Regulation of PCD in the Nervous System during Metamorphosis
The ecdysteroids couple neuronal PCD with other meta-morphic changes. It is important to note that the ecdys-teroid cue for triggering metamorphic neuronal death can be either a rising or a falling titer, depending upon developmental stage. For example, the decline in circulating levels of 20E that occurs at the end of adult development prior to adult eclosion is the cue for PCD of abdominal motoneurons at this time, and treatment with exogenous 20E blocks this PCD (Truman and Schwartz, 1984). By contrast, it is the pre-pupal rise in circulating ecdyster-oids that is responsible for the larval—pupal transition death of the proleg motoneurons in abdominal ganglia A5 and A6 (Weeks and Ernst-Utzschneider, 1989; Weeks et al., 1992). The response of the proleg motoneurons to the steroid signal, however, is segment-specific. Homologous neurons in abdominal ganglia A3 and A4 persist through the pupal stage and adult development, but then undergo PCD within 24 hours of adult eclosion (Zee and Weeks, 2001). These responses to 20E, as well as the segment specificity of response at different stages in development, are retained when individual proleg motoneurons are cultured in vitro, providing evidence for the cell-autonomous, target-independent nature of these PCDs (Streichert et al., 1997; Hoffman and Weeks, 1998).
While ecdysteroids regulate gene expression in the Manduca nervous system (see, for example, Garrison and Witten, 2010) the target genes that mediate neuronal PCD have not been identified. In Drosophila, the expression of the ecdysteroid receptor A (EcR-A) has been directly correlated with the occurrence of post-eclosion neuronal death in the CNS and transcriptional activation of the reaper and hid death genes (Robinow et al., 1993; Jiang et al., 2000). It is not known if a similar relationship prevails in Manduca neurons, although autoradiographic evidence has demonstrated that Manduca motoneurons fated to die at the start of adult life display nuclear concentration of radiolabeled ecdysteroids (Fahrbach and Truman, 1989).
Evidence that other signals fine-tune timing of the death of neurons during metamorphosis comes from several sources. Adult Manduca sexta emerge from their pupal cuticle in an underground pupation chamber, and then must dig to the surface before inflating their wings. Adult moths forced to continue digging for hours beyond the time this behavior would normally cease exhibited delayed death of abdominal motoneurons (Truman, 1983). In addition, transection of the ventral nerve cord prior to adult eclosion blocks the death of specific moto-neurons in ganglia posterior to the point of transection, even in moths in which the levels of 20E undergo a normal decline (Fahrbach and Truman, 1987).
A well-studied example of a spared abdominal moto-neuron is MN-12. Subsequent to ventral nerve cord transection, this supernumerary member of the adult abdominal ganglion maintains its normal central arborizations and electrophysiological properties, implying that the cell death program has been completely blocked in the absence of a descending signal (Fahrbach et al., 1995; DeLorme and Mesce, 1999). Treatment of cultured abdominal ganglia with extracts prepared from ventral nerve cord restores the normal pattern of cell death to MN-12, but the active factor in the extracts remains to be identified (Choi and Fahrbach, 1995). Other examples of motoneuron death, such as the death of the accessory planta retractors (APRs) at the larval-pupal transition, however, are unaffected by cutting of the connectives prior to the normal time of death (Weeks and Davidson, 1994). This suggests that the phenomenon of intergangli-onic cell death signaling affects only a subset of neurons.
Because of the scattered and episodic nature of neuro-nal death during metamorphosis, and the unavailability of transgenic Manduca for analysis, little is known about the molecular mechanisms of neuronal PCD in this species.
