Beetle diversity so characterizes Earth that instead of telling future extraterrestrial colleagues we come from the “blue planet,” we might better state that we come from the “beetle planet.” Beetles comprise 25% of all described animals and plants, single-handedly making them the primary contributor to the world’s known biodiversity. The 350,000 described beetle species are members of the largest order of life on Earth, Coleoptera.
Familiar beetles are known by various names including fireflies, ladybugs, june bugs, and weevils. The vast number of beetle species is reflected by a bewildering array of anatomical and biological diversity in the order. Coleoptera are represented in nearly all bio-geographic regions and nonmarine habitats. Most adult beetles can fly; when not in use, however, the delicate flight wings are usually concealed beneath protective shell-like elytra, permitting beetles to utilize diverse resources and engage in a broad range of activities that otherwise would be restricted to either winged or wingless insects. Most beetles are herbivores, fungivores, or predaceous carnivores in the larval and adult stages. Many are considered to be serious pests of our homes, forests, crops, and stored products, whereas some beneficial species are regularly employed as biological control agents. Countless curious youngsters, including Charles Darwin, Alfred Russel Wallace, and Henry Walter Bates, have started their broader studies of biology through beetle collecting, as beetle species often are consistently found in specific sorts of habitats.
The technical name, Coleoptera, was coined by Aristotle to signify the hardened, shield-like forewings (coleo = shield + ptera = wing). Although several other insect orders possess hardened forewings, beetles are considered to be a monophyletic assemblage based on their sum of shared evolutionary derivations that include the following:
1. A holometabolous life cycle, wherein the larval stages are devel-opmentally separated from the adult by the pupal stage.
2. Possession of hardened forewings, called elytra, that abut medially. Flight is powered predominantly by the metathoracic wings, which are folded longitudinally and usually transversely to lie under the elytra when the beetle is walking or at rest. The mesothoracic scutellum is usually visible as a triangle situated medially between the bases of the two elytra.
3. A prothorax that is distinct from, and most often freely articulating with, the following mesothorax. The meso- and metathoracic segments are fused to form the pterothorax.
4. A generally depressed body shape, whereby the legs are situated on the ventral surface of the body. The leg bases, or coxae, are recessed into cavities formed by heavily sclerotized thoracic sclerites.
5. Abdominal sternites that are much more heavily sclerotized than the tergites. These sternites may close tightly against the lateral edges of the elytra, protecting the hind body from the attentions of predators and parasitoids.
6. Antennae usually with 11 or fewer segments.
7. Terminal genitalia that are not visible when in repose; that is, the male aedeagus and the female ovipositor are retracted into the abdominal apex when not in use.
Insects in several other orders may appear superficially similar to beetles. For example, various Hemiptera in the superfamily Pentatomoidea possess an enlarged triangular scutellum and heavily sclerotized forewings. However, these bugs can be distinguished by their beak-like suctorial mouthparts, whereas beetles retain the more generalized mandibulate mouthparts seen throughout orders such as Odonata, Orthoptera, and Hymenoptera. In addition, the forewings of Hemiptera always retain an apical membranous portion, whereas beetle forewings are consistently sclerotized throughout their length. Also, Dermaptera, or earwigs, exhibit quadrate forewings, looking much like the foreshortened elytra of staphylinoid, or rove beetles. Earwigs, however, exhibit a radial wing-folding mechanism versus the transverse folding system of beetles, retain the presence of abdominal cerci, represented by large tong-like forceps at their abdominal apex, and do not undergo complete metamorphosis incorporating the pupal stage.
BEETLE DIVERSITY
Although beetles share characters supporting their common evolutionary origin, remarkable variations have evolved from the basic beetle theme. For example, adult body size ranges from the 0.4-mm-long Nanosella fungi ptiliid feather-winged beetles of North America to the 200-mm-long Titanus giganteus cerambycid long-horned beetles of South America. A rough estimate based on maximum dimensions for adult length, breadth, and depth puts the disparity in volume at a factor of 2.8 X 107. Life cycles also can vary in extraordinary ways, depending on the larval food resources used for development. The mushroom-inhabiting aleocharine staphylinid Phanerota fasciata completes three instars in 3.2 days at room temperature. Even more impressive, Anisotoma—round fungus beetles of the family Leiodidae—can complete larval development on ephemeral slime mold fruiting bodies in as little as 2 days, making them arguably the fastest developing beetles yet recorded. Conversely, C. V Riley, the first entomologist of the U.S. Department of Agriculture, reported that a larva of the dermestid carpet beetle, Trogoderma inclusum. survived for 3.5 years in a tight tin box. These larvae feed on the dried proteinaceous matter in animal remains, and even if Riley’s larva had started with a tin full of insect specimens, the feat of solitary confinement is remarkable. Trogoderma larvae can even molt to a smaller size under starvation conditions, and then regain size by progressively molting when food returns. Stan Beck found that mature larvae molted retrogressively eight times during a year of starvation, dropping from an initial weight of 9.24 mg to a final, svelte 1.38mg (an 85% weightloss!).
Dramatic variation in reproductive capacity is also observed across the Coleoptera. An abundant plant pest such as the chrysomelid northern corn rootworm, Diabrotica barberi, can colonize cornfields and build populations quickly, since each female lays on average nine clutches of eggs, spaced 6 days apart, totaling 274 eggs over the reproductive period. At the opposite extreme we once again find the diminutive, feather-winged Ptiliidae. In eight species of Bambara ptifiids from Sri Lanka, the males produce spermatozoa that range in length from 220 to 600 |im; the largest size being more than two-thirds the length of the adult male producing them. After mating, these giant sperm pack the female spermatheca, with up to 28 spermatozoa recorded filling this structure. The length of the female sper-mathecae of various Bambara species is consistent within species and varies in proportion to the length of the complementary male sperm,whereas the diameter of the spermathecal duct varies in proportion to the diameter of the sperm. The female also invests heavily in her progeny, maturing one relatively giant egg in her abdomen at a time. The highly complementary male spermatozoa and female spermath-ecae ensure reproductive isolation because of biomechanical incompatibilities associated with any attempted interspecific mattings.
Beetles are among the earliest diversifying groups of the Holometabola. They form a branch on the Tree of Life together with the Neuropteroidea (Megaloptera, Raphidioptera, and Neuroptera). The order Coleoptera is divisible into four major lineages, which are recognized as the suborders Archostemata, Adephaga, Myxophaga, and Polyphaga (Table I). Present-day diversity among the four coleopteran suborders is highly skewed toward the Polyphaga. Taking the numbers of beetle species estimated for Australia, John Lawrence and Everard Britton calculated that Archostemata (9 species) make up 0.03% of the Australian beetle fauna, Adephaga, with 2730 species comprise 9.6%, Myxophaga, with 2 species (0.007%), and, with 25,600 species, Polyphaga, dominates at 90.4% of the fauna. Extrapolating these figures to the estimated world total of 350,000 described beetle species suggests that Polyphaga would account for more than 300,000 species.
Consensus concerning the phylogenetic relationships among all four suborders has yet to be achieved. Recent summaries of morphological data and separate efforts using molecular sequence data reach different conclusions based on the character types and sets of taxa included. Most studies agree that the Archostemata are the sister group to the other three suborders. The position of Myxophaga remains ambiguous, though Beutel and Haas’s comprehensive morphological analysis places them as the sister group to Polyphaga.
The burgeoning discoveries of beetle diversity throughout the course of modern scientific endeavor have begged the question, “Why?” The noted geneticist J. B. S. Haldane, in a lecture on the biological aspects of space exploration, stated that “the Creator, if he exists, has a special preference for beetles, and so we might be more likely to meet them than any other type of animal on a planet that would support life.” No single answer provides the definitive biological explanation for the present-day preponderance of beetle diversity. A number of answers are consistent with the pattern of diversity, with some better supported by the comparative totals of species in the different suborders and the major families.
First, the origin of Coleoptera, relatively early in the Triassic compared with other holometabolous orders, provided ample time for diversification. Having been in existence throughout the breakup of Pangaea, which started in the Jurassic, distinct beetle biotas have evolved in place on the various continental fragments of that supercontinent. A remarkably high percentage of the early coleopteran lineages persist today.
Second, beetle diversification has been explained as the result of a successful body plan incorporating protective elytra and a flexibly articulating prothorax. Although beetles are generally not regarded as fast or agile fliers, representatives of various beetle families have routinely colonized the most remote island systems in the world. In many families, the outward appearance and function of the walking beetle has been maintained, while the metathoracic flight wings have been reduced to nonfunctional straps or vestigial flaps. This brach-ypterous condition eliminates the possibility of winged dispersal by individuals and is associated with increased speciation and ende-mism, most often in ecologically stable, geographically isolated montane, desert, or island habitats.
Third, as representatives of the Holometabola, the larval and adult beetle life stages have been morphologically decoupled via the intervening pupal stage. Larvae may exhibit morphological specializations not observed in the adult stages, and may live in particular microhabitats not primarily occupied by the adults.
TABLE IClassification of Beetle Suborders, Series, Superfamilies, and Families of the Order Coleoptera |
||
| Suborder Archostemata | 53. Cneoglossidae | 112. Endomychidae |
| Cupedoidea | 54. Dryopidae | 113. Erotylidae |
| 1. Ommatidae | 55. Elmidae | 114. Helotidae |
| 2. Crowsoniellidae | 56. Eulichadidae | 115. Hobartiidae |
| 3. Cupedidae | 57. Heteroceridae | 116. Kateretidae |
| 4. Micromalthidae | 58. Limnichidae | 117. Laemophloeidae |
| 5. Jurodidae | 59. Lutrochidae | 118. Lamingtoniidae |
| Suborder Adephaga | 60. Psephenidae | 119. Latridiidae |
| Caraboidea | 61. Ptilodactylidae | 120. Monotomidae |
| 6. Amphizoidae | Elateroidea | 121. Myraboliidae |
| 7. Aspidytidae | 62. Artematopodidae | 122. Nitidulidae |
| 8. Carabidae | 63. Brachypsectridae | 123. Passandridae |
| 9. Dytiscidae | 64. Cantharidae | 124. Phalacridae |
| 10. Gyrinidae | 65. Cerophytidae | 125. Phloeostichidae |
| 11. Haliplidae | 66. Drilidae | 126. Priasilphidae |
| 12. Hygrobiidae | 67. Elateridae | 127. Propalticidae |
| 13. Meruidae | 68. Eucnemidae | 128. Protocucujidae |
| 14. Noteridae | 69. Lampyridae | 129. Silvanidae |
| 15. Trachypachidae | 70. Lycidae | 130. Smicripidae |
| Suborder Myxophaga | 71. Omalisidae | 131. Sphindidae |
| 16. Hydroscaphidae | 72. Omethidae | 132. Tasmosalpingidae |
| 17. Lepiceridae | 73. Phengodidae | Tenebrionoidea |
| 18. Sphaeriusidae | 74. Plastoceridae | 133. Aderidae |
| 19. Torridincolidae | 75. Rhagophthalmidae | 134. Anthicidae |
| Suborder Polyphaga | 76. Rhinorhipidae | 135. Archeocrypticidae |
| Staphyliniformia | 77. Telegeusidae | 136. Boridae |
| Hydrophiloidea | 78. Throscidae | 137. Ciidae |
| 20. Histeridae | Derodontiformia | 138. Chalcodryidae |
| 21. Hydrophilidae | Derodontoidea | 139. Melandryidae |
| 22. Sphaeritidae | 79. Derodontidae | 140. Meloidae |
| 23. Synteliidae | 80. Jacobsoniidae | 141. Mordellidae |
| Staphylinoidea | 81. Nosodendridae | 142. Mycetophagidae |
| 24. Agyrtidae | Bostrichiformia | 143. Mycteridae |
| 25. Hydraenidae | Bostrichoidea | 144. Oedemeridae |
| 26. Leiodidae | 82. Bostrichidae | 145. Perimylopidae |
| 27. Ptiliidae | 83. Dermestidae | 146. Prostomidae |
| 28. Scydmaenidae | 84. Endecatomidae | 147. Pterogeniidae |
| 29. Silphidae | 85. Ptinidae | 148. Pyrochroidae |
| 30. Staphylinidae | Cucujiformia | 149. Pythidae |
| Scarabaeoidea | Lymexyloidea | 150. Ripiphoridae |
| 31. Belohinidae | 86. Lymexylidae | 151. Salpingidae |
| 32. Diphyllostomatidae | Cleroidea | 152. Scraptiidae |
| 33. Geotrupidae | 87. Acanthocnemidae | 153. Stenotrachelidae |
| 34. Glaphyridae | 88. Chaetosomatidae | 154. Synchroidae |
| 35. Glaresidae | 89. Cleridae | 155. Tenebrionidae |
| 36. Hybosoridae | 90. Metaxinidae | 156. Tetratomidae |
| 37. Lucanidae | 91. Phloiophilidae | 157. Trachelostenidae |
| 38. Passalidae | 92. Phycosecidae | 158. Trictenotomidae |
| 39. Pleocomidae | 93. Prionoceridae | 159. Ulodidae |
| 40. Ochodaeidae | 94. Mauroniscidae | 160. Zopheridae |
| 41. Scarabaeidae | 95. Melyridae | Chrysomeloidea |
| 42. Trogidae | 96. Thanerocleridae | 161. Cerambycidae |
| Sciritiformia | 97. Trogossitidae | 162. Chrysomelidae |
| Scirtoidea | Cucujoidea | 163. Disteniidae |
| 43. Clambidae | 98. Agapythidae | 164. Megalopodidae |
| 44. Decliniidae | 99. Alexiidae | 165. Orsodacnidae |
| 45. Eucinetidae | 100. Biphyllidae | 166. Oxypeltidae |
| 46. Scirtidae | 101. Boganiidae | 167. Vesperidae |
| Elateriformia | 102. Bothrideridae | Curculionoidea |
| Dascilloidea | 103. Byturidae | 168. Anthribidae |
| 47. Dascillidae | 104. Cavognathidae | 169. Attelabidae |
| 48. Rhipiceridae | 105. Cerylonidae | 170. Belidae |
| Buprestoidea | 106. Coccinellidae | 171. Brentidae |
| 49. Buprestidae | 107. Corylophidae | 172. Caridae |
| Byrrhoidea | 108. Cryptophagidae | 173. Curculionidae |
| 50. Byrrhidae | 109. Cucujidae | 174. Nemonychidae |
| 51. Callirhipidae | 110. Cyclaxyridae | 175. Urodontidae |
| 52. Chelonariidae | 111. Discolomatidae | |
Fourth, the early diversification of beetles in the Jurassic placed many lineages in prime position to exploit ecological opportunities associated with the Cretaceous diversification of flowering plants. Many of the largest families of Polyphaga (e.g., Buprestidae, Scarabaeidae, Chrysomelidae, Cerambycidae, and Curculionidae) include lineages that are intimately associated with angiosperms. These host plant associations are based on the use of various portions of the particular species or sets of species of flowering plants as larval or adult food. In addition, many other beetle groups use fungi as a food source, and fidelity to fungi of particular types is not atypical. The ability to specialize along with their larval and adult hosts has clearly been associated with extensive speciation across the Coleoptera.
EVOLUTIONARY HISTORY
The earliest beetle-like insects represented in the fossil record are known from Lower Permian (270 mya) deposits in Moravia, Czech Republic, and the Ural Mountains of Russia. These insects, classified in the family Tshekardocoleidae, order Protocoleoptera, resemble present-day species of the archostematan families Ommatidae and Cupedidae. They differ from true beetles in having 13-segmented antennae, elytra with more fully developed venation and more irregular longitudinal ribbing, and an abdomen and ovipositor extending beyond the apex of the elytra (Fig. 1).
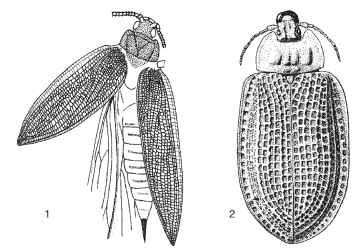
FIGURES 1-2 Fossil beetles. (1) Moravocoleus permianus (Tshekardocoleidae: Protocoleoptera, Permian). (© Czech Geological Survey.) (2) Notocupes picturatus (Cupedidae: Coleoptera, Triassic).
The oldest true Coleoptera in the fossil record date back about 230 million years to the Triassic. These fossils exhibit the coleopteran 11-segmented antennae, have more regular longitudinal ribbing on the elytra, and possess internal genitalia (Fig. 2). The earliest fossil beetle faunas have been described from Queensland in Australia, South Africa, and central Asia. All four lineages now recognized as suborders appear to have been extant by the end of the Triassic. The presumed basal suborder, Archostemata, was represented by species assignable to the extant families Ommatidae and Cupedidae, plus others belonging to families not lasting past the Mesozoic. The Triassic Adephaga included species sharing enlarged hind coxal plates such as are seen in present-day Haliplidae, as well as species attributed to Carabidae, the ground beetles, and a closely related family, Trachypachidae. Definitive myxophagans are not represented by Triassic fossils, but various lines of evidence suggest that they must have existed then. In these faunas, the currently dominant suborder Polyphaga included representatives of the extant families Staphylinidae and Elateridae. These earliest beetles inhabited a world made up of forked-leaved pteridosperms, lycopods, cycads, gingkos, and early conifers. The large animals of these communities included therapsid reptiles and dinosaurs; however, neither birds nor true mammals had yet evolved.
During the Jurassic (210-145 mya), known family-level beetle diversity increased dramatically. About 35 families and 600 species of beetles are known from this period. Among the Adephaga, first appearances are documented for the whirlygig beetle family Gyrinidae and the predaceous diving beetle family Dytiscidae. In both families, the predaceous habit would be considered to be the ancestral condition. Among Polyphaga, the major families Scarabaeidae, Tenebrionidae, and Curculionidae are first documented. Other earliest occurrences include members of the scavenging water beetles (Hydrophilidae), carrion beetles (Silphidae), jewel beetles (Buprestidae), ovoid bark-gnawing beetles (Trogossitidae), sap beetles (Nitidulidae), tumbling flower beetles (Mordellidae), false blister beetles (Oedemeridae) and three additional families representing the weevil superfamily Curculionoidea (Nemonychidae, Belidae, and Caridae). Of these, Scarabaeidae, Oedemeridae, Mordellidae, and Curculionidae are strictly phytophagous or saprophagous.
The diverse present-day assemblage of Chrysomeloidea also is thought to have appeared in the Jurassic, although definitive fossil evidence is lacking. Chrysomeloids use a broad diversity of plant hosts, ranging from cycads to conifers to angiosperms. Based on a phylo-genetic hypothesis derived from extant species, the basal chrysomel-oid lineages are associated with primitive conifers (Araucaria spp.) and cycads (Fig. 3) . Curculionoidea, the sister group to chrysomel-oids, also exhibits this ancestral association with conifers and cycads. The larvae of present-day Oedemeridae are borers in conifers. Thus it appears that at least several lineages of phytophagous Coleoptera were in place before the evolutionary advent of the angiosperms.
The Cretaceous witnessed initiation of the most recent round of southern landmass fragmentation, via the opening of the southern Atlantic Ocean and the isolation of New Zealand. South America and Antarctica plus Australia became progressively isolated from Africa, although they maintained contact with one another. Beetle families responded to this pattern of vicariance, with relictual distributions of several extant taxa supporting their origin during this time (Fig. 4 ). Continuing vicariance of the southern portions of Gondwana continued into early Tertiary, with progressive isolation of Australia, and finally the separation of Antarctica and South America at the start of the Oligocene (38 mya). This last event permitted formation of the circum-Antarctic current, helping plunge the world into a latitudi-nally zonated climate similar to that of today.
Preservation of beetles in amber has provided unparalleled levels of information about extinct taxa. The deposits of Baltic amber dated at 35-50 mya, and Dominican amber dated 15-40 mya, open windows onto the transition from the tropical world of the Eocene to the climatically zonated world of today. Most often, amber fossils (Fig. 5) indicate historically broader distributions for taxa presently known from only one continent (Fig. 6) . This range contraction, continuing from the Eocene until the present day, suggests one explanation for the current latitudinal pattern of biodiversity. Many of the tropically adapted groups of organisms, of which beetles count significantly, have been progressively excluded from higher latitudes through the advent of cool to cold higher latitude climes, followed by the dramatic climatic perturbations associated with Pleistocene glaciation. G. Russell Coope goes so far as to argue that Pleistocene glaciation halted to speciation of beetles in the temperate zones most influenced by the glaciation. His argument is based on a simple fact: As he and his students studied sub-fossil beetle bits interred in wetland peats throughout various portions
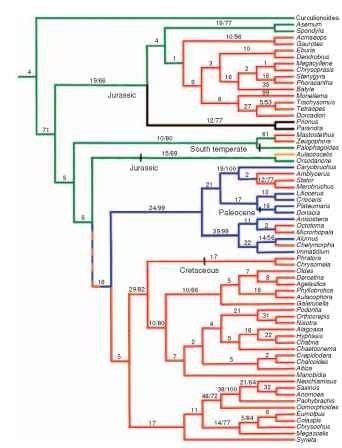
FIGURE 3 Strict-consensus estimate of the phylogeny of Chryso-meloidea and outgroups, with host groups mapped onto the clado-gram. Numbers of synapomorphies/bootstrap values exceeding 50% shown along branches. Colors indicate major host group attributable to common ancestor of each group (green, Coniferae; mustard, Cycadales; red, dicotyledonous angiosperms; blue, monocotyledonous angiosperms; black, do not feed on living plants). Approximate ages of Mesozoic and early Tertiary fossils are only indicated where known, since almost all subfamily groups are present in the mid-Tertiary fossil record.
of Europe and North America, they found that all species taken from deposits younger than Pliocene could be identified as currently extant. These findings contrast starkly with those from tropical island systems, where speciation may have occurred in far younger areas. In Hawaii, for example, cave-adapted carabid beetles with reduced eyes and elongate legs have evolved from fully eyed, short-legged, epigean ancestors on the younger volcanoes of East Maui and Hawaii Island, which respectively broke the ocean surface no earlier than 750,000 and 430,000 years ago. Numerous Hawaiian beetle radiations in the Carabidae, Anobiidae, Nitidulidae, Cerambycidae, and Curculionidae demonstrate the many rapid and extensive bouts of speciation that occur in newly evolving tropical island communities.
