Summary
The germ-lines of more than 35 species from five orders of insects have been genetically transformed, using vectors derived from Class II transposable elements. Initially the P and hobo vector systems developed for D. melanogaster were not applicable to other species, but four transposons found in other species, Hermes, Minos, Mosl, and piggy-Bac, were found to be widely functional in most insects. Genetic marker discovery and development have been equally important to vector development. Originally, cloned eye-color genes from Drosophila that complemented existing mutations in other insects were used, but now more widely applicable dominant-acting fluorescent protein genes are effective transformation markers and reporters for gene expression. Transformation technology is advancing at a fortuitous time when genomics is providing resources necessary for transgenic strain development in pest species to control their population size and behavior. Transposon-based transformation methods are also advancing insertional mutagenesis techniques, such as enhancer traps and transposon tagging, to facilitate the gene discovery and functional analysis that provides these resources. Together, efficient and routine methods for transposon-mediated germ-line transformation and genomics analysis should provide tools critical to the advancement of our understanding and control of insect species.
Introduction
The ability to create genetically transformed organisms has played a central role in the history of modern genetics; in particular, to our understanding of gene expression and development. Indeed, the pioneering transformation experiments of Pneumoccus by Griffith (1928), and subsequent systematic analyses by Avery, MacLeod, and McCarty (1944), which showed transformation from a "rough" to a "smooth" bacterial cell wall phenotype, were instrumental in defining DNA as the inherited genetic material. The importance of these initial transformation experiments to prokaryotic genetic analysis was widely appreciated, and continued studies by many other laboratories laid the foundation for modern molecular biology.
The importance of "transformation" technology to eukaryotic genetic studies was apparent, and several of the initial attempts to create transgenic animals were performed in insects, though the means of achieving and assessing insect transformation were not straightforward. The primary reasons why these early attempts to create transgenic insects were largely unsuccessful were the inability to isolate and reproduce individual genetic elements that could be used as transformation vectors and markers, and the lack of efficient means of introducing DNA into germ cells. Most of the initial studies of insect transformation relied on soaking embryos or larvae, with visible mutant phenotypes, in solutions of genomic DNA from wild type individuals in hopes of reverting the mutant phenotype. The first experiments performed in Bombyx and Ephestia met with some success, where mutant wing color pattern phenotypes were reverted in some organisms, though inheritance was inconsistent and transformation events could not be unequivocally confirmed (Caspari and Nawa, 1965; Nawa and Yamada, 1968; Nawa et al., 1971). Similar results were obtained in studies of Drosophila (Fox and Yoon, 1966, 1970), and for all of these initial experiment it is most likely the observed phenotypic changes resulted from extrachromo-somal maintenance of introduced DNA in the somatic tissue by an unknown mechanism. A different approach involving the microinjection of wild type genomic DNA into embryos homozygous for a recessive eye-color mutation (vermilion) resulted in transformants with a reversion to the normal red eye-color phenotype. While the reversion event was genetically mapped away from the mutant locus, a thorough molecular analysis to verify a transformation event, before the lines were lost, was not achieved and so the nature of the phenomenon observed in this experiment remains unexplained (Germeraad et al, 1976).
In the mid-1970s a turning point in insect science occurred with the extension of molecular genetic analysis to Drosophila melanogaster. These early studies and subsequent studies not only provided many of the tools and reagents necessary for developing and critically assessing genetic transformation in insects, but also emphasized the need for a technology that would facilitate a more complete understanding of the genes being isolated using recombinant DNA methods. One technology that was clearly needed was a means to stably integrate DNA molecules into the chromosomes of germ cells, resulting in heritable germ-line transformation. The simple introduction of raw linearized DNA into pre-blastoderm embryos in the hope of fortuitous recombination into host chromosomes was clearly not reliable. Interest was growing, however, in the use of mobile genetic elements as vectors for DNA integration, including retrotransposons and transposons that were being isolated in Drosophila for the first time. Foremost among these was the P transposable element, isolated from certain mutant alleles of the white gene. The subsequent testing and success of transformation mediated by the P element in the Drosophila germ-line proved to be a dramatic turning point in the genetic analysis of an insect species (see Figure 1 for the general germ-line transformation scheme). The eventual impact of this technology on understanding genetic mechanisms in all eukaryotic systems cannot be understated. The success with P in Drosophila gave hope that this system could be straightforwardly extended to genetic manipulation of other insect species, and especially those highly important to agriculture and human health. While there was reason for optimism, we now realize that this was a naive expectation, given what we now understand about the natural history of P elements relative to other Class II transposable elements – in particular, its extremely limited distribution and its dependence on species-specific host factors. The inability of P to function in non-drosophilids, however, was a motivating force to more completely understand transposon regulation, and the identification and testing of new vector systems. These included other transposable elements, as well as viral and bacterial vectors.
