; this strategy prevents the loss of the egg if the host happens to molt shortly after oviposition. Insertion of the egg through the epidermis of the host has evolved in a few groups (e.g., Phorocera). An alternative strategy used by some taxa is to lay many eggs on partially consumed plants; when a potential host returns to continue feeding, the eggs are consumed along with the plant material. A few eggs survive maceration by the mandibles, and the larvae hatch within the host’s foregut. Other genera broadcast their eggs, and the larvae burrow selectively into soil or rotting wood where they actively seek a host insect. Most often, tachinid flies attack only one species or a narrow spectrum of hosts; however, a small number (e.g., Compsilura) have been reared from some 200 different animal host species.