Hormone-dependent neuronal PCD is blocked by treatment with inhibitors of transcription or translation (Weeks et al., 1993; Fahrbach et al, 1994; Ewer et al., 1998; Hoffman and Weeks, 1998). In support of the hypothesis that PCD of Manduca neurons requires de novo protein synthesis, a two-dimensional gel electrophoresis analysis of the Manduca abdominal ganglia revealed changes in protein expression patterns associated with newly-eclosed adults, a period of massive PCD. These changes included expression of novel proteins (Montemayor et al., 1990). In cultured proleg motoneurons, inhibition of caspase activity blocked PCD (Hoffman and Weeks, 2001), but ultrastructural studies of the APR motoneurons and the motoneurons that innervate the ISMs indicate that neu-ronal death in Manduca during metamorphosis may be autophagic rather than apoptotic, or combine features of both PCD programs (Stocker et al., 1978; Kinch et al., 2003).
Immunolabeling studies, in which the distribution of several death-associated gene products (initially identified from a screen of dying moth muscle; see section 12.5.4.3 and Table 1) was examined in the segmental ganglia, failed to reveal a reliable correlation of enhanced ubiq-uitination- or multicatalytic proteinase-immunoreactivity within dying neurons (Fahrbach and Schwartz, 1994; Hashimoto et al., 1996), despite association of these gene products with PCD in insect skeletal muscles (Haas et al., 1995; Jones et al., 1995). This neuron-muscle discrepancy may reflect that the basal levels of these components are sufficient to mediate neuronal PCD, but are inadequate for the destruction of the giant muscle fibers. By contrast, apolipophorin III is upregulated both by dying neurons and by degenerating muscles in Manduca, a finding that suggests that this molecule has functions in PCD in addition to its role in lipid transport (Sun et al., 1995).
PCD of Muscles during Metamorphosis
The ISMs of Manduca (Figure 1) are the major abdominal muscles of the larva, pupa, and pharate adult. The ISMs are divided into separate pairs of bilaterally symmetric bundles, each of which attaches to the cuticle at the inter-segmental boundaries. These muscles form in the embryo, and span eight of the abdominal segments in the larva. The ISMs provide the propulsive force for hatching and subsequent larval locomotion. Following pupation, the muscles in the first two and last two abdominal segments die and rapidly disappear. The muscles in the middle four segments persist throughout metamorphosis, and are used for the defensive and respiratory movements of the pupa. Following adult eclosion, the remaining ISMs undergo PCD during the subsequent 30 hours. While the basis for this segmental fate determination has not been examined, presumably it is established early in embryogenesis as a result of the actions of segmentation and homeotic genes (Bejsovsc and Wieschaus, 1993; DiNardo et al, 1994; French, 2001; Sanson, 2001).
Table 1 Genes Differentially Expressed in Condemned Manduca ISMs
|
Process |
Gene |
Response |
Reference |
|
Proteolysis |
Polyubiquitin |
Induced |
Schwartz et al. (1990b) |
|
14-kDa E2 ubiquitin conjugase |
Induced |
Haas et al. (1995) |
|
|
18-56 (Sug1) 26S proteasome ATPase |
Induced |
Sun et al. (1996) |
|
|
28.1-kDa subunit catalytic subunit of 20S proteasome |
Repressed |
Low et al. (2000) |
|
|
S6 (TBP7, MS73) 26S proteasome ATPase |
Induced |
Jones et al. (1995) |
|
|
S6′ (TBP1) 26S proteasome ATPase |
Induced |
Low et al. (2000) |
|
|
S7 (MSS1) 26S proteasome ATPase |
Induced |
Low et al. (2000) |
|
|
S10b (SUG2) 26S proteasome ATPase |
Induced |
Low et al. (2000) |
|
|
Transcription |
E75B |
Repressed |
Low et al. (2005) |
|
Translation |
Acheron (putative RNA binding protein) |
Induced |
Valavanis et al. (2007) |
|
elFIA Translation-Initiation Factor |
Induced |
Low et al. (2005) |
|
|
Oskar (maternal effect protein) |
