Cuticular Enzymes and Sclerotization
The study of cuticular enzymes has to a large extent been concerned with characterization of enzymes assumed to play a role in cuticular sclerotization, mainly those involved in oxidation of catechols, and there has been a tendency to neglect the possibility that enzymes not directly involved in sclerotization may play a role in cuticular metabolism. Enzymes such as glucose oxidase, catalase, and superoxide dismutase have been reported from locust cuticle (Candy, 1979), but have not been studied in much detail. Catechol oxidation is an important step in sclerotization, as well as in wound healing and immune responses in insects, and it is often difficult to decide whether a given cuticular phenoloxidase activity is involved in sclerotization or whether its main role is to take part in defense reactions. It is therefore necessary to be careful when interpreting the observations reported for cuticular enzymes.
orfAo-Diphenoloxidases
After being transported from the epidermal cells into the cuticle the catecholic sclerotization precursors may encounter different oxidative enzymes, such as o-diphenoloxidases, laccases, and peroxidases, capable of oxidizing them to quinones, but the relative roles of the enzymes are still uncertain. Insects contain inactive pro-enzymes for o-diphenoloxidases both in hemolymph and in cuticle, which can be activated by a cascade of processes involving limited proteolysis and initiated by wounding or by the presence of small amounts of microbial cell-wall components (Ashida and Dohke, 1980; Ashida and Brey, 1995). The o-diphenoloxidases are able to oxidize a wide range of o-diphenols, but not p-diphenols; they can hydroxylate monophenols, such as tyrosine and tyramine, to o-diphenols, and they are readily inhibited by thioureas and sodium diethyldithiocarbamate. They have been isolated and characterized from soft, non-sclerotizing cuticles, such as larval cuticle of Bombyx mori (Ashida and Brey, 1995; Asano and Ashida, 2001a), sclerotizing pupal cuticle of Manduca sexta (Aso et al., 1984; Morgan et al., 1990), and blowfly and fleshfly larval cuticles (Barrett, 1987a, 1987b, 1991). The amino acid sequences of o-diphenoloxidases from various insect species have been deduced from the corresponding DNA sequences (Fujimoto et al, 1995; Hall et al, 1995; Kawabata et al, 1995). The insect o-diphenoloxidases resemble diphe-noloxidases (tyrosinases) from other organisms, but differ with regard to substrate specificity and amino acid sequence (Sugumaran, 1998; Chase et al., 2000).
The established sequences of insect prophenoloxidase genes indicate that the gene products do not possess an N-terminal signal peptide sequence (Sugumaran, 1998), in contrast to what is observed for most proteins destined for export from cells. It appears that the prophenoloxi-dases are released by cell rupture before they are transformed into active enzymes (Kanost and Gorman, 2008). The silkmoth B. mori has genes for two o-diphenoloxidase proenzymes, and the products of both genes are present in both hemolymph and cuticle of B. mori larvae; the proenzymes appear to be synthesized in hemocytes, and can be transported into the cuticle via the epidermal cells. A difference between the cuticular and hemolymphal pro-enzymes is that some methionine residues, which in the hemolymphal proenzymes are unmodified, are oxidized to methionine sulfoxides in the cuticular proenzymes. When activated, the cuticular enzymes have nearly the same substrate specificity as the hemolymphal enzymes; a difference between the two groups of proenzymes is that the oxidized cuticular form cannot be transported back across the epidermal cell layer, indicating that the epidermal transport of the proenzymes is a one-way traffic, from hemolymph to cuticle (Asano and Ashida, 2001a, 2001b).
The enzymatic properties of the diphenoloxidases purified from hemolymph and from pharate pupal cuticle of M. sexta are very similar, suggesting a close relationship between the enzymes (Aso et al., 1985; Morgan et al., 1990), and it seems probable that they, like the B. mori enzymes, are derived from the same gene(s).
Both cuticular and hemolymphal o-diphenoloxidases become very sticky when activated; they tend to aggregate and to stick to any available surface and macromolecule, thereby hindering diffusion of the active enzymes from the site where they were activated. The o-diphenoloxidase activity in the larval cuticle of the blowfly Lucilia cup-rina was localized to epicuticular filaments (Binnington and Barrett, 1988). Some activity was also observed in the procuticle, but only when the cuticle had been damaged beforehand, and the activity was limited to the close neighborhood of the wound, indicating that wounding is needed to activate the enzyme, and that the active enzyme remains in the vicinity of the wound.
Using immunocytochemical methods, the prophenol-oxidase in larval cuticle of B. mori could be demonstrated both in the non-lamellate endocuticle, where it was randomly distributed, and in an orderly arrayed pattern in the lamellate endocuticle; it appeared to be absent from the cuticulin layer and the epidermal cells (Ashida and Brey, 1995).
The role of the cuticular o-diphenoloxidases in the scler-otization process is problematic; their presence as inactive proenzymes, which have to be activated, their close relationship to the hemolymphal phenoