Collembola or springtails comprise one of the most widespread and abundant groups of terrestrial arthropods. They are found everywhere, to the utmost reaches of multicel-lular animals in the Antarctic and Arctic and in all habitats except
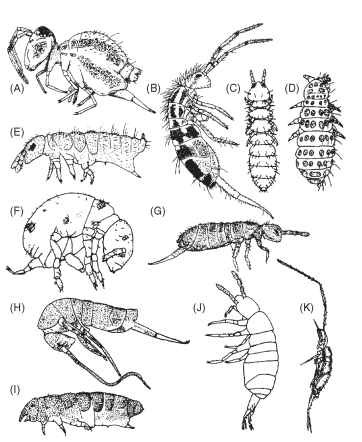
FIGURE 1 Variety of Collembola forms (not to scale). (A) Sminthuridae. (B) Entomobryidae. (C) Onychiuridae. (D) Neanuridae. (E) Hypogastruridae. (F) Neelidae. (G) Isotomidae. (H) Tomoceridae. (I) Odontellidae. (J) Oncopoduridae. (K) Paronellidae.
the open oceans and deep areas of large lakes. These all-wingless hexapods range in adult size from 0.2 to over 17 mm. Their small size generally results in their being overlooked, but they display an enormous range of body forms (Fig. 1), habitats, and habits. While most feed on fungi, bacteria, and decaying vegetation, some are carnivores, others are herbivores, and a number are fluid feeders. There are many commensal but no parasitic forms. They are most common in soils and leaf litter, but many species live in vegetation, littoral and neustonic habitats, caves, and ice fields or glaciers. Collembola have been classified with the insects but are now generally considered to belong to a separate class, in line with the Diplura and Protura. There are approximately 8000 described species belonging to about 29 families ( Table I ).
ANATOMY
All Collembola are primitively wingless hexapods. All have three thoracic segments and six or fewer abdominal segments, including a tel-son consisting of a dorsal and two ventral valves surrounding the anus.
There are typically four antennal segments, each with musculature (this distinguishes them from true insects, with three, and Diplura, with many antennal segments). Collembola vary enormously in form and somewhat in internal anatomy, but all lack Malpighian tubules and most have paired labial nephridia that empty into the ventral groove at the base of the labium. One universal and unique feature is the ventral tube or collophore (Fig. 2)—a distally weakly paired projection from the first abdominal segment with membranous,
TABLE IFamilies and Numbers of Species of Collembola |
|
| Family | Number of species |
| Suborder Poduromorpha | |
| Hypogastruridae | 650 |
| Odontellidae | 120 |
| Brachystomellidae | 130 |
| Neanuridae | 1350 |
| Onychiuridae | 530 |
| Poduridae | 1 |
| Tullbergiidae | 210 |
| Isotogastruridae | 5 |
| Suborder Entomobryomorpha | |
| Isotomidae | 1500 |
| Coenaletidae | 2 |
| Actaletidae | 10 |
| Entomobryidae | 1620 |
| Microfalculidae | 1 |
| Paronellidae | 370 |
| Cyphoderidae | 130 |
| Oncopoduridae | 50 |
| Tomoceridae | 130 |
| Suborder Symphypleona | |
| Arrhopalitidae | 120 |
| Collophoridae | 8 |
| Mackenziellidae | 1 |
| Sminthurididae | 150 |
| Katiannidae | 200 |
| Sturmiidae | 2 |
| Spinothecidae | 6 |
| Dicyrtomidae | 200 |
| Bourletiellidae | 240 |
| Sminthuridae | 250 |
| Suborder Neelipleona | |
| Neelidae | 30 |

FIGURE 2 Typical Collembola anatomy.
sometimes eversible, distal vesicles. Probable functions include imbibition, excretion, respiration, and adhesion to smooth surface. Collembolan mouthparts are said to be entognathous, being concealed by the head capsule, and typically adapted for chewing. The mandible usually has apical teeth and a molar plate, and the maxilla varies greatly and bears a number of complex lamellae.
In some Neanuridae and a few other groups, the mouthparts are simplified and the mandible may be lost (in connection with adaptation for specialized, including liquid, diets). In other Neanuridae the mandibles and maxillae show an inexplicable complexity (Fig. 3) and

FIGURE 3 Collembolan mouthparts: (A) typical mandible and (B) maxilla; (C) reduced mandible and (D) maxilla of Cyphoderidae; (E) piercing and sucking mandible and (F) maxilla of Neanura; (G-I) various mandibles of Neanuridae and (J-L) various maxillae of Neanuridae.
diversity of form equal to that seen in any other order of insects. The mouth opening is connected to the anterior surface of the ventral tube by a ventral groove through which fluids may flow.