The development of routine methods for insect gene-transfer was probably delayed by a decade due to attention being focused exclusively on the P element. Yet this delay has resulted in a more varied toolbox of vectors and markers that now allow nearly routine transformation for many important species, and the potential for transformation of most insects (see Handler, 2001). Indeed, some of the tools developed for testing the P element, in particular embryonic mobility assays, are now routinely used for initial tests for function of other vectors in an insect species before more laborious and time-consuming transformation experiments are attempted.
The creation of this varied toolbox first related to the potential need for different vector and marker systems for different insect species. We now realize that the future of genetic analysis will depend on multiple vector and marker systems for each of these species, since genomics and functional genomics studies will require multiple systems for DNA integration and reporters for gene expression.
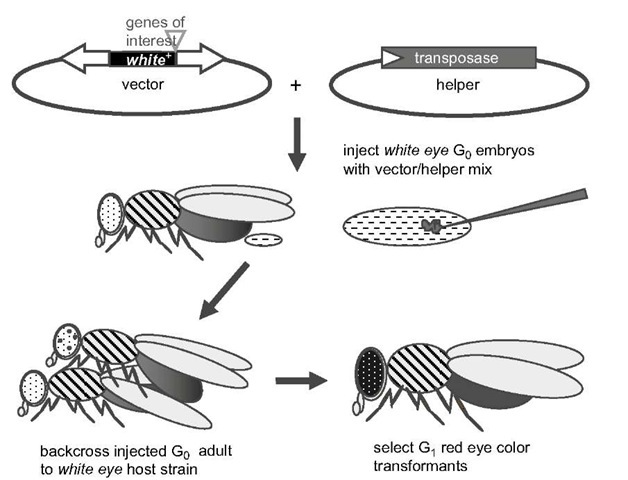
Figure 1 Diagram of the germ-line transformation method using a transposon-based vector marked with a wild type white eye+ gene, and a transposase helper, in a mutant white eye- host strain. Both vector and helper are non-autonomous in that the vector has a non-functional transposase, and the helper has one or both terminal sequences deleted. Vector and helper plasmids are mixed in 3 : 1 to 5 : 1 proportions at a total concentration of < 1 jg/ml in an injection buffer, and microinjected into preblastoderm we- host G0 embryos. G0 adults (which may show mosaic ommatidial pigmentation) are backcrossed to we-host strain flies with G-, progeny screened for complete eye pigmentation. Transgenic mutant-rescue eye pigmentation may be weaker than wild type eye due to position effect suppression. See text for more complete methods.
Indeed, germ-line transformation is essential for the insertional mutagenesis and functional genomics studies that are critical underpinnings for both assessing genomic architecture and relating sequences to gene expression. Notably, the continuing functional analysis of the Drosophila genome now relies on the vectors and markers, described in this topic, that were first developed for non-drosophilid insect species.
P Element Transformation
P Element
The use of transposable element-based vectors for Dro-sophila transformation followed the discovery of short inverted terminal repeat-type elements similar to the Activator (Ac) element discovered in maize by McClinotck (see Federoff, 1989). The first such element to be discovered in insects was the P element, the factor responsible for hybrid dysgenesis that occurred in crosses of males from a P strain (containing paternal [P] factors associated with hybrid dysgenesis) with females from an M strain (devoid of P factor) (Kidwell et al., 1977). The identification of P sequences resulted from the molecular analysis of P-induced white mutations that occurred in dysgenic hybrids (Rubin et al., 1982). While the initial
P elements isolated as insertion sequences were incomplete, non-autonomous elements, complete functional elements were later isolated and characterized by O’Hare and Rubin (1983).