Fossil-based estimates for the origin of Coleoptera and its subgroups provide conservative, minimum ages for these events; however, phylogenetic studies utilizing molecular evolution models and dating methods by Alfried Vogler and his colleagues suggest much earlier dates. According to this work, the origin of true beetles is pushed back to about 285 mya, in the Lower Permian, and the first appearance of all four beetle suborders also is pushed back to the Permian.
ADULT SPECIALIZATION
It is impossible to argue for or against the proposition that possession of elytra has helped beetles’ evolutionary success, because possession of elytra is a defining character of “beetleness,” and beetle
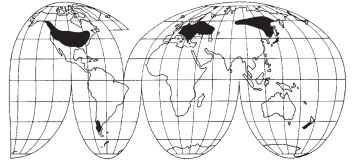
FIGURE 4 World distribution of Derodontidae. Areas supporting species include North America, Europe, Siberia, Japan, the Valdivian forest of Chile, and the South Island of New Zealand.
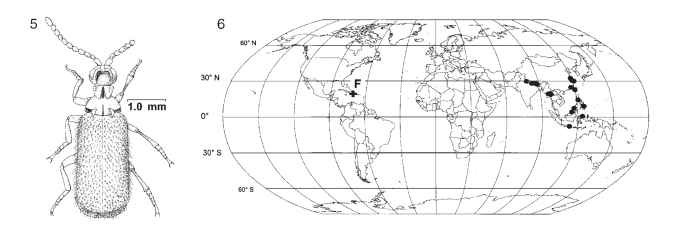
FIGURES 5-6 Protopaussus pristinus (Carabidae), described from Dominican amber. (5) Reconstruction of adult, dorsal view. (6) Distribution of Protopaussus: F, P. pristinus fossil; • localities of extant Protopaussus.
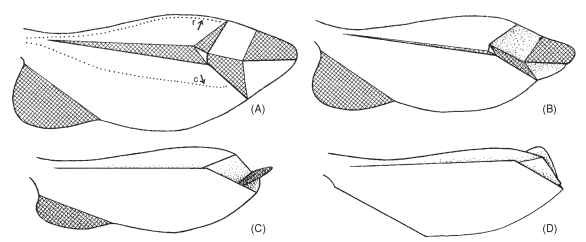
FIGURE 7 Paper model of right wing of Cantharis sp. arranged to demonstrate wing folding. Cross-hatched areas face ventrally in fully folded wing. In the extended wing (A) the principal veins—radius (r) and cubitus (c)—are apart by muscular action from the wing base. When this action ceases, the wing apex automatically folds (B, C) until wing is fully folded (D).
families vary so dramatically in their diversity. For example, one of the earliest evolving beetle groups, the Cupedidae, is currently represented by only 26 species worldwide. Thus possession of elytra is only one step among many leading to successful diversification of Coleoptera. Nonetheless, functional study of the beetle body plan illustrates many instances in which “beetleness” has predisposed lineages to enter and proliferate in particular habitats.
Most generally, the organization of beetle bodies that has permitted entry into confining, laminar microhabitats involves (1) thick hard cuticle on the head and prothorax, (2) a prothorax flexibly articulating with the pterothorax, and (3) a pterothorax topped by elytra that cover folded flight wings and soft, expansible abdominal tergites. Carabid beetles utilize wedge-pushing locomotion to move through leaf litter and under loose tree bark. In these beetles, a rounded projection on the base of the hind femur impinges on the metatrochanter, which articulates only in a horizontal plane with the immobile hind coxa. Pulling the hind leg forward pushes the apex of the femur away from the body, thereby elevating the carabid’s dor-sum (the wedge). This upward motion is then followed by a thrust of the hind legs, forcing the beetle body forward (the push). Using this mechanism, rhysodine carabid beetles can move through dying or even living wood without leaving a trace; the wood simply closes up behind them! Their goal in this unlikely activity is foraging on the amoeboid plasmodia of slime molds (Myxomycetes).
For beetles to both fly and move through confining spaces, their wings must be stowed under the elytra while walking or wedge pushing, yet quickly unfurled for flight. All wing folding is controlled through muscles attached to the wing base; as long as tension is applied so that the radial and cubital veins are pulled apart, the wing surface remains flattened. However, relaxation of this tension brings natural folds into play so that, with the wing apex folding in upon itself (Fig. 7), the medius comes to lie above the radius posterior (Fig. 8 ).
Numerous variations on wing folding have evolved depending on elytral configuration. In the archostematan Cupedidae, the wing apex rolls up longitudinally (Fig. 9). Wing folding can proceed even, given the evolutionary reduction of wing venation observed in tiny beetles such as Microsporidae or Ptiliidae (Figs. 10 and 11). In
addition to the folding characteristics of the wings, setose binding patches occurring on the wing surface, inner elytral surface, and abdominal terga are used to manage the wing folding, thus ensuring safe stowage of the wing membranes (Fig. 12).
The generalized thickening of cuticle characteristic of archostem-atans, adephagans, and many polyphagans results in an adult insect that is highly constrained in internal volume. Abdominal sutures between ventrites allow these segments to move against one another so that well-fed or gravid individuals will exhibit an abdomen extending beyond the elytral apex. Nonetheless, longitudinal abdominal extension can be minimized and external structural integrity maintained if the body is allowed to expand in another direction. Beetles accomplish this increase in body volume through dorsoventral expansion of the abdomen.
In a newly eclosing beetle, the lateral reaches of the abdominal tergites lie both between and below the lateral portions of the abdominal sternites. The tergites and sternites are joined by extensive membranes, within which the spiracles are situated. These membranes may stretch, and the tergites may move dorsally relative to the stationary lateral margins of the sternites, dramatically increasing the volume of the abdomen. This volumetric expansion is accomplished without any compromise to the external armor represented by the cuticle. The soft, flexible abdominal tergites are protected by the elytra, except when the beetle is flying. At this time the soft membranes and flexible tergites are vulnerable to attack by predators or parasites.
In the floricolous, day-flying Buprestidae and scarab beetles of the subfamily Cetoniinae, flight is undertaken without significant separation or lifting of the elytra, with the metathoracic wings extended under the lateral elytral margins. In the buprestids, this posture allows the aposematic coloration of the elytra to be visible both in flight and at rest. In other polyphagans and the Archostemata, the elytra are held at an angle during flight, beating synchronously with the flight wings, and thereby providing some degree of aerodynamic lift.
Given the need to exchange oxygen and carbon dioxide at a liquid interface on the surfaces of the tracheolar cells, respiration represents the major activity through which an insect can lose water. This source of water loss is of particular importance for an animal of small body volume. The beetle’s respiratory system opens via large metathoracic spiracles and up to eight pairs of abdominal spiracles, all of which open onto the subelytral cavity. Thus, in addition to controlling gas exchange via the spiracular openings, a beetle can modulate respiration by the position of the abdominal venter relative to the elytra. Reduction of the elytra to the quadrate condition seen in Staphylinidae has resulted in secondary exposure of the abdominal spiracles.
Beetles have invaded freshwater aquatic habitats several times during their evolutionary history. In all instances, adult aquatic beetles retain the spiracular respiratory system of their terrestrial relatives, requiring that they regularly have access to atmospheric gases. The subelytral space provides the means to hold an air bubble while the beetle is active underwater. This bubble can be replenished by periodic surfacing of the beetle, during which the tip of the abdomen breaks the water surface, permitting exchange of gases. Because of the makeup of our atmosphere (79% nitrogen and 21% oxygen), the subelytral air bubble serves as a compressible gill, permitting extended underwater sojourns. As the beetle uses oxygen, more oxygen diffuses into the bubble from the surrounding water. The carbon dioxide produced through the beetle’s respiration, being highly soluble in water, quickly leaves the bubble. Because nitrogen dissolves slowly into the water, there is a gradual reduction in bubble size. The beetle can use up to eight times as much oxygen as was in the original bubble before being required to surface to replenish its air supply. Swimming beetles using these simple subelytral compressible gills include various Adephaga (e.g., Haliplidae and Dytiscidae).
In other families of the aquatic realm, oxygen is supplied to the subelytral bubble by a plastron composed of microfuge hairs or other columnar evaginations of the cuticle that are close together along their outer surface, excluding water with their surface-filming qualities. Oxygen diffuses into the plastron without any change in plastron gas volume, allowing the beetle to remain indefinitely below the water surface. Nonetheless, plastron respiration can work only in highly oxygenated water, so beetles with plastrons are usually found in moving waters. Plastron breathers also are less active than the adephagan compressible gill breathers, because the plastron cannot provide the high levels of oxygen required for intense activity. This type of structure has evolved repeatedly in the order, being found in the Hydrophilidae, Dryopidae, Elmidae, and some Curculionidae.
LARVAL SPECIALIZATION
Among the four suborders of Coleoptera, life histories of the pre-daceous Adephaga most closely resemble those of the beetles’ phylo-genetic sister group, the neuropteroid orders. Adephagan larvae are generally campodeiform, that is, elongate and slightly dorsoventrally flattened, with long thoracic legs and a posteriorly tapered, dorsally sclerotized abdomen (Fig. 13). They typically have anteriorly directed mouthparts that often include elongate, sickle-shaped mandibles with a reduced mola (Fig. 14) . The legs are six-segmented (coxa, trochanter, femur, tibia, tarsus, and claws) as in the Megaloptera, Raphidioptera, and Neuroptera. The ninth abdominal tergite usually bears a pair of dorsolateral appendages (urogomphi) that may be short and unsegmented, or longer and variously segmented. These are secondarily evolved structures of the Coleoptera, and not homologous with the cerci of, for example, the orthopteroid orders. Adephagan larvae usually develop through three instars before pupation.
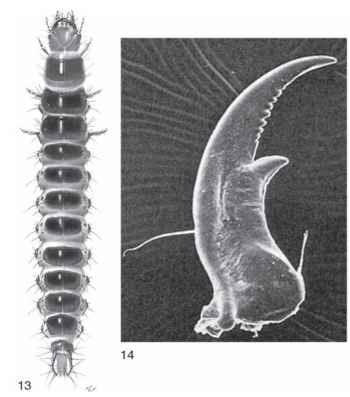
FIGURES 13-14 (13) Abaris bigenera mature larva (Carabidae), dorsal view (larval length, 8.3 mm). (Image © F. L. Fawcett.) (14) Right larval mandible, ventral view, Platynus sp. (Carabidae). Note absence of basal mola and presence of large retinacular tooth and serrate incisor.
The larvae of Archostemata deviate from this generalized configuration by representing the syndrome that has evolved repeatedly in taxa characterized by the larval wood-boring habit. In these groups, the larvae are lightly sclerotized, more or less tubular, with stout mandibles, a dorsal cephalic endocarina, shortened or reduced legs, and various ampullae on the thoracic and abdominal segments (Fig. 15 ). The archostematan family Micromalthidae exhibits probably the most
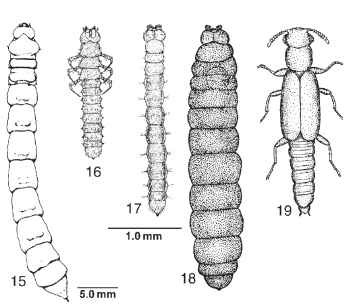
FIGURES 15-19 (15) Tenomerga concolor mature larva (Cupedidae), dorsal view. [From Lawrence, J. F. (1991). Order Coleoptera. In "Immature Insects," Vol. 2 (F. W. Stehr, ed.), Fig. 34.67a. Kendall/Hunt, Dubuque, IA. Figures 16-19, Micromalthus debilis (Micromalthidae), dorsal view. (16) Triungulin first instar larva. (17) Cerambycoid larva. (18) Pedogenetic larva. (19) Adult female. (Drawings, Figs. 16-19, courtesy of the copyright holder, the Royal Entomological Society, London, U.K.)
bizarre set of larval forms and associated life cycle seen in Insecta. The campodeiform first instar is an active triungulin. It molts to become a legless, feeding cerambycoid larva, which in turn may undergo four types of molt. It may pupate directly to become an adult diploid female. Alternatively, it may develop into one of three kinds of larviform reproductive: a thelytokous pedogenetic female that parthenogenetically produces viviparously a number of diploid triungulins; an arrhenoto-kous pedogenetic female that lays a single egg, from which hatches a stump-legged curculionoid larva that in turn devours the mother, pupates, and then emerges as an adult haploid male; and an amphitok-ous pedogenetic female, which may produce either form. The hormonal controls of this system are not known, although production of the various larval types seems to be affected by environmental conditions.
The demographic consequences of this life cycle include the ability to quickly multiply and to use available rotting wood in the production of numerous dispersive adults. The triungulin larvae (Fig. 16) can expand the infestation to adjacent portions of the rotten log or timber. The cerambycoid larvae (Fig. 17), more typical of other archostematan larvae, can efficiently feed in confined galleries in rotting wood. The pedogenetic form (Fig. 18) can itself produce many more triungulins, enhancing the rate of increase of the population. The adults (Fig. 19) are produced in massive numbers, with these winged colonists establishing new colonies. Natural infestations have been reported in large Quercus (oak) or Castanea (chestnut) logs across the beetles' native range in northeastern North America. Other human-associated infestations have been reported from timbers deep in a South African diamond mine, and in thick oak paneling used to line the vaults of the Federal Reserve Bank in New York City.
The small suborder Myxophaga is characterized by adults and larvae of extremely small size, with both larvae and adults living interstitially in riparian areas, where they feed on algae. As opposed to the Archostemata and Adephaga, the larval legs are five-segmented, with the tarsus and claws fused into a single segment, the tarsungulus. The abdomen may or may not bear urogomphi on the ninth tergite. Like many other beetle species that feed on small par-ticulate matter (pollen, spores, conidia, etc.), the larval mandibles bear a basal mola. Because they are aquatic in all stages, the adults bear a plastron, and the larvae may breathe by means of a plastron that covers the spiracles or via vesicular gills (i.e., a balloon-like expansion of the spiracular peritreme with an apical opening).
It is in the order Polyphaga that divergence of larval and adult lifestyles becomes evolutionarily significant. Among basal polypha-gans in the superfamilies Staphylinoidea and Hydrophiloidea, larval anatomy remains generally of the campodeiform type, although mouthparts may be specialized for feeding on fungal food through development of broadly papillate molar regions on the mandible (Figs. 20 and 21). As in the Myxophaga, the larval leg has five segments. Aquatic forms may bear lateral gills on the thorax or abdomen (Fig. 22). Urogomphi of various configurations may also be present.
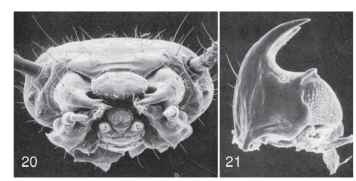
FIGURES 20-21 Anisotoma errans larva (Leiodidae). (20) Head capsule, anterior view. (21) Right mandible, ventral view. Note large asperate mola at base. [From Newton, A. F., Jr. (1991). Leiodidae. In "Immature Insects" (F. W. Stehr, ed.), Vol. 2, pp. 327-329, Figs. 34.152a and 34.154. Kendall/Hunt, Dubuque, IA.
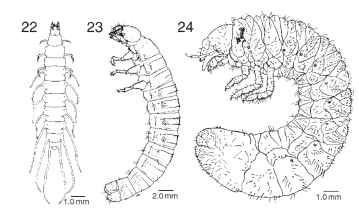
FIGURES 22-24 Beetle larvae. (22) Berosus metalliceps(Hydrophilidae), dorsal view.
The larvae of the superfamilies Dascilloidea (Fig. 23), Byrrhoidea, and Bostrichoidea exhibit a dorsally convex body configuration that has evolved into the much more exaggerated C-shaped grub characteristic of the Scarabaeoidea (Fig. 24). Scarab grubs can develop in a variety of microhabitats. Primitive scarabaeoids such as stag beetles (Lucanidae) and bess beetles (Passalidae) develop as sapropha-gous larvae in rotting wood. Larvae of the Geotrupidae and scarab subfamilies Scarabaeinae and Onthophaginae develop in mammalian herbivore dung where they also feed on fungi. Flowering plant roots are fed on by larvae of species in the more highly derived scarab subfamilies Melolonthinae, Rutelinae, and Dynastinae. Many species in these subfamilies are of economic concern, because they feed on commodities such as corn, small grains, vegetable crops, grasses, turf, fruits, and nursery stock. The C-shaped larval configuration results in an increased abdominal capacity relative to the head and thoracic fore body. This increased capacity is directly connected to the scarab larva's penchant for feeding on large amounts of food in order to pupate at a large size. Scarabs are well represented among the largest beetles, with the impressive Goliathus beetles of Africa and Asia attaining the greatest body mass of any beetle known.
A C-shaped larva has evolved independently in another phytophagous group with concealed larval stages, the Curculionidae. The cur-culionid sister group, the Chrysomeloidea, is primitively characterized by larval stages superficially similar to those of Dascilloidea, that is, larvae of moderately convex dorsal habitus (Fig. 25). Primitive weevils retain evidence of thoracic legs (Fig. 26); however, all evidence of thoracic appendages has been evolutionarily erased in higher weevils (Fig. 27). As phytophagous weevils have specialized, taxa have moved from being internal feeders to foraging on the external surfaces of their hosts. External feeders such as the lesser clover-leaf weevil gain a foothold on their host plant through ventral abdominal ampullae (Fig. 28), analogous to the prolegs of Hymenoptera and Lepidoptera. A parallel transition from hidden feeders to exposed foliage feeders has also evolved in the weevil sister group, the Chrysomeloidea.
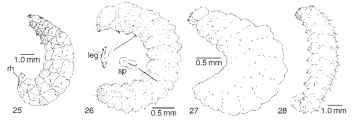
FIGURES 25-28 Larvae of phytophagous Chrysomeloidea and Curculionoidea. (25) Donacia sp. (Chrysomelidae), lateral view, sharp respiratory horns (rh) insert into underwater stems of water lily, providing air to the spiracles located at base of horns. (© New York Entomological Society.) (26) Neocimberis pilosus (Nemonychidae), lateral view (sp, spiracle). (27) Apion griseum (Apionidae), lateral view. (28) Hypera nigrirostris (Curculionidae), lateral view.
The Cerambycidae comprise one basal division of the chrysomel-oids, with all their larvae internal feeders. The Palophaginae represent the earliest divergent lineage of Chrysomelidae, based both on late Jurassic fossils (>145mya) and phylogenetic analysis of living species. Larvae of this subfamily attack the male strobili of Araucaria (Coniferales: Araucariaceae).
The subfamily Aulacoscelinae represents another early-diverging chrysomelid lineage. Larvae of this group are internal feeders on cycads. From this syndrome of hidden feeding, leaf beetle larvae have evolved to live on open plant tissues of many of the world's angiosperms. Where plants have evolved the ability to incorporate secondary chemical compounds in their tissues, herbivorous chrysomelids have evolved to use these chemicals to recognize food and stimulate oviposition. They have also evolved the ability to sequester these broadly toxic chemicals into their tissues to gain protection from predators. Today it is commonplace to observe brightly colored larvae and adults of protected leaf beetles congregated on exposed plant surfaces, serving as a communal warning to predators regarding their unpalatability.
The wood-boring larval body plan of the Archostemata is well represented in the Polyphaga, having independently evolved in the Buprestidae (Fig. 29), Eucnemidae, and Cerambycidae (Figs. 30 and 31). Larvae in all these families can bore through freshly dead or dying wood by using their well-developed, anteriorly directed mandibles. Laterally expanded thoracic segments or abdominal ampullae serve to anchor these larvae in their tunnels, facilitating purchase by the mandibles on the wood surface. Leg reduction has proceeded during diversification of cerambycid borers, with larvae of more basally divergent subfamilies such as the Prioninae and Lepturinae having shortened thoracic legs (Fig. 30), whereas larvae of the highly derived subfamily Lamiinae (Fig. 31) are legless.
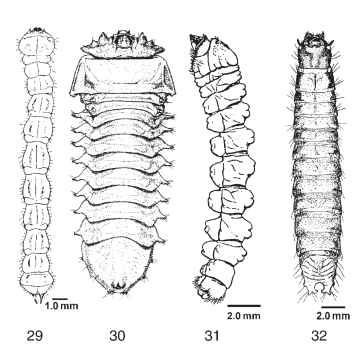
FIGURES 29-32 Larvae of wood-boring beetles. (29) Agilus anxius (Buprestidae), dorsal view. (30) Unidentified lepturine larva (Cerambycidae), ventral view, scale unknown.(31) Platyzorilispe vari-egata (Cerambycidae), lateral view. (32) Hemicrepidius mem-nonius (Elateridae), dorsal view.
Where wood-boring beetles have gone, similarly shaped predatory beetles have followed. These tubular larvae in the Elateroidea and Cleroidea may be highly sclerotized in general, and bear well-sclerotized head capsules and/or urogomphal plates (Fig. 32) that armor them appropriately for their habitats (e.g., under bark, within wood-boring beetle galleries). Elaterid larvae have diverse feeding habits, with many groups being phytophagous or saprophagous. However, all forms, regardless of food habit, imbibe their food as an extraorally predigested liquid.
Other elateroid larvae, such as fireflies (Lampyridae) and soldier beetles (Cantharidae), lack the heavy armor of the concealed gallery feeders, and prey on other arthropods among leaf and ground litter. These larvae use grooved mandibles to suck up the liquefied contents of their prey. In Elateridae, and independently in Phengodidae and Lampyridae, larval and adult stages have evolved the ability to produce light using organs composed of modified cuticular cells. The significance of larval luminescence has been variously explained. For example, night-active Pyrearinus larvae in the elaterid subfamily Pyrophorinae use light organs to attract flying insect prey to Brazilian termite mounds where they make their home. Phengodid larvae of the genus Phrixothrix possess medial photic organs on the head that use red light to illuminate potential prey. But they also possess lateral abdominal light organs that emit green light. These abdominal light organs are homologous with those of Lampyridae and most likely serve to advertise that the larvae are chemically protected. Increasingly complicated light communication systems have evolved in the adult stages of various phengodid and lampyrid taxa.
Cucujoidea and Tenebrionoidea are diverse superfamilies whose larval forms blend imperceptibly into each other morphologically and biologically. It is in these groups that saprophagous and myco-phagous feeding habits are associated with extensive larval diversification. Primitive larvae of both superfamilies are similar and typical of Polyphaga in many ways (e.g., five-segmented legs, urogomphi, and moderate degree of sclerotization) Evolutionary trends in one are often mirrored in the other. Cucujoid and tenebrionoid larvae are usually small to moderate in size, and somewhat dorsoventrally compressed. Many are cryptozoic, occurring in leaf litter, under bark, in fungus, or in rotting wood, where they feed on fungi or on fungus-altered plant matter. Groups specialized for feeding on spores, conidia, loose hyphae, or other small particles exhibit various specializations correlated with microphagy. Most notably, these include a well-formed mandibular mola and prostheca (Fig. 33).
Extreme dorsoventral compression of the body has occurred repeatedly in response to the selective pressures of occupying subcortical and interstitial leaf litter habitats. Sometimes (Fig. 34 - the body form is simply flattened, with a reorientation of the head to a protracted, prognathous condition and a migration of the leg articulations to more lateral positions. Flattening, however, may be accompanied by an additional transition to an onisci-form (or pie-plate-shaped) body through extensive development of tergal flanges, resulting in a broadly oval body outline in some Cerylonidae, Corylophidae (Fig. 35), Discolomidae, and Nilioninae (Tenebrionidae). Larvae specialized for life under bark, in fungi, or in rotting wood typically have short, stout, unarticulated, and unseg-mented urogomphi. The apex is typically recurved to point dorsally. This form of urogomphi is thought to help larvae move about in cramped habitats.
Several tenebrionoid and cucujoid groups experienced parallel transitions to a parasitic lifestyle, including Meloidae, Rhipiphoridae, and some Cucujidae and Bothrideridae. The Rhipiphoridae provide a glimpse at parasitism involving both externally and internally feeding
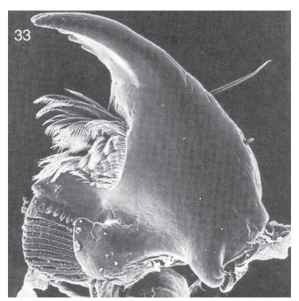
FIGURE 33 Left larval mandible, ventral view, of Anchorius lin-eatus (Biphyllidae), showing basal mola (lower left) and prostheca with comb hairs; mandible width, 0.16 mm.
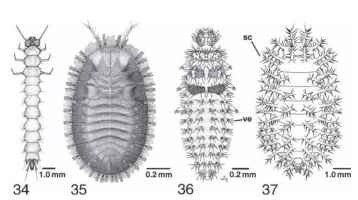
FIGURES 34-37 (34 and 35) Flattened beetle larvae, dorsal view. (34) Dendrophagus americanus (Cucujidae). [From Lawrence, J. F. (1991). Order Coleoptera. In "Immature Insects" (F. W. Stehr, ed.),Vol. 2, Fig. 34.527. Kendall/Hunt, Dubuque, IA. IA(36 and 37) Larvae of Coccinellidae, dorsal view. (36) Predaceous Stethoris histrio. (37) Phytophagous Epilachna varives-tis: sc, scolus; ve, verruca.
stages. In Rhipiphorinae, the triungulin first instar locates and attaches itself to an adult of a suitable hymenopteran host. After being carried back to the host's nest, the triungulin detaches itself and searches for a host larva. Once a host has been located, the larva burrows inside, where feeding continues (endophagy) until the parasitoid becomes greatly swollen. As the host larva reaches maturity, the parasitoid exits from its thorax, switching to feed externally (ectophagy), eventually killing it.
In Rhipidiinae, the reverse sequence of internal and external feeding occurs: the triungulin locates a cockroach as a potential host, inserts its head and thorax into a membranous region on its venter, and begins to feed while most of its body remains outside the host. Later, the larva transforms into a less mobile, legless form and moves entirely inside the host, where it begins to grow rapidly. Near the end of its development, the larva molts to a form with legs and emerges from the host to pupate.
Cucujoid and tenebrionoid taxa that are adapted for external feeding typically have a more eruciform (caterpillar-like) shape resulting from elongation of the legs, reorientation of the head to a more hypognathous position, and dorsoventral inflation to a more cylindrical shape. These external feeders also tend to exhibit defensive modifications. Aposematic coloration is common in these groups. Tergal and pleural armature, which is absent or modest in most cucujoids and tenebrionoids, becomes exaggerated in some predators (e.g., Stethoris, Coccinellidae; Fig. 36), surface feeding herbivores (e.g., the coccinel-lid genus Epilachna; Fig. 37) , and fungus feeders (e.g., the erotylid genus Aegithus), to form various structures such as setose, tuberculate verrucae, and complexly branched scoli.
Within Cucujoidea and Tenebrionoidea, there is a recurring evolutionary transition from mycophagy/saprophagy to a lifestyle of true phytophagy as a borer in healthy herbaceous stems or wood. This entire sequence can be observed within individual families (e.g., Melandryidae), where there is a range of larval feeding that extends from boring in fungus sporophores to boring in fungus-infested wood and finally to boring in sound wood. Cucujoid and tenebrionoid wood borers tend to have fleshy bodies with conspicuous sclerotized plates usually restricted to the anterior end of the body. The head capsule tends to be prognathous and often bears a median endoca-rina, an internal keel on the dorsum associated with the development of especially powerful mandibular muscles.
Predatory larval forms have arisen repeatedly within Cucujoidea and Tenebrionoidea, most notably in the Coccinellidae. Accompanying this trophic transition is a suite of morphological changes to produce a campodeiform body (Fig. 36). The head typically has a more prognathous orientation. The mandibles are more prominent and lack a mola.
The beetle pupa is adecticous and usually exarate (i.e., the mandibles are fixed in position, and the head and thoracic appendages are free). Several groups have independently evolved the obtect condition; among them staphylinine Staphylinidae, Ptiliidae, and Coccinellidae, and the hispine Chrysomelidae. If the pupa rests concealed in a pupal chamber, it lies on its dorsum elevated from the substrate by numerous thoracic and abdominal setae. Pupae may be enclosed in a cocoon made of silk (aleocharine Staphylinidae, Tenebrionidae, and Curculionidae), fecal material (Passalidae and some Scarabaeidae), or the larval fecal case (cryptocephaline Chrysomelidae).
Exposed pupae, as in Coccinellidae, Chrysomelidae, and Erotylinae (Erotylidae), may remain attached to their host plant or fungus via the sloughed-off last larval cuticle, which encircles the anal portion of the pupa. Such exposed pupae may be protected by defensive secretions remaining in the shed larval skin. Beetle pupae retain the ability to move the abdomen by using the flexible abdominal intersegmental membranes. Sclerotized processes on opposing margins of the abdominal segments, called gin traps, have been suggested as defensive devices used to pinch and drive off mites and other predators.
ECOLOGICAL SPECIALIZATION
One means of estimating the ground-plan feeding habits of Coleoptera uses observations of extant taxa, interpreted in the context of phylogenetic hypotheses for the various lineages making up the order. By this method, we would deduce that the most primitive beetles were either saprophagous wood borers as larvae, such as extant Archostemata, or that they were campodeiform predators, such as the Adephaga and the sister group to Coleoptera, the Neuroptera + Raph-idioptera + Megaloptera. Examining the fossil record of Coleoptera as well as suggestive damage to fossil plants of the Triassic and Jurassic formations containing the earliest beetle fossils provides a second means of making such an estimation. By this method, we find that archostematans and primitive weevils predate fossils of all other types, suggesting that the earliest feeding habits were either saprophagous or herbivorous. Of course, fossil evidence of predation is not likely to be preserved, nor interpretable as such if it were. These two viewpoints, phylogenetic and paleontological, represent the diversity of opinion about how the first beetles lived their lives.
The two viewpoints can be reconciled if we view fossil data drawn from the various periods in light of phylogenetic estimates based on a diversity of taxa and characters. To do this, we must assume that the lifestyles of recent taxa represent those of their related fossil relatives. By this reasoning, it is very apparent that herbivorous taxa have constituted a majority of the major life-forms, measured by their recognition as genera, for coleopteran faunas from the Jurassic and Tertiary to recent times (Fig. 38). Even before the advent of the flowering plants, more than half the variety of beetle life-forms had evolved to focus their feeding attentions on plants. We can investigate the impact of the origin and diversification of angiosperms on beetle diversity by looking at beetle sister taxa in which one group is restricted to gymnospermous plants, whereas its sister is found on angiosperms. Brian Farrell examined lineages within the Chrysomeloidea and Curculionoidea. He found angiosperm-feeding taxa to be far more rich in species today than their gymnosperm-feeding sister groups. Clearly, angiosperm feeding has enhanced the species-level diversification of beetles living on them.
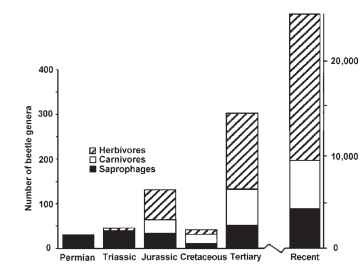
FIGURE 38 The number of beetle genera of each of three trophic levels from Permian to recent epoch. Permian genera represent Protocoleoptera.
In addition to internal feeding on stem tissue, and feeding on saprophagous growth in decaying cambium, the angiosperms offer floral resources unavailable from gymnosperms. Adult beetles of many families characterized by phytophagous, saprophagous, or scavenging larvae may be found feeding in or on flowers, associated exudates, or pollen. Melolonthine and cetoniine scarab beetles, whose larvae are subterranean root feeders or rotten wood feeders, respectively, often feed on flowers. Dermestid beetle larvae scavenge dead animal matter, and then move to flowers to feed on pollen after they have eclosed as adults. Once a female dermestid has fed, she becomes negatively phototactic and searches for cavities containing animal remains, where she will oviposit. Other families well represented among the pollen-feeding adults include Buprestidae, Lycidae, Nitidulidae, Mordellidae, Rhipiphoridae, Meloidae, Anthicidae, and Cerambycidae. Meloids and rhipiphorids not only feed at flowers but also oviposit there, with their hatching triungulin larvae waiting in the flower to climb on passing bees and wasps, which they parasitize. Feeding on hard pollen grains is facilitated by possession of mandibles bearing a well-developed mola. Such mandibles are also associated with fungal feeding, and families such as the Nitidulidae, Tenebrionidae, and Oedemeridae contain species representing both adult-feeding habits; individual oedemerid species have been reported to feed on both fungi and pollen.
Coleopteran relationships with fungi are widespread throughout the order and diverse in form. Approximately 25 extant families of beetles are primarily mycophagous. Greatly unappreciated, however, are the less obvious trophic relationships between fungi and many beetles that are ostensibly saprophagous or phytophagous. Many beetles eat plant tissue only after it has been partially broken down by fungi. Some harbor endosymbiotic fungi that allow digestion of plant tissue or provide essential nutrients. Others are thought to ingest and acquire fungal enzymes that are essential for their existence as herbivores. John Lawrence estimated that as many as half of all beetle families either are truly mycophagous or feed on plant matter that has been altered by fungal enzymes.
Ancient Greeks believed fungi were merely homes of insects. A rich insectan fauna often dwells in larger fungi, and much of it comprises mycophagous and predaceous beetles. Through evolutionary time few fungus groups have escaped the interest of beetles. Mycophagous families seem to be especially concentrated in the polyphagan superfamilies Cucujoidea, Tenebrionoidea, and Staphylinoidea. However, fungivory arose repeatedly in various other lineages within the order as well.
Fungi are tremendously diverse physically, chemically, behaviorally, and ecologically. Mushrooms, woody conks, puffballs, truffles, yeasts, smuts, rusts, and molds present separate special challenges as food sources. In addition, a single fungus often represents a composite of resources. For example, a single polypore shelf on a log may provide a delicate layer of spore-bearing tissue on the underside, a hard, woody context, and an area where its hyphae penetrate decaying wood. Some mycophagous beetles have a broad range of acceptable hosts; however, many are more selective, feeding only on some portions of fruiting structures from a few species at a particular stage of development or decay. Host specificity tends to be narrower for immature stages. Specialization of beetles has occurred in response to the various resources and challenges that fungi present.
Woody polypore shelves offer large, persistent sources of food for mycovores. There are many different strategies for the use of the soft spore-bearing tissue of wood polypore fungi. Species of Ellipticus (Erotylidae) have robust mandibles capable of gouging off chunks of hymenium and its supporting tissues (Fig. 39). Larvae of Holopsis (Corylophidae) have found another method of tapping this resource.
They use a slender, snout-like elongation of the head to graze on the inner surface of individual spore tubes (Fig. 40). The Nannosellinae (Ptiliidae) exhibit another evolutionary solution, namely, miniaturization: fully grown adults, only 0.4 mm in length, crawl inside individual spore tubes to feed directly on the soft spore-bearing tissue.
Specialists on fleshy mushrooms [e.g., Oxyporus (Staphylinidae)] face different challenges. Unable to fly around to look for new mushrooms, larvae must complete their feeding on their ephemeral host before it decays. Many of the fungus beetles that specialize on soft mushrooms exhibit greatly accelerated development. Their mandibles are more blade-like and are capable of slicing through large chunks of soft fungal tissue.
Beetles preferring small, scattered fungal spores or conidia as food often have a suite of features related to their microphagous habits. The mouthparts tend to be brushy and capable of sweeping tiny particles from the substrate into their mouth. These modifications often involve the maxillae, but in larval Dasycerus (Staphylinidae) the mandibular apices are modified for this function as well (Fig. 41). The mandibular mola is also commonly modified in spore feeders. Spores are ground between opposing molar surfaces on each mandible, with an action much like that of a millstone grinding wheat into flour. Nosodendridae, which feed partially on yeasts that occur in sap fluxes, use their brushy mouthparts to filter the fungal cells from the fluid (Fig. 42).
Less exploitative symbiotic relationships with fungi are also widespread and diverse within Coleoptera. The best-studied examples of mutualism with fungi are the relationships occurring in the bark and ambrosia beetles (Platypodinae and Scolytinae of the Curculionidae). Perhaps the most familiar case is that of Dutch elm disease. At the corners of this ” ecological triangle” are the bark beetles (Scolytus spp.), the fungus (Ceratostomella ulmi), and the host elm trees (Ulmus spp.). Adult beetles nibble on tree twigs and thereby inoculate them with fungal spores. Following the germination of the spores, the fungus attacks the tree and ultimately kills it. The beetles prefer to oviposit on recently killed Ulmus trees, many of which were recent victims of C. ulmi. Upon hatching, their larvae bore about, feeding on fungus-infested wood. The final link in the cycle is completed when newly emerging adults pick up fungal spores as they move around the gallery before flying off to dine on some living elm twigs.
A broad range of variants stems from the basic pattern observed in Dutch elm disease. In some cases the link between the fungus and the beetles weakens to the point of being merely incidental. In Lymexylidae and at least some Platypodinae, the relationship is a tighter, obligatory one in which the beetles farm a fungus to feed their brood. The wood of the host tree is important to the beetle only as a substrate for the fungal garden. In these evolutionarily linked relationships, the beetles often have specialized pockets called mycangia on their body to aid in the transportation of spores or conidia to new substrates (Figs. 43 and 44- . Mycangia sometimes have associated glands that help to keep the fungal tissue viable until it is needed to start a new garden. Also, there is a tendency for these ambrosia fungi to be less invasive and destructive to the tree, instead staying near the galleries in which they are cultivated. Neither the fungi nor the beetles in these closer relationships can exist independently.
Another solution to digestion of plant matter is seen in some Cerambycidae, Anobiidae, and Passalidae. Instead of using fungi to externally convert plant matter to digestible food, they rely on endo-symbiotic yeasts and bacteria to accomplish the feat internally. Although yeasts and bacteria are common inhabitants of the gut in many insects, the relationship between some yeasts and beetles is one of obligatory symbiosis. Endosymbiotic yeasts may be harbored in the lumen of the gut, in diverticula (Fig. 45), or in specialized cells in the cytoplasm called mycetocytes. Clusters of mycetocytes can form small organs called mycetomes. Yeasts may permit the breakdown of cellulose and xylose and provide various nutrients to their host. In the drugstore beetle, Stegobium paniceum, endosymbiotic yeasts are credited with providing riboflavin, niacin, pyridoxine, pantothenic acid, folic acid, and biotin.
To perpetuate endosymbiotic relationships, the gut of offspring must be charged with endosymbionts early in development. Yeasts are passed from adult beetles to larvae in various ways. The egg cho-rion may be inoculated with yeast so that the young are charged upon
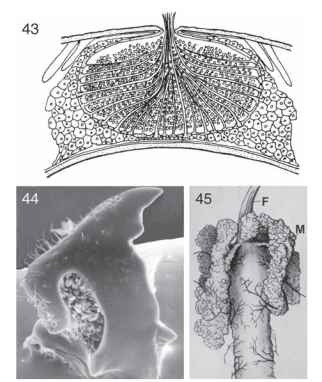
FIGURES 43-45 Beetle mycangia and mycetome. (43) Scolytoplatypus sp. (Curculionidae), transverse section of front part of adult pronotum, showing mycangial cavity filled with spores. (44) Eurysphindus hir-tus (Sphindidae), left adult mandible, dorsal view, showing spores of myxomycete inside dorsal cavity that is presumed to serve as a mycangium. (45) Foregut (F) and anterior portion of midgut of Lixus sp. larva (Curculionidae), showing mycetomes (M).
chewing out of egg and ingesting the chorion. In some Cucujidae, Silvanidae, Lyctidae, and Curculionidae, yeasts migrate into the egg within the female before the chorion is secreted. A third method of yeast transmission results following migration into the testes of the male. The yeast and sperm then enter the egg through the micropyle.
Formerly classified as fungi and studied by mycologists, the Myxomycetes are now recognized as protozoan animals. Despite their phylogenetic position, Myxomycetes are similar to fungi in some respects, and as a result beetle-myxomycete interactions share parallels with beetle-fungus interactions. In the plasmodial stage, Myxomycetes flow around their environment, consuming bacteria. Rhysodine Carabidae and Cerylonidae feed, at least facultatively, on the plasmodial stage. When these colonial protozoans amass as plas-modia to form a sporocarp, they take on many fungus-like features. This stage has attracted specialist beetles from no fewer than seven families: Leiodidae (Fig. 46), Staphylinidae, Clambidae, Eucinetidae, Cerylonidae, Sphindidae, and Latridiidae. Pits in the mandibles of Sphindidae, an entirely myxomycophagous family (Fig. 44), and the venter of slime-mold-feeding latridiid species have been found to house myxomycetan spores.

FIGURE 46 Anisotoma basalis (Leiodidae) feeding on a Stemonitis myxomycete fruiting body.
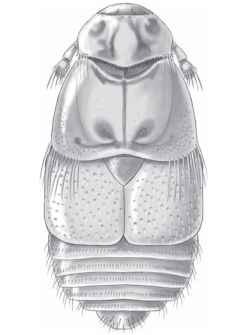
FIGURE 47 Platypsyllus castoris (Leiodidae), parasitic on beaver (Castor spp.). (Illustration courtesy of Ainsley Seago.)
Whereas other holometabolous insect orders such as the Hymenoptera and Diptera include parasitoid lineages of great diversity, the Coleoptera have not diversified to any great extent via parasitism on animal hosts. In addition to meloid and rhipiphorid hymenopteran parasites, parasitism of single host individuals has been infrequently observed. Aleocharine Staphylinidae parasitize the pupae of higher flies (order Diptera, suborder Cyclorrhapha). Within the Carabidae, the bombardier beetles, or Brachinini, parasitize the pupae of Dytiscidae, Gyrinidae, Hyrdophilidae, and carabid beetles of the genus Amara. Carabid beetles of the genus Lebia parasitize chrysomelid leaf beetles. Lebia beetles imitate various alticine flea beetle species with which they co-occur. The quick-jumping alticines are protected from predatory birds by their ability to disappear via a jump, suggesting that the Lebia have evolved a similar appearance through mimetic evolution. Coccinellid predatory larvae approach the specialization seen in some parasitoids, as some of the smaller species require only one to several sternorrhynchan prey individuals to complete larval development. Nonetheless, these species can switch prey species depending on the density of various hosts.
Platypsyllus castoris beetles of the family Leiodidae are specialists on beavers, with both the flattened, highly modified adults (Fig. 47) and the larval stages living in the animals’ fur. Related leiodids in the subfamily Leptininae live on the bodies of rodents, though they exhibit much less extreme body forms, and a lower level of host specificity, than the beaver beetles. The highly specific host relationship of Platypsyllus probably evolved from a more general predaceous habit. Such nest inquilines are found in a variety of lineages within the Staphylinidae, with adults and larvae variously preying on flea larvae or other nest-associated scavengers.
INTRASPECIFIC INTERACTIONS
The newly eclosed adult beetle faces the various tasks of dispersing from the pupal habitat, finding a mate, mating, finding a suitable larval habitat, ovipositing, and possibly guarding or facilitating the development of its young, all the while avoiding natural enemies. To beetles, flight may be a rare event. Many species undergo only a nuptial flight from the larval habitat to a new habitat, where mating and oviposition occur. Others may move from a breeding habitat to a drier micro-habitat for overwintering, and thence back to the breeding habitat the next spring, making three flight periods in their lifetime. Others such as the floricolous cerambycid long-horned beetles, buprestid jewel beetles, herbivorous Chrysomelidae, and Sternorrhyncha-feeding Coccinellidae may fly more or less continuously during their adult life span as they move from plant to plant. Beetle flight always requires the unfolding of the flight wings. Typically, beetles will climb some sort of prominence, use their antennae as ” windsocks ” and orient their body so that their initial liftoff is against the wind, and then open their elytra and unfold their flight wings prior to takeoff. During the nuptial flight, beetles are likely to be reproductively incompetent. In some scarab beetles, vast amounts of air are swallowed prior to flight, resulting in a distended gut unsuitable for feeding.
Mate finding may be facilitated by aggregation on host plants. The crushed leaves of host- and nonhost-plant species are attractive to both male and female scarab beetles. Adult emergence occurs as synchronous mass flights. Once near or on the host plants, female pheromones attract male scarabs. Ruteline scarabs utilize sex phe-romones derived via fatty acid biosynthesis, whereas scarabs in the not distantly related Melolonthinae utilize amino acid derivatives and terpenoid compounds. The compounds of different classes are released from glands on different parts of the body: ruteline phe-romones from epithelial cells lining inner surfaces of the apical abdominal segments, for example, or melolonthine pheromones from eversible glands on the abdominal apex. Pheromones used in the other beetle superfamilies span these scarab pheromone classes (e.g., terpenoids in the Curculionidae, fatty-acid-derived aldehydes and acetates in the Elateridae, and esters in the Dermestidae).
Beetles use the other sensory modalities in mate finding, outdoing diversity observed in any other insect order. The anobiid death-watch beetle acquired its ominous name through the predisposition of its males to bang their head capsules on host wood, telegraphically inquiring whether a receptive female is in the vicinity. This rapping was thought to foretell an imminent death. A male initially taps an average of five times, and if a female responds with a single tap, he moves a short distance and taps once. If he determines that the second female tap is fainter than the first, he turns at various angles to attempt to approach the female. Males receiving no returning female tap to their five-tap overture move greater straight-line distances between tapping bouts, searching greater expanses of wooden habitat for a responsive female (Fig. 48 ).
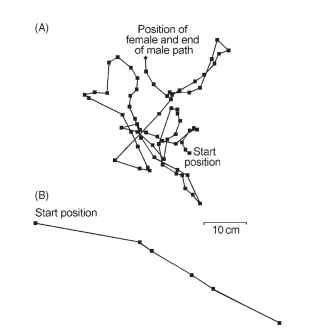
FIGURE 48 Paths taken by male deathwatch beetle, Xestobium rufovillosum (squares indicate positions at which male stopped to tap). (A) Female responded to male head tapping and was successfully found by male. (B) Male tapped in absence of any responding female
The use of light for mate finding has been evolutionarily refined in the Elateroidea, with the Lampyridae using flashing signals produced in abdominal light organs to engage in complex male-female dialogue before mating. These light organs are modified fat body cells with transparent outer surfaces, backed with highly reflective uric acid crystals. The light is highly efficiently produced via the oxidation of luciferin by the enzyme luciferase in the presence of adenosine triphosphate (ATP) and oxygen, producing oxyluciferin, carbon dioxide, and light. Male flashes are composed of species-specific series of flashes of varying duration, composition, and in some instances intensity. Males of different species fly in different patterns and at different heights, while females respond with a simpler flash that encodes species identity by the response delay to the male flashing sequence, by the flash duration, and in several species by a multiple-flash sequence. This sexual communication has been co-opted as a predation mechanism in Photuris fireflies. Males and females of these adult-feeding lampyrids use a typical male-female light dialogue to mate, whereupon the female’s nervous system is affected so that she sends species-specific mating responses coded for sym-patric, smaller Photinus species. Photinus males who venture too near the faux-Photinus female responses sent by the Photuris females are eaten.
Males and females may undertake various types of precopulatory behavior before mating. These may involve the sensing of species-specific alkene aphrodisiacs related to cuticular hydrocarbons, as in aleo-charine Staphylinidae. Males or females may stridulate as part of their behavioral repertoire. In Meloidae, a male will climb onto the dorsum of the female and antennate her head, palps, or antennae. In Eupompha meloids, the males draw the antennae of the females along a longitudinal sulcus on the male vertex (Fig. 49). Genitalic insertion by the male is successfully attempted only after antennation of the female. The passage of a nuptial gift of the highly toxic compound cantharidin has been incorporated into mating behavior in the pyrochroid fire beetles. In Neopyrochroa flabellata, the female samples an exudate from a transverse sulcus on the male vertex. If the exudate contains the terpenoid cantharidin (better known as the mammalian “aphrodisiac” Spanish fly), the male successfully mates, whereupon he transfers to the female, along with his sperm, about half the cantharidin stored in the accessory glands of his reproductive tract. The female translocates this cantharidin from her spermatheca to the developing eggs, which are thus chemically protected from predation by cantharidin-sensitive predators. Although meloids are known to produce cantharidin, transfer of this compound during meloid mating has not been documented. Conversely, although pyrochroids utilize this chemical in their mating behavior, they do not seem to be able to synthesize it, and the natural source of cantharidin that facilitates their behavior remains to be discovered.
FIGURE 49 Eupompha fissiceps (Meloidae), male antennating female while rubbing his tarsi under her head; male antennae bring female’s antennae alternately into his cephalic sulcus.
Precopulatory behavior may involve more than a male and a female, especially in species in which male-specific structures have evolved in elaborate fashion. The enlarged male mandibles of stag beetles, Lucanidae, and prominent horns on the heads and pronotum of scarab beetles are used by males to joust for advantageous mating sites with females. Dynastine and other scarabs seek out branches of shrubs and low trees upon which to mate. Males competitively maneuver for the top position on the branch, which is favored by females entering the fray for mating.
Copulation occurs with male dorsal to the female, the male grasping the female with the fore- and midlegs, and sometimes the mandibles, as in the tiger beetles or cicindeline Carabidae. The male aedeagus is inserted into the female gonopore. An aedeagal internal sac may be everted to place the male’s gonopore near the entrance to the female spermatheca, and a spermatophore may be passed that encloses the sperm. Most beetles exhibit a monotrysian female reproductive tract; that is, the eggs pass out of the same structures used for copulation. However, in the dytiscid water beetles, a ditry-sian configuration has evolved whereby copulation and oviposition occur via parallel, though connected, passages in the female.
The necessity for mating and copulation has been obviated in various groups of Curculionidae, Chrysomelidae, and Carabidae through thelytokous parthenogenesis. Species may be composed entirely of parthenogenetic populations, or such populations may be restricted to peripheral portions of the range. Parthenogenesis may also be associated with polyploidy, especially in weevils.
Although most mated female beetles oviposit into appropriate microhabitats where the larvae will develop, some families are characterized by eggs being laid in masses (e.g., Coccinellidae). Some tortoise shell chrysomelid females, Cassidinae, will lay eggs in a mass and then hover over the mass through hatching and the early days of the larvae. Female pterostichine carabids of the genera Abax and Molops similarly guard their eggs, although only until hatching. Females of several sta-phylinid species of Oxyporus , voracious mycovores with large sicklelike mandibles, have been reported to oviposit several eggs within a cavity in a soft mushroom, and then stay with the larvae as they quickly develop to pupation over 3-6 days. Females of the erotylid genus Pselaphacus also provide maternal care to their young as they herd tight balls of their writhing larvae across fruitings of their fungal host.
Ovoviviparity, or the holding of eggs until larvae hatch, has evolved several times across the Coleoptera. Typically it occurs in beetles occupying marginal environments dangerous to egg development. Chrysomelid females of montane or subarctic species hold developing eggs in the reproductive tract while basking on sun-drenched leaves to hasten egg development before larviposition. In Pseudomorpha hubbardi carabid beetles, females hold developing eggs until the larvae can be deposited, whereupon the larvae complete development as inquilines in an ant nest.
Male and female cooperative brood rearing has evolved repeatedly in various groups of Coleoptera. The long-known and oft-revered dung-rolling Scarabaeidae provision nest burrows with rolled dung balls, upon which the eggs are laid. Females undertake this activity alone in some species, whereas the sexes work together in others. Burrows may be dug before dung balls are cut from mammalian dung pats, requiring navigation from the dung pat to a predetermined burrow location, or the burrow may be dug after the dung ball has been constructed.
In the Australian Cephalodesmius armiger, males and females pair up and divide duties. Males actively forage for decomposing leaves, flowers, fruit, and seeds, which they bring back to the nests where the females remain. The female works the plant materials into a compressed ball, to which she adds her fecal material. The microbiological action of fungi from her feces causes fermentation in this external rumen after larval brood balls have been made from the mass. As the larvae develop, they feed on the brood ball from the inside out. When the thickness of the walls of the brood ball drops to about 2 mm, the increased volume of larval stridulations sensed by the female stimulates her to add more decaying material to the brood ball. Four to ten brood balls are made per nesting pair. When the larvae finish their development, the female seals the brood ball with a combination of larval and female feces, the larva having ejected its fecal material through cracks in the brood ball before pupation. Both parents die before adult emergence of their young. The new adult beetles feed on the walls of the brood ball, inoculating their gut with the fungi used by the mothers to produce fermentation in the external rumen.