Collembola are equipped maximally with 8 + 8 ommatidia but often have a supplementary light sensory organ between the antennae on the dorsum of the head. A few Collembola possess rudimentary trachea; however, respiration is normally through their thin cuticle and the membranous surface of the ventral tube. The reproductive system consists of paired ovaries or testes opening on the venter on the fifth abdominal segment. Collembolan legs consist of one or two apparent subcoxal segments, a coxa, femur, trochanter, fused tibiotar-sus, and distal, normally four bladed, unguis. An opposable smaller lamellate unguiculus is usually present.
Most Collembola have a forked ventral jumping apparatus or fur-cula on the fourth abdominal segment, consisting of a single basal manubrium and paired distal dentes and mucrones. It is held in place by the latch-like tenaculum on the third abdominal segment. When the tenaculum releases, the furcula catapults the animal, as much as 10 cm. All Collembola are covered with setae but their number, size, and structure vary greatly from group to group. The cuticle of Collembola is extremely varied and often has elaborate surface structures.
FOSSIL HISTORY
The first fossil Collembola occur in the 400 million-years-old Rhynie chert deposits of the Devonian, although there are secondary fossil hints of earlier Collembola occurrence. These fossils display very modern collembolan features, including typical entognathous, chewing mandibles; ventral tube; and, probably, a furcula. The single described species —Rhyniella praecursor—has been placed in a variety of families, including recently Isotomidae; however, all family placement must be considered very tentative and it is likely that one or two additional species are in this deposit. A single specimen of a very probable member of the family Entomobryidae was found in Permian shale of South Africa but extensive collembolan fossils are limited to amber of the Cretaceous, Oligocene, Miocene, and Pliocene. Collembola represent only a small fraction of the hexapods found in amber, and they are absent from many amber deposits; however, there are over 70 specimens from late Cretaceous Canadian amber, 78 from mid cretaceous Burmese amber, over 160 from the Baltic Eocene amber, about 130 from Miocene amber of Chiapas and the Dominican Republic, and 16 from Pliocene Japanese amber. The Cretaceous material has only one specimen from an extant genus and most specimens can be placed in one of 19 extinct genera. All the remaining amber specimens can be placed in extant genera and in a few cases in extant species. Since the Eocene, generic extinction appears to have been absent, a unique feature among hexapods well represented in Eocene deposits.
VARIETY OF BODY FORMS
Although the generally considered primitive Collembola (Fig. 2) display most of the features described above, most genera differ from this. All families have some forms with reduced numbers of eyes, and Neanuridae, Hypogastruridae, and Isotomidae (Fig. 1E and 1G) often have reduced or no furcula. The Neanuridae (Fig. 1D) often have large spines on the body as well as spectacularly complex mouthparts. Indeed these are so complex and varied (Fig. 3) that species can be identified by their mouthparts alone. The Onychiuridae (Fig. 1C) all lack eyes and almost all lack pigment and a furcula. They are characterized by the presence of pseudocelli through which defensive toxic and/or repulsive fluids are secreted. These along with the Hypogastruridae, Poduridae, and Neanuridae have well-developed, seta bearing, first thoracic segments; the remaining families all have greatly reduced, nonsetaceous, first thoracic segments (Fig. 2 } , and some families have fusion of abdominal segments. The Neelidae and Symphypleona have the first four abdominal segments fused and more or less fused with thoracic segments. Some Entomobryidae and Symphypleona (as well as most Tomoceridae) have antennal subseg-mentation, giving the appearance of more than four antennal segments. The largest species are found in the Neanuridae, Entomobryidae, and Tomoceridae, often reaching 5 mm and occasionally over 10 mm in length, but the Neelidae and Mackenziellidae rarely reach 1 mm.