P is 2907 bp in length with 31-bp terminal inverted repeats (ITR) and 11-bp subterminal inverted repeats that occur approximately 125 bp from each terminus (see Figure 2). Other repeat sequences exist within P, but their functional significance, if any, remains unknown. A defining signature for P, as with other transposable elements, is the nature of its insertion site, which consists of an 8-bp direct repeat duplication. The extensive use of P for transformation and transposon mutagenesis has shown the element to have a distinctly non-random pattern of integration. It is now clear that P elements are blind to a significant fraction of the genome, and new gene vectors are being employed in Drosophila to complement these limitations. P elements and all transposable elements currently used as insect gene vectors belong to a general group of transposable elements known as Class II short inverted terminal repeat transposons (see Finnegan, 1989). These elements transpose via a DNA intermediate, and generally utilize a cut-and-paste mechanism that creates a duplication of the insertion site. These are distinguished from Class I elements, or retrotransposons, that transpose via reverse transcription an RNA intermediate, and may or may not have long terminal inverted repeats.
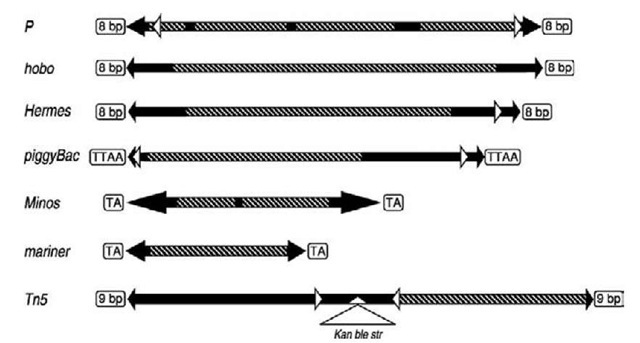
Figure 2 Diagram of transposable elements currently in use for the germ-line transformation of insect species. The left arms represent the 5′ termini, and right arms represent the 3′ termini. Transposon sizes and specific internal elements are shown in relative positions but are not at precise scale. Major structural elements include duplicated insertion sites (open boxes); inverted terminal repeat sequences (black arrowheads); internal subterminal repeat sequences (white arrowheads); transposase coding region (boxed diagonals); and intron sequences (black boxes). The Tn5 element is a composite transposable element consisting of two functional elements flanking three antibiotic resistance genes.
The original use of P for germ-line transformation was accomplished by inserting a marker gene into the element so that it did not disrupt activity of the terminal sequences or the transposase gene. The rosy+ gene was inserted at the 3′ end of the transposase-coding region, but upstream of the 3′ subterminal inverted repeat sequence. Plasmids containing this vector were injected into preblastoderm (syn-citial) embryos homozygous for ry- so the P vector could transpose into germ cell nuclei. Germ-line transformation events were identified in the following generation (G1) by virtue of reversion of the mutant ry- eye-color phenotype to wild type. These experiments not only proved the feasibility of transposon-mediated transformation, but also permitted structure-function relationships within the P element to be determined (Karess and Rubin, 1984). The P transcriptional unit was found to be composed of four exons separated by three introns. Further analysis determined the cause of P’s germ-line only activity to be due to the absence of splicing of the third intron in somatic cells. The absence of third-intron splicing in the soma results in production of non-functional truncated transposase polypeptides in these tissues (Rio et al., 1986).