Like the nest-building scarab, beetles of the silphid carrion beetle genus Nicrophorus raise their young on a concealed, highly desirable resource, a decaying carcass. Adult Nicrophorus actively fly long distances searching for a carcass. If a male discovers one, he emits a pheromone that attracts a female, with mating occurring on the carcass. Male and female then cooperatively bury the carcass by digging underneath it, and maneuver the corpse into a ball. Their activities isolate the corpse from competing silphids and insulate it from microorganisms in the soil. After repeated mating, the female lays eggs in the surrounding soil. Upon hatching, the larvae crawl to the carcass, attracted by olfactory cues and adult stridulation. The adults precondition part of the carcass for larval feeding by chewing on it. They first feed the young larvae by regurgitating predigested carrion. Older larvae feed on their own, developing in 1-3 weeks, during which the female stays on the carcass. Upon maturation, the larvae crawl into the adjoining soil to pupate, and the female leaves to search for a new carcass. Variations on this scenario include more than one pair of adults supported by a larger carcass, mated females raising their larvae alone when they discover a carcass without a resident male, and larger Nicrophorus species usurping a carcass through killing the original colonizing adults and their larvae.
True sociality, wherein more than one generation of adult lives together, and reproduction is restricted to a portion of the individuals, is likely to be rare in Coleoptera. This behavior has been reported only twice, and conclusive studies to completely document interactions among adults and larvae have not been fully documented for either family, represented by the wood-inhabiting bess beetles (Passalidae) and the ambrosia beetle Austroplatypus incompertus (Curculionidae: Scolytinae). There is no doubt that adults and larvae live together, and that fungi are passed from generation to generation. For this arrangement to qualify as eusociality, the existence of individuals that assist reproductives but do not themselves reproduce, at least during a portion of their life, must be documented.
INTERSPECIFIC INTERACTIONS
Beetles exhibit defensive behavior that is mostly rooted in the attributes of their cuticle. Many beetles living an exposed portion of their life cycle on vegetation will use the “drop-off” reflex if disturbed (i.e., simply close the legs and tumble off the leaf or branch and fall to the ground, where their often cryptic coloration helps protect them from visually oriented predators). The drop-off reflex can be combined with thanatosis, in which the beetle lies still with legs appressed to the body. Alternatively, the legs may be held at irregular positions by muscular tetanus (catalepsy), or the individual may roll up into a ball with the antennae, mouthparts, and legs hidden from view. More brightly colored species do not use the drop-off reflex. Chrysomelid flea beetles have enlarged hind femora containing strong tibial extensor muscles; a cuticular femoral spring releases the stored energy, launching them into the air. Elateridae are commonly known as click beetles due to the familiar sound that is produced when their specialized thoracic locking mechanism is released, usually catapulting the beetles far away from threats.
Defensive chemical secretions that protect beetle adults from predators have evolved numerous times. Toluquinone is a defensive constituent common to several major terrestrial families (Carabidae, Staphylinidae, and Tenebrionidae), suggesting that this was one of the earliest defensive secretion types to have evolved. Since quinones are used in the tanning process of new cuticle, they would have been evo-lutionarily available in large quantities in well-sclerotized ancestral lineages of these families. Their tanning nature is not restricted to insect cuticle, as attested by the darkly stained fingertips of anyone who picks up an oozing Eleodes tenebrionid beetle.
Perhaps the most famous defensive chemical reaction in beetles is observed in the crepitating bombardier beetles of the carabid tribe Brachinini. These beetles, like other carabids, possess pygidial defensive glands that empty from the lateral edges of the intersegmental membranes between the seventh and eighth abdominal segments; Brachinine bombardier beetles plus carabid beetles of several other tribes (Metriini and Paussini) eject a combination of hydroquinones plus hydrogen peroxide held in one chamber of the gland, and catalases plus hydrogen peroxidase held in a second chamber. These chemicals combined result in an explosive ejection of hot (100°C) secretion, with liberation of the oxygen of H2O2, thus reducing hydroquinone to qui-none, with the released O2 propelling the spray (Fig. 50) .
FIGURE 50 Cross section of pygidial defense gland of Brachinus bombardier beetle adult (Carabidae): L, secretory lobes; B, collecting vesicle; M, sphincter muscle; E explosion chamber; G, ectoder-mal glands that secrete catalase; O, outlet. Vesicle B contains mixture of hydroquinone and hydrogen peroxide, exploded by catalase, when it passes into E.
In addition to quinone compounds, beetles have evolved to use a variety of other defensive chemicals. The more recently evolved carabid beetle groups spray formic acid, a chemical also utilized as a defensive agent by their omnipresent antagonists, the ants, or Formicidae. Brightly colored or starkly patterned beetles are candidates for chemical protection via defensive gland secretions. The buprestid jewel beetles are often colored in black and yellow stripes to appear like the Hymenoptera with which they cohabit in various flowers. Jewel beetles are highly protected by bitter chemicals named buprestins. Not only are these chemicals distasteful to mammals (viz., organic chemists!), but ants reject sugar solutions laced with buprestins. Jewel beetles form mimetic complexes with lycid beetles, themselves protected by defensive secretions composed of various substituted parazines, reportedly among the most powerful odorous substances known (Fig. 51).
Various other beetle families regularly contribute members to lycid-based mimicry rings, including Cerambycidae, Meloidae, and
FIGURE 51 Five Australian beetles and a moth forming part of a mimicry ring: (A) Metriorrhynchus rhipidius (Lycidae), (B) Eroschema poweri (Cerambycidae), (C) Tmesidera rufipennis (Meloidae), (D) Rhinotia haemoptera (Belidae), (E) Stigmodera nas-uta (Buprestidae), (F) Snellenia lineata (Lepidoptera: Oecophoridae). (Images provided by copyright holder, CSIRO Entomology, Canberra,ACT, Australia.)
Oedemeridae. Given that the meloids and oedemerids can synthesize cantharidin, it is likely that most beetles in such rings are distasteful, making Mullerian mimicry the dominant basis for such common color patterns (Fig. 51). Other mimicry rings center on the dangerously toxic Paederus staphylinid beetles (Fig. 52 ), the cuticle of which exudes pederin. When such a beetle is scraped or crushed, contact with the pederin released results in human whiplash dermatitis (Fig. 53 ).

FIGURES 52-53 (52) exhibiting warning coloration observed in many species of this genus. (Image provided by copyright holder, CSIRO Entomology, Canberra, ACT, Australia.) (53) Dermatitis linearis on human forearm at 66 h after an adult Paederus beetle had been crushed on volunteer’s skin. Paederus cruenticollis (Staphylinidae)
INTERACTIONS WITH HUMANS
Throughout history, humans have had diverse interactions with, and perceptions of, beetles. Coccinellid beetles were once perceived to have a close association with the Virgin Mary, hence their common name “ladybugs.” Ancient Egyptians recognized dung beetles (Scarabaeidae) as a symbol of Ra, the sun god, because of parallels between the beetles’ behavior and cosmic activities credited to the deity. Much as the scarabs rolled dung balls across the desert, Ra was thought to guide the sun across the sky each day. The symbolism of sacred scarabs has continued until today, as scarab images are still incorporated into jewelry, signifying good luck to the buyer or wearer.
The mystery and aesthetic beauty of beetles has been captured in paintings, sculptures, dances, poems, songs, and other art forms. Beetles have been used by many cultures for decoration. The brilliant metallic elytra of Buprestidae serve as natural sequins on textiles, and as biological gems in jewelry. In some cultures, beetle horns are included in jewelry because they are thought to increase sexual potency.
Live stag beetles (Lucanidae) are prized as pets in Japan, where a considerable amount of study has been given to their care in captivity. In Thailand the practice of “fighting” male Hercules beetles (Scarabaeidae) is a traditional source of entertainment. With a referee controlling the action, two males are introduced into an arena. When a female is placed nearby, her mating pheromones trigger the combatants to engage each other. The match ends and a victor is declared when one male becomes exhausted or backs down from the advances of his opponent. In Central America local craftsmen blur the distinction between “pet” and “jewelry” by gluing rhinestones, glass beads, and a small chain to the dorsal surface of zopherid beetles. When the tiny chain is pinned to clothing, the tethered beetle becomes living jewelry.
Entomophagy, the eating of insects, is common in many parts of the world, and beetles often make up part of the menu. Larvae of palm weevils (Curculionidae) are considered to be a delicacy on the islands of the South Pacific. Similarly the fleshy, sausage-like larvae of various long-horned beetles (Cerambycidae) and scarabs are relished by people around the world. Mealworms, the larvae of some tenebrionid beetles, are easily reared and have become standard fare for culinary demonstrations of entomophagy.
Beetles attract the most attention when they become economic pests of agriculture, horticulture, and forestry. Two families, the snout beetles (Curculionidae) and the leaf beetles (Chrysomelidae), include many serious pest species. In the middle to late 1800s, the Colorado potato beetle, Leptinotarsa decimlineata (Chrysomelidae), abruptly expanded its range across North America and then colonized Europe and neighboring regions. Great efforts were made to thwart the invader each time it appeared, but ultimately the beetles succeeded. Throughout the 20th century an epic battle was waged against the notorious boll weevil, Anthonomus grandis grandis (Curculionidae), in the Cotton Belt of the southern United States, where it inflicted great financial losses. A sustained and coordinated effort to control this pest succeeded in eradicating the boll weevil from portions of several states by the turn of the millennium.
Predaceous ladybugs are often used in biological control to suppress populations of sternorrhynchan crop pests (i.e., aphids and scales). In the first successful biological control introduction, an Australian ladybug, Rodolia cardinalis, suppressed the cottony cushion scale (Hemiptera) on citrus crops in California. Phytophagous beetles have been employed to control weeds. In the 1960s, the cattle-rearing industry in Australia faced a dilemma, because cows are not native to
the continent, no natural bovine dung entomofauna was available to use their feces. Therefore cow patties persisted for months, during which time they served as breeding grounds for pestiferous horn flies. After careful study, Australian entomologists introduced South African Onthophagus dung beetles (Scarabaeidae). The measure was successful, and the problem quickly abated.
Perhaps the least appreciated human-beetle interactions are those in which human population pressure inflicts a negative impact on beetle populations. Coleopteran diversity is largely attributable to their specialization for particular geographic locales, microhabitats, and food. As human populations grow and people alter the Earth for their needs, destruction of spatially restricted resources is an inevitable result, leading to extinction of species associated with those resources. Ironically, a characteristic that helped Coleoptera to attain the astounding degree of diversity that it exhibits today also predisposes many beetle species to anthropogenic extinction (Fig. 54 ).

FIGURE 54 The jewel scarab, Chrysina cusuquensis, known only from a restricted fragment of forest in northern Guatemala.