HABITATS AND HABITS
Most Collembola in temperate and arctic zones live in the soil or ground litter, but there are several groups, most notably the Sminthuridae, that largely inhabit vegetation. In tropical regions Collembola are abundant in trees and epiphytic plants. In rain forests, they are rare in soils but abundant in trees. Collembola are abundant in many caves and are frequent in marine littoral zones. They are also common in the interstitial sand regions of marine beaches and the surface of standing fresh water. In all these examples there are many species specialized for these habitats. Collembola have recently been discovered at depths up to 20 m in both fresh and salt water, but nothing is known of the habits of such forms. Many species are found in bird and mammal nests, and microcav-ernicole habitats are frequently exploited but such forms show no particular specializations, being also found either in litter or in soil habitats. Ant and termite nests are frequently occupied, and one family, the Cyphoderidae, consists largely of species limited to and highly adapted for life in these habitats. Some of the most striking examples of presumed commensalism occur in the genus Axelsonia (Isotomidae), of which one species lives in the gill chamber of land crabs, and in the family Coenaletidae, of which all species are confined to the shell of terrestrial hermit crabs.
The forms living in the different habitats often display a suite of morphological characteristics correlated to their habitat. Thus, forms that have reduced furcula, reduced or no eyes, weak pigment, and reduced pointed tenent hairs are characteristically found in soil. Forms with no eyes or pigment; well-developed furcula; elongate, slender untoothed ungues; and reduced, pointed tenent hairs (troglomorphic) are almost always cave dwelling. Almost all species with well-marked color patterns and well-developed furcula are either litter or vegetation dwelling (Fig. 4).

FIGURE 4 Collembolans discovered in various habitats in Reading, UK. (A) Podura aquatica (Poduridae), from the surface of a garden pond. (B) Kalaphorura burmeisteri (Onychiuridae), from soil. (C) Dicyrtoma fusca (Dicyrtomidae), from leaf litter. (D) Entomobrya nicoleti (Entomobryidae), under surface debris.
REPRODUCTION AND DEVELOPMENT
Fertilization is internal; however, exchange of sperm occurs in a variety of fashions. Sexual receptivity is associated with adult molting and in some species pheromones to facilitate aggregation of sexes. The sperm is produced in a packet, often with a stalk holding it above the substrate. In some groups (most Onychiuridae) these packets are produced randomly and fertilization occurs by accidental contact of the female with the packet of sperm. In a number of species the packets are produced only in the presence of females, but the most elaborate procedures are seen in Podura aquatica and the Symphypleona. Here, often, there are elaborate courtship and maneuvering associated with fertilization. This is often accompanied by modifications in male anatomy, which ensure the appropriate species response and/or positioning for sperm packet uptake. Most of these species are brightly colored and patterned, which may also be associated with species recognition. In these forms, sexual dimorphism is the rule and often extreme. This is also true of many marine littoral species, but in these, the method of sperm transfer is still unknown and the function of the dimorphic structures (usually male) is unclear.
In some members of the family Isotomidae secondary sexual characters alternate with molts, being expressed in stages in which the animals are sexually receptive and not expressed in stages in which they are not receptive. In most Collembola there is little or no sexual dimorphism and sexes can be separated only by the difference in their genital openings. Both males and females occur in most species but parthenogenesis is common, especially in some genera of the Tullbergiidae.
Development is direct, with the young generally very similar to the adults except for the absence of sexually associated features and body ratios and some aspects of the setae clothing. The main exception to this generalization is in the Tomoceridae, whose juveniles have been assigned to genera different from those of the adults. Collembola continue to molt after reaching sexual maturity and some species can molt very large numbers of times (the record is 52). They stop reproducing at some point and later molts result in reduced rather than increased size. Although some Collembola have been known to live more than 5 years in captivity, their life span in the wild is undoubtedly much shorter.
UNUSUAL FEATURES
One remarkable feature of some members of the family Onychiuridae is that some male-only specialized setae on the venter of the abdomen achieve full development only several molts after sexual maturity. Their function is unknown.
Many species of Collembola, almost entirely of the families Isotomidae and Hypogastruridae, go through a period of reduced activity, wherein they develop a unique morphology, often associated with the development of heavy abdominal spines and wrinkled surface and reduced mouthparts and digestive systems. When this is associated with particular ecological conditions (most commonly drying or elevated temperature), it is termed ecomorphosis: feeding ceases and the structural changes are usually striking. The cessation of the causal conditions results in a quick molt and return to normal anatomy and activity. When these conditions are part of a regular cycle the process is called cyclomorphosis.