While the original P vector allowed efficient transformation, the presence of a functional transposase gene within the vector made the system self-mobilizable (autonomous) and inherently unstable, allowing potential excision or transposition of the original insertion event. Subsequent vector development resulted in a binary system in which the transposase coding region was either deleted from the vector or made defective by insertion of a marker gene. The ability of the transposase to act in trans allowed transposase to be provided on a separate plasmid (helper) that could facilitate vector integrations when co-introduced with the vector-containing plasmid into the same nucleus (Rubin and Spradling, 1982). Integrations would remain stable if the helper did not integrate, but the original helpers, such as pn25.1, were autonomous P elements themselves that could integrate along with the vector. While helper integration was diminished by injecting several-fold higher concentrations of vector plasmid, this possibility was only eliminated with the creation of defective helpers having one or both of their terminal sequences deleted (known as "wings-clipped" helpers). The first of these was pn25.7wc, which was immobilized by deleting 3′ terminal sequences (Karess and Rubin, 1984). This prototype vector system served as a model for binary systems of non-autonomous vector : helper elements used for all the transposon-based transformation systems currently in use.
A notable characteristic of P elements was not only their discontinuous intraspecific distribution (P and M strains), but also their discontinuous interspecific distribution. Based on distribution patterns, it has become apparent that P elements were recently introduced into D. melanogaster from D. willistoni by an unknown mechanism (Daniels and Strasbaugh, 1986). Regardless of the mechanism, since the 1950s P elements have thoroughly invaded wild populations of D. melanogaster (Anxolabehere et al., 1988), and without the existence of M strain laboratory stocks that were removed from nature before this time, the development of P vectors may never have been realized. This is due to the repression of P mobility in P-containing strains that was first observed in hybrid dysgenesis studies, which also showed that movement was not repressed in M strains devoid of P. The basis for P-strain repression appears to be due to a number of factors, including repressor protein synthesis, transposase titration by resident defective elements, and transcriptional control of transposase gene transcription (Handler et al., 1993a; Simmons et al., 2002; Castro and Carareto, 2004; Jensen et al., 2008). As will be discussed, other vector systems in use have thus far been shown to be widely functional in several orders of insects, and the presence of the same or a related transposon in a host insect does not necessarily repress vector transposition. In this and several other aspects, the P vector system appears to be the exception rather than the rule for transposon-mediated gene transfer in insects.
P Vectors and Markers
Regardless of regulatory differences between P and other transposon vector systems currently in use, methods developed for P transformation of Drosophila serve as a paradigm for all other insect vector systems (see Figure 1). Those familiar with Drosophila transformation will be in a good position to attempt these methods in other insects. Current techniques developed for other insect species are variations on a theme, though, as we describe, considerable modifications have been made. Several comprehensive reviews are available for more specific details on the structure, function, and use of P for transformation in Drosophila, which are highly relevant to the understanding and use of other vector systems (see Karess, 1985; Spradling, 1986; Engels 1989; Handler and O’Brochta, 1991; Venken and Bellen, 2005, 2007). Particularly useful are the topics and methods manual by Ashburner (1989a, 1989b) that review the various vectors, markers and methodologies used for Drosophila transformation, as well as early techniques used to manipulate Drosoph-ila embryos. This information is especially applicable to other insect systems.
The first consideration for transformation is the design of vector and helper plasmids, and the marker system used for transformant selection. The first P vectors and helpers were actually autonomous vectors, which was probably a useful starting point, since the actual sequence requirements for vector mobility and transposase function were unknown. As noted, the first non-autonomous helper had a 3′ terminal deletion that prevented its transposition, providing greater control over vector stability. However, this source of transposase was inefficient until it was placed under hsp70 regulation that allowed transposase induction by heat shock (Steller and Pirrotta, 1986). All other vector system helper constructs have similarly taken advantage of heat shock promoters, mostly from the D. melanogas-ter hsp70 gene, but other hsp promoters have been tested, including those from the host species being transformed. Other constitutive promoters such as those from the genes for actin and a1-tubulin have proven successful for helper transposase regulation, and will be discussed further on.
While sufficient transposase production is critical for transposition, the structure of the vector is equally important, and, for some, very subtle changes from the autonomous vector can dramatically decrease or eliminate mobility. These variations include critical sequences (typically in the termini and subtermini), and placement and amount of exogenous DNA inserted within the termini. For some vectors, the amount of plasmid DNA external to the vector can affect transposition rates. Subsequent to the initial test of several P vectors, the terminal sequence requirements for P mobility were determined to include 138 bp of the 5′ end and 216 bp of the 3′ end. While the inverted repeat sequences within these terminal regions are identical, the adjacent sequences were found not to be interchangeable in terms of vector mobility (Mullins et al., 1989). Of interest was the discovery that the strongest binding affinity for the P transposase was at sequences approximately 50 bp internal to the terminal repeats (Rio and Rubin, 1988; Kaufman et al., 1989). While the minimal sequences required for mobility may be used in vectors, typically the rate of mobility decreases with the decreased length of terminal sequence. Specific sequences may be required for binding of transposase or other nuclear factors, and conformational changes needed for recombination may be dependent upon sequence length and position.
P-vector mobility was also found to be influenced by the amount of exogenous DNA inserted between the termini, with transformation frequency diminishing with increasing size. Initial tests with 8-kb vectors marked with rosy yielded transformation frequencies of approximately 50% per fertile G0, while use of 15-kb vectors resulted in 20% frequencies (see Spradling, 1986). Larger vectors could transpose, but frequencies approached 1% or less.
Of equal importance to creating an efficient vector system is having marker genes and appropriate host strains that will allow efficient and unambiguous identification or selection of transgenic individuals. Indeed, the genetic resources available for Drosophila also provided cloned wild type DNA and appropriate mutant hosts for use in visible mutant-rescue marker systems that made testing P transformation possible. As noted, the first of these used the ry+ eye-color gene, but this required a relatively large genomic fragment of nearly 8 kb. The white (w) eye-color gene was then tested, but this required a genomic sequence that was longer than ry, and resultant transformation frequencies were relatively low (Hazelrigg et al., 1984; Pirrotta et al., 1985). New w markers, known as mini-white, which had the large first intron deleted, decreased the marker insert to 4 kb, resulting in much more efficient transformation, and placing the mini-white marker under hsp70 regulation increased efficiencies further (Klemenz et al., 1987). Use of white markers, especially in CaSpeR vectors (Pirrotta, 1988), has been a mainstay of Drosophila transformation, yet expression of the w gene in particular is subject to position effect variegation/suppression (PEV) that typically diminishes eye pigmentation. PEV, indeed, was originally discovered as a result of translocating w+ proximal to heterochromatin (Green, 1996), and it routinely manifests itself in w- flies transformed with w+. This effect has been observed with use of eye-pigmentation markers in several other insect species as well.
Other markers based upon chemical selections or enzymatic activity were also developed for Drosophila, though none have found routine use. These included alcohol dehydrogenase (Adh) (Goldberg et al., 1983) and dopa decarboxylase (Ddc) (Scholnick et al., 1983), which complemented existing mutations; and neomycin phos-photransferase (NPT or neo) (Steller and Pirrotta, 1985), P-galactosidase (Lis et al., 1983), organophosphorus dehydrogenase (opd) (Benedict et al., 1995), and dieldrin-resistance (Rdl) (ffrench-Constant et al., 1991), which are dominant selections not requiring pre-existing mutations (see ffrench-Constant and Benedict, 2000)
P Transformation of Non-Drosophilids
Given the straightforward procedures for transforming Drosophila with P elements, there were high expectations that the system would function in other insects. The ability to test this was facilitated by the development of the neomycin (G418)-resistance marker system (Steller and Pirrotta, 1985), and neomycin resistance-containing P vectors were widely tested in tephritid flies and mosquitoes (see Walker, 1990; Handler and O’Brochta, 1991). Unfortunately, the neo-resistance system was generally unreliable, and recovery of resistant individuals that were not transgenic was common. In three mosquito species, however, neomycin-resistant transgenic insects were recovered, but they arose from rare transposition-independent recombination events (Miller et al., 1987; McGrane et al., 1988; Morris et al, 1989). Other dominant chemical resistance markers, including opd and Rdl, which had some success in Drosophila, were also tested, but no transformation events could be verified in other insects. A major limitation of these experiments was that, given the numerous variables involved, it was impossible to determine which components in the system were failing. This limitation led to efforts to systematically determine whether the transposon vector system was indeed functional in host embryos, which resulted in the development of rapid transposon mobility assays as described below. The first of these assays tested P excision in dro-sophilid and non-drosophilid embryos, revealing that P function decreased in drosophilids as a function of relat-edness to D. melanogaster, with no function evident in non-drosophilids (O’Brochta and Handler, 1988; Handler et al., 1993b). These results were the first indication that for transposon-mediated germ-line transformation to succeed in non-drosophilids, new vector systems would have to be created from existing and newly discovered transposon systems.
Excision and Transposition Assays for Vector Mobility
Assessing the ability of an insect gene vector to function in a particular species can be challenging. The procedures required to create a transgenic insect using transposable element-based gene vectors require a great deal of technical skill, and the ability to perform basic genetic manipulations. Depending on the insect, its generation time, and its amenability to being reared in the laboratory, the process of genetic transformation can be quite lengthy. At the early stages of developing non-drosophilid transformation technology, there was little experience in manipulating and injecting the embryos of the various non-drosophilid species of insects. In addition, the genetic markers available to select for or recognize transgenic insects were limited, and none could confidently be expected to function optimally in the species being tested at that time. Consequently, early efforts to test the functionality of potential gene vectors by attempting to create transgenic insects required simultaneous success in dealing with a number of daunting challenges. The failure of these efforts to yield a transgenic insect could not, unfortunately, be ascribed to the failure of any one particular step in the process (see Handler and O’Brochta, 1991). These efforts, therefore, did not represent an isolated test of the gene vector, since failure to obtain a transgenic insect may have been due to a failure in DNA delivery, expression of the genetic marker, or the failure of the transposable element vector system. Technology development under these conditions was very difficult. What was needed was an experimental system that permitted the activity of the transposable element system to be assessed in the species of interest, independent of any prospective genetic marker system and DNA delivery system. Such a system was developed for investigating the mobility properties of the D. melanogas-ter P element, and was very adaptable to other transpos-able element and insect systems (see Figure 3).
The system developed for P elements involved trans-fecting Drosophila cells with a mixture of two plasmids; one containing a P element inserted into the coding region of the lacZa peptide of a common cloning vector, and a second containing the P element transposase gene under the regulatory control of a strong promoter (Rio et al., 1986). Transient expression of the transposase gene resulted in the production of transposase, catalyzing the excision of P elements from the "excision indicator plasmids." Subsequent recovery of the injected plasmids from the cells, followed by their introduction into an appropriate strain of E. coli, permitted plasmids that had lost the P element through excision to be recognized by virtue of their restored lacZa peptide coding capacity. This transient P element excision assay was readily adaptable to use in Drosophila embryos through the process of direct microinjection of preblastoderm embryos, and it played a critical role in assessing the functionality of the P element system in a variety of drosophilid and non-drosophilid insect systems (O’Brochta and Handler, 1988).
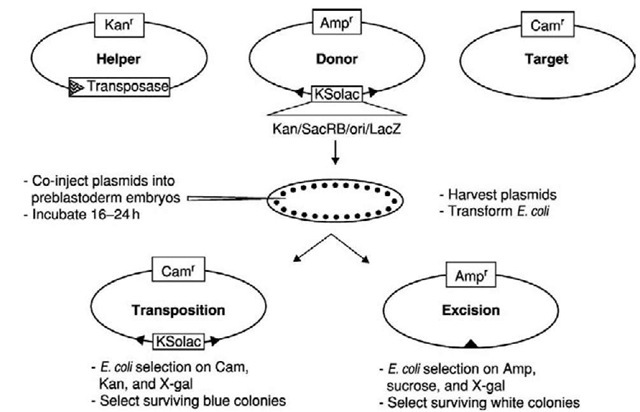
Figure 3 Plasmid-based transposable element mobility assays. A mixture of three plasmids is co-injected into preblastoderm embryos to ensure incorporation into nuclei. After approximately 24 hours, the plasmids are extracted from the embryos and introduced into E. coli. Transient expression of the transposase gene on the helper plasmid in the developing embryos results in the production of functional transposase. If the transposase catalyzes excision and transposition of the element, excision will result in the loss of element-specific markers on the donor plasmid. In the example shown, sucrose sensitivity, p-galactosidase activity, and kanamycin resistance are lost, and others could be used. Transposition results in the target plasmid acquiring all of the element-specific markers. In this example, the target plasmid is from a Gram-positive bacteria and is incapable of replicating in E. coli unless it acquires the origin of replication present on the element. Assays can be completed in 3 days, and rates of movement of 0.001% or greater are routinely detectable.
As originally configured, the excision assay only permitted the identification and recovery of excision events that resulted in the restoration of the open reading frame of the lacZa peptide reporter gene. Various modifications in this basic assay were adopted that permitted precise and imprecise excisions to be identified and recovered (O’Brochta et al., 1991). For example, marker genes, such as the E. coli lacZa peptide coding region, E. coli supF, sucrase (SacRB) from Bacillus subtilis, and streptomycin sensitivity, were incorporated into the transposable element (O’Brochta et al., 1991; Coates et al., 1997; Sun-dararajan et al., 1999). Plasmids recovered that lacked marker gene expression were usually excision events. Further refinements of the excision assay involved the use of transposable element-specific restriction endonuclease sites as a means for selecting for excision events. Digesting plasmids recovered from embryos with restriction endonucleases with sites only in the transposable element was a very powerful method of physically removing plas-mids that had not undergone excision from the pool of plasmids recovered from embryos and used to transform E. coli. Each restriction site was essentially a single dominant genetic marker, and therefore transposable elements with multiple restriction sites provided a very powerful system for selecting against plasmids that had not undergone excision (D. O’Brochta, unpublished).
Continued development of element mobility assays led to assays in which inter-plasmid transposition could be measured. These assays involved the co-injection of a transposase-encoding helper plasmid, an element "donor" plasmid, and a "target" plasmid. Typically, the target plas-mid contains a gene whose inactivation results in a selectable phenotype. For example, the SacRB gene has been used because its inactivation eliminates sucrose sensitivity. If the donor element also contains unique genetic markers, then transposition events would lead to a recom-binant plasmid with a new combination of a variety of markers (Saville et al., 1999). Perhaps the most powerful transposition assay developed for assessing transposable elements in insect embryos involved the use of a genetic marker cassette containing a plasmid origin of replication, an antibiotic resistance marker, and the lacZa peptide coding region, in combination with a target consisting of a Gram-positive plasmid (pGDV1) (Sarkar et al., 1997a). pGDV1 contains an origin of replication that cannot function in E. coli, although it does have a chlorampheni-col resistance gene that is functional in this species. Transposition of the marked transposable element into pGDV1 converts it into a functional replicon in E. coli. Because of the absolute cis-dependence of origins of replication, and the complete inability of pGDV1 to replicate in E. coli, transposition events can be readily detected even at low frequencies.
Transient mobility assays are now a standard for defining vector competence in insect embryos, and in particular when assessing a vector in a species for the first time. For this application, transposition assays provide the most information relevant to the potential for successful germ-line transformation, and can be used as a system to test helper construct function. As noted below, however, there may be differing constraints on plasmid and chromosomal transpositions for particular transposons. The use of these assays for analyzing transposon function is discussed in more detail in the relevant sections below.
Embryonic assays also provide an essential test system for assessing potential transgene instability by mobilizing or cross-mobilizing systems within a host genome, which is critical information for the risk analysis of transgenic insects being considered for release. The importance of excision assays for this purpose became evident by the hobo excision assays in M. domestica that revealed the existence of the Hermes element (Atkinson et al., 1993), and the subsequent assays that defined the interaction between the two transposons (Sundararajan et al., 1999). Since cross-mobilizing systems do not always promote precise excisions, assays that reveal imprecise as well as precise excisions are most sensitive for this purpose. Since successful transposition may depend on precise excision, transposition assays may only reveal the existence of mobilizing systems that have a high level of functional relatedness.