Repressed |
Zhang et al. (2007) |
|
|
Signal transduction |
Small Cytoplasmic Repeat Protein SCLP) |
Induced |
Kuelzer et al. (1999) |
|
G coupled receptor GPR85 |
Induced |
Zhang et al. (2007) |
|
|
Calmodulin-dependent calcineurin A1 subunit |
Induced |
Zhang et al. (2007) |
|
|
Death Associated LIM-Only Protein (DALP) (insect |
Induced |
Hu et al. (1999) |
|
|
ortholog of Hic-5) |
|||
|
Metabolism |
Apolipoprotein III |
Induced |
Sun et al. (1995) |
|
Low MW lipoprotein PBMHPC-23 |
Induced |
Zhang et al. (2007) |
|
|
Hydroxy acid oxidase 1 |
Induced |
Zhang et al. (2007) |
|
|
Contractile protein |
Actin |
Repressed |
Schwartz et al. (1993b) |
|
Myosin heavy chain |
Repressed |
Schwartz et al. (1993b) |
|
|
Myosin light chain |
Repressed |
Zhang et al. (2007) |
|
|
Myosin Regulatory Light Chain isoforms 1 and 2 |
Repressed |
Zhang et al. (2007) |
|
|
Calponin 1 |
Repressed |
Zhang et al. (2007) |
|
|
Troponin 1 |
Repressed |
Zhang et al. (2007) |
The nuclear changes that accompany ISM death display none of the features of apoptosis (Schwartz et al., 1993a) (Figure 1). The chromatin does not become electron-dense, but remains dispersed throughout the nucleoplasm. In addition, agarose gel electrophoresis of ISM genomic DNA fails to reveal apoptotic ladders. Ultrastructurally, there is an increase in autophagic vesicles, and the death of these cells is accompanied by autophagy (Lockshin and Beaulaton, 1974, 1979).
Following PCD of muscles in many animals, the cell corpse is phagocytosed by neighboring cells or circulating macrophage-like cells (Hart et al., 2008). A classic example of this phenomenon is found in amphibian metamorphosis, where the massive tail musculature is lost during the transition from larva to adult (Weber, 1964; Watanabe and Sassaki, 1974). During this process, muscle fibers become decorated with macrophages that contain identifiable remnants of skeletal muscle debris (Metchnikoff, 1892; Nishikawa et al., 1998). While dying muscles in insects are sometimes phagocytosed by circulating hemocytes (Crossley, 1968), this is not universally so (Jones et al., 1978). In particular, the death of the ISMs following adult eclosion in moths does not attract macrophage-like cells, or rely on phagocytosis for resolution (Beaulaton and Lockshin, 1977) (Figure 1). In fact, estimates of ISM volumes and hemocyte numbers in adult Manduca suggest that removal of dying cells in these animals would require at least an order of magnitude greater number of phagocytic cells than has been shown to reside in the hemolymph (Jones and Schwartz, 2001).
While the ISMs of adult moths are not phagocytosed, Rheuben (1992) observed an intimate association between phagocytic hemocytes and the sarcolemma during the death of mesothoracic muscles in pupae. The phagocytes were well spaced along the fibers, and appeared to degrade the basal lamina. One difference between the ISMs and the mesothoracic fibers is that the latter are not completely degraded during development. Instead, they act as scaffolds for myoblasts that remodel the fibers during formation of adult muscle fibers. Phagocytes may play a more significant role in tissue remodeling rather than cell death.
Endocrine control of ISM death Timing of ISM death must be coordinated with other metamorphic events, or the animal might suffer disastrous consequences. For example, premature loss of the ISMs in moths would leave the animal trapped within the pupal cuticle and locked in either a cocoon or an underground chamber. Delays in ISM death might have other deleterious consequences, including depriving the adult of nutrients required for gametogenesis. As described in section 12.4.4, the titer of ecdysteroids serves as an endogenous developmental time reference that can be used by the different organs of the pupa to coordinate developmental decisions, including the timing of ISM death.
Early reports suggested that the cessation of motoneuron activity was the proximal trigger for ISM death (Lockshin and Williams, 1965b). Subsequent studies demonstrated that the timing of ISM death in Antheraea polyphemus was not altered by silencing motoneuron activity with the sodium channel blocker tetrodotoxin or by removal of the entire ventral nerve cord (Schwartz and Truman, 1983, 1984a). Instead, in this species, the trigger for cell death is the peptide eclosion hormone (EH) (Schwartz and Truman, 1984a, 1984b). EH acts via cGMP; the description of its role in ISM death represented the first study demonstrating that cGMP met all of Earl Sutherland’s requirements for identifying a second messenger for action of a hormone (Sutherland, 1972; Schwartz and Truman, 1984b). The capacity of EH to act on the ISMs is itself under the control of circulating ecdysteroids, as a decline in 20E regulates both the timing of EH release and the capacity of the ISMs to respond to this trigger (Truman, 1984; Schwartz and Truman, 1984a). The possible role of other insect peptides, such as ecdysis-triggering hormone (ETH), in PCD has not been explored.
Physiology of ISM death The size of the ISMs, and the coordinated nature of the developmental changes that take place in this tissue during metamorphosis, facilitate examination of the physiological changes that accompany naturally occurring muscle atrophy and PCD. Under laboratory conditions, metamorphosis in Manduca is complete in 18 days, with adult eclosion taking place late on day 18. On day 15 of adult development the mass of the ISMs begins to decline, and during the next 3 days ISMs lose 40% of their mass. This pre-eclosion program of atrophy is non-pathological, and the muscles retain almost all of their normal physiological responses, including force/cross-sectional area and sensitivity to calcium ions in skinned fiber preparations (Schwartz and Ruff, 2002). These observations suggest that the reduction in muscle mass observed during the atrophy phase reflects a generalized enhancement of protein turnover rather than selective destructive of contractile proteins, despite the fact that entire contractile bundles are lost during this phase (Lockshin and Beaulaton, 1979).
The ISMs of Manduca begin PCD coincident with adult eclosion. At this time, the ISMs begin to lose mass at a rate of approximately 4% per hour (Schwartz and Ruff, 2002). By 24 h post-eclosion, reliable resting potentials can no longer be recorded (Lockshin, 1973). While there are few changes in the organization of the contractile apparatus during the atrophy phase, the post-emergence period is marked by profound sarcomere disruption (Lockshin and Beaulaton, 1979). Whole filaments disappear rapidly, with a preferential loss of thick filaments relative to thin filaments (Beaulaton and Lockshin, 1977). During this same period mitochondria are lost, autophagic vacuoles form, and the T tubule system swells. As a consequence, the muscle fibers rapidly weaken, even when force is normalized to cross-sectional area. This is true for twitches, and for tetanus and caffeine-induced contractions (Schwartz and Ruff, 2002). There are also defects in the ability of the contractile apparatus to respond to free calcium in both intact muscles and skinned fiber preparations.
Patterns of gene expression during PCD of ISMs The primary biochemical mechanism that mediates the atrophy phase appears to be an increase in the ubiquitin-proteasome pathway, which allows protein catabolism to outstrip synthesis (Haas et al., 1995). There is a transient increase in polyubiquitin expression in the ISMs on days 15 and 16 of pupal-adult development during the early phases of atrophy that is controlled by the falling ecdysteroid titer (Schwartz et al., 1990b).
Lockshin (1969) demonstrated that ISM PCD in silk-moths is blocked by inhibitors of RNA or protein synthesis, suggesting a requirement for de novo gene expression. These results are similar to those reported for PCD in other tissues, including the insect nervous system, as well as in other metamorphosing taxa such as amphibians (Weber, 1965).
To identify genes that may mediate ISM death, Schwartz and colleagues utilized a differential screening approach using cDNA libraries constructed from Day 18 Manduca ISM mRNA (Schwartz et al., 1990). Even though the ISMs are dying, the abundance of most transcripts was unchanged during the last days of pupation. However, a few transcripts were found that dramatically induced or repressed with the commitment of the cells to die. The cloning of these differentially expressed genes resulted in the first identification of death-associated gene expression from any organism (Schwartz et al., 1990b). Among the genes that are repressed when the ISMs become committed to die are actin and myosin heavy chain (Schwartz et al., 1993b) (Table 1; Figure 3). These transcripts are among the most abundant in the muscle during larval and pupal life, but begin to disappear late on day 17 (the day before adult eclosion), and are almost undetectable by late day 18 when the animals eclose.
One mechanism for reducing transcript abundance is transcriptional repression. A complementary mechanism is enhancement of transcript degradation. In this regard, it was found that there is a transient increase in endogenous ISM RNase activity on day 17, which facilitates removal of transcripts prior to induction of new gene expression (Cascone and Schwartz, 2001). Coordinated control of transcription and degradation may allow the muscles to rapidly shift developmental programs from homeostasis to death. While the molecular mechanism that mediates this global change in transcript abundance has not been determined, microRNAs are potential regulators. MicroRNAs bind to sequences within the 3′ untranslated region (UTR) of target mRNAs and regulate transcript stability and translatability (Fabian et al., 2010). In the case of Manduca, the stability of ISM transcripts could be transferred to ectopic reporter mRNAs by swapping the 3′ UTRs (Cascone and Schwartz, 2001). In other models, such as Drosophila, microRNAs can exert a profound effect on developmental processes (Jones and Newbury, 2010). Specific microRNAs, such as Let-7C, control the timing of intersegmental muscle cell death following adult eclosion (Sokol et al., 2008).
A small number of induced cell death-associated genes were identified from the ISM screen (Schwartz et al., 1990b). Some encoded known proteins, including poly-ubiquitin (Schwartz et al., 1990b) and several proteasome subunits (Sun et al., 1996), while others encoded novel proteins (Hu et al., 1999; Kuelzer et al., 1999; Valavanis et al. , 2007). More recently, additional PCD-associated genes expressed in ISMs have been identified either via proteomics (Low et al., 2000, 2005; Zhang et al., 2007) or by direct examination of proposed candidates in both Manduca and Bombyx (Table 1). The following section will focus on the genes identified from the molecular screen.
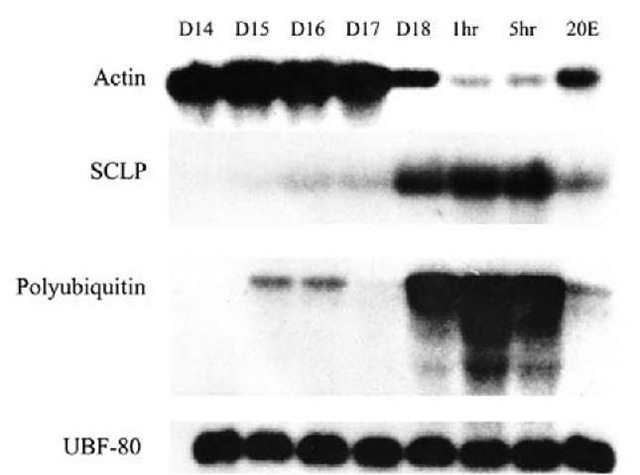
Figure 3 The ISMs begin to atrophy on day 15 of pupal-adult development and then initiate programmed cell death coincident with adult eclosion late on day 18. By 5 hours post-eclosion, the muscles have lost significant mass and physiological function. Most genes are constitutively expressed, like the ubiquitin fusion 80 (UBF-80) gene, which plays a role in ribosome biogenesis. While most genes are constitutively expressed within the ISMs, independent of developmental stage, a small number are induced or repressed with the commitment to die. Actin mRNA goes from being one of the most abundant transcripts within the ISMs prior to eclosion to all but disappearing in the dying cells. Conversely, Small Cytoplasmic Leucine Rich Repeat Protein (SCLP) is almost undetectable in the muscles prior to day 18 and is then dramatically induced with the commitment to die. Polyubiquitin is transiently induced on day 15, coincident with the onset of atrophy, and is then expressed at very high levels on day 18. All of the death-associated changes in transcript abundance can be prevented with injection of 25 ng of 20-hydroxyecdysone (20E) on day 17. D, day of pupal-adult development; h, hours post-eclosion; 20E, 20-hydroxyecdysone.
Ubiquitin is a 76 amino-acid peptide that is the most highly conserved protein present in all eukaryotes. At the protein level, insect and human ubiquitins are identical (Rechsteiner, 1988). The post-translational covalent attachment of ubiquitin to selected lysine residues on substrate proteins serves as a molecular tag to target proteins to specific fates within the cell (Salomons et al., 2010). The addition of single ubiquitin moieties directs proteins to specific subcellular locations, while addition of multiple head-to-tail ubiquitin chains promotes binding to the 26S proteasome. This multisubunit protease then releases the ubiquitin, unfolds the substrate, and rapidly degrades it to small peptides.
As mentioned above, there is a transient increase in polyubiquitin expression that correlates with ISM atrophy. Polyubiquitin mRNA then accumulates to prodigious levels on day 18, along with the coordinated expression of both 20S and 26S proteasome subunits (Schwartz et al., 1990b; Dawson et al., 1995; Haas et al., 1995; Jones et al., 1995; Takayanagi et al., 1996; Low et al., 1997). This enhancement in ubiquitin-dependent proteolysis is presumably adaptive, because the ISMs are not phagocy-tosed, and therefore require a cell-autonomous mechanism for the liberation of cellular constituents (Jones and Schwartz, 2001). The ubiquitin-proteasome pathway is presumably serving roles in both cell death signal trans-duction and large-scale protein turnover (Broemer and Meier, 2009). In Manduca, injection of day-18 pharate adults with proteasome inhibitors (hemin and N-acetyl-leu-leu-norleucinal) delayed ISM death (Bayline et al., 2005).
Other known genes are induced in the dying ISMs, but their role in PCD is unknown. For example, the abundance of apolipoprotein III (apoLp-III) is dramatically induced, at both the RNA and protein levels, in both the ISMs and a subpopulation of neurons undergoing PCD (Sun et al., 1995). ApoLp-III is synthesized predominantly in the fat body, and normally facilitates lipid transport in the hemolymph by associating with lipophorin. Given that the ISMs do not express lipophorin, the role of ApoLp-III in PCD is currently mysterious.
The majority of cDNAs isolated in the ISM screen encoded novel proteins of unknown function. An example is SCLP (small cytoplasmic leucine-rich repeat protein), which is induced at both the RNA and protein levels in condemned ISMs expressed on day 18 (Kuelzer et al., 1999). This small protein is composed of multiple leucine-rich repeat protein-protein interaction motifs, and likely serves as a signal transduction protein. Ectopic expression of SCLP in different tissues in Drosophila did not result in an overt phenotype (Kuelzer et al., 1999).
Two of the novel genes identified in the ISM screen do have vertebrate homologs. DALP (death-associated LIM-only protein) contains one perfect and two imperfect LIM domains (Hu et al., 1999), structural motifs that consist of paired zinc fingers that mediate protein-protein interaction (Retaux and Bachy, 2002). DALP is induced on day 17, well in advance of the other death-associated cDNAs from Manduca ISMs. Expression of the DALP protein is likely restricted to the ISMs, as it was not detected in flight muscle, fat body, Malpighian tubules, the ovary, oocytes, or the male sexual accessory gland. As with SCLP, the function of DALP was explored using transgenic flies (Hu et al., 1999). Ectopic expression of Manduca DALP in the abdominal ISMs of fly pupae resulted in the disorganization of the contractile apparatus and subsequent muscle atrophy. Targeted mutations in the LIM domain blocked muscle atrophy, suggesting that the observed effects of ectopic DALP expression were dependent on expression of the intact functional protein.
Further insights into the function of DALP were gained by examining the effects of expressing Manduca DALP in the mouse myoblast C2C12 line (Hu et al., 1999). This muscle satellite cell line has been extensively used as a model for examining muscle differentiation and PCD in mammals (Schwartz et al., 2009). C2C12 cells can be maintained as a stable, non-transformed line that, when incubated in a low serum medium, ceases cycling and differentiates into multinucleated myotubes (Yaffe and Saxel, 1977). Expression of DALP blocked the differentiation of C2C12 cells into myotubes by blocking induction of MyoD, a basic helix-loop-helix muscle transcription factor required for differentiation. Effects of DALP were overcome by co-transfecting cells with an expression vector driving production of MyoD. In addition to blocking differentiation, DALP enhanced the probability of cell death. Identical results were obtained with C2C12 cells transformed to express Hic-5 (hydrogen peroxide-inducible clone-5), the mammalian ortholog of Manduca DALP. These data show that DALP and Hic-5 are likely conserved proteins that function as negative regulators of muscle differentiation and survival in insect and mammalian cells.
Another of the novel cell death associated genes from Manduca may play a role in human pathogenesis. Acheron (Achn) contains three Lupus antigen (La) repeats, nuclear localization and export (NLS and NES) signals, and an RNA recognition motif (Valavanis et al., 2007). In fact, Achn defines a new subfamily of Lupus antigen (La) proteins that appears to have branched from authentic La protein relatively late in metazoan evolution. In mammalian cells, Achn (also known as La related protein 6, or LARP6), binds to the 5′ untranslated region of collagen mRNA and facilitates translation (Cai et al., 2010). While its role in ISM death has not been explored, Achn plays an essential role in myogenesis in zebrafish (Wang et al., 2009).
In C2C12 myoblasts, Achn acts upstream of MyoD and is required for these cells to either differentiate or undergo apoptosis following loss of growth factors (Wang et al., 2009). Other studies have explored the role of Achn in regulating integrin-extracellular matrix interactions required for myogenesis. Both control C2C12 myoblasts and those engineered to express ectopic Achn expressed the fibronectin receptor integrin a5P1 in the presence of growth factors and the laminin receptor a7P1 following growth factor withdrawal. Expression of the laminin receptor was blocked in cells expressing either Achn anti-sense or dominant-negative Achn. Control cells and those expressing ectopic Achn undergo sequential and transient increases in both substrate adhesion and migration before cell fusion. Blockade of Achn expression reduced these effects on laminin but not on fibronectin. Taken together, these data suggest that Achn may influence differentiation in part via its control of cell adhesion dynamics (Glenn et al., 2010).
A recent study has demonstrated that Achn is expressed in the myoepithelial cells of the mammary gland (Shao et al., 2011). Microarray and immunohistochemical analysis of tissues from patients with breast cancer have demonstrated that Achn expression is significantly elevated in some basal-like tumors, the most aggressive of the breast cancers. Ectopic expression of Achn in MDA-MB-231 breast cancer cells induced a number of pheno-typic changes that are associated with malignancy and metastasis, including enhanced cell proliferation, lamel-lipodia formation, greater invasive activity, and elevated expression of the metastasis-associated proteins MMP-9 and VEGF. In xenograph studies using athymic mice, MDA-MB-231 cells expressing ectopic Achn displayed enhanced angiogenesis and an approximately five-fold increase in tumor size relative to control cells. These data support the hypothesis that Achn enhances human breast tumor growth and vascularization, and may represent a target for diagnostics and therapeutics.