A number of Collembola are also capable of anhydrobiosis, that is, they can become completely dry without dying. In some (but not all) instances these animals form small ball-like capsules around themselves before entering this state. If wetted, the animal resumes normal activity in an hour or two. Recent studies with sand dune Collembola suggest that this capacity may be more widespread than currently established. Another unusual feature of Collembola is the ability of some species to live very long periods without food. This characteristic appears to be best developed in some cave forms, and in several instances animals reproduced after not being fed for 30 weeks. The longest survival was a specimen of Onychiurus, which lived over a year without food and was then accidentally killed.
ECOLOGY AND ROLES IN ECOSYSTEM
Because Collembola are found in all habitats, from the coldest to the hottest supporting multicellular life, and from treetops to the deepest soil layers supporting multicellular animals, it is clear that their responses to various abiotic conditions must vary enormously. Humidity is usually the most important factor in determining Collembola distribution. High humidity is seldom a problem for Collembola but desiccation is often serious. Collembola resist desiccation by moving into microenvironments of high humidity (under stones or into deeper soil layers) and/or limiting activity to nights and by morphological adaptations (such as cuticular thickening, ornamentation, and scales). Some species, as already discussed, change form radically and cease feeding, while others go into anhydrobiosis. Many species lay eggs that are much more resistant to drying and they survive desiccation in this stage, often accompanying this with short postembryonic life cycles.
Collembolans have vastly different temperature tolerances and preferences, ranging from a species of Sminthurides found in volcanic vents with temperatures as high as 48°C to an Antarctic species shown to survive temperatures below -30°C. Survival (and activity) in low temperatures has been studied extensively. Some Collembola are primarily inhabitants of glaciers and ice fields and others are dominant members of the arthropod faunas of high latitudes. Winter-active Collembola in temperate climates often build up large numbers under snow and on suitable warm days pour out onto the snow in vast numbers as snow fleas. Extreme cold tolerance always involves supercooling with the accumulation of cryoprotective substances.
Oxygen requirements of Collembola also vary enormously. The greatest tolerances discovered are in the Antarctic Cryptopygus ant-arcticus, which has a 30% survival rate after 30 days in pure nitrogen atmosphere. In many Collembola, respiration when submerged is via air films surrounding the animals as a result of their hydropho-bic cuticle, but this apparently is not necessary in all forms. In many forms the eggs are more resistant to immersion than in other stages.
Collembola, even in uniform soils, are never randomly distributed, but show strong clumping because of pheromones or local food abundance or simply as a result of limited dispersion after founding events and subsequent population growth.
Competition between Collembola species in cultures has in at least a few instances shown that there is no evidence for competitive exclusion, even under long-term, clearly competitive conditions. In addition it has been shown that interactions between two species can be either positive or negative depending upon the nature of the interaction (airborne allomones, substrate-transmitted allomones, or direct contact).
While most soil- and litter-inhabiting Collembola feed primarily on decaying vegetation and fungi (and appear to be general feeders), experimental studies have shown that, given a choice, they may be very selective as to both the decay state and nature of the vegetation and the species of fungi. A number of Collembola are occasionally or primarily (and in a few species exclusively) carnivores, different species feeding on a variety of organisms, ranging from rotifers to other Collembola. Probably the most commonly eaten prey is nema-todes. Vegetation-inhabiting Collembola eat primarily unicellular algae, pollen, and soft parts of vegetation and fungal spores. Many Collembola are coprophagic, feeding largely on arthropod feces. Some littoral species appear to feed largely on diatoms or unicellular algae, and forms with piercing-sucking mouthparts feed largely on fungal hyphae juices. Thus their primary role in the environment is that of reducer; however, another major role is that of prey. The ability to jump is the major defense mechanism of Collembola; however, many Poduromorpha, particularly those with the furcula short or absent, have body fluids that are repellent to predators, and they may release these by reflex bleeding when attacked. Most carnivorous soil organisms feed on Collembola, and many beetles, ants, and wasps are specialized for feeding on them.
HUMAN INTERACTIONS
Collembola rarely interact overtly with humans. There are few agricultural pests and, except for the introduced Lucerne flea (Sminthurus viridis) in Australia, which is a pest in pastures and horticultural crops, these are of little economic importance. There are no parasitic Collembola and they are not known to transmit any diseases. Mass emergences occur and may cause a temporary problem with household infestation but they are generally short-lived there. The true household Collembola are unobtrusive and generally overlooked. Collembola play an important role in the development and maintenance of healthy soils, but this is not generally appreciated. Here they are usually abundant and may reach densities up to a trillion per square meter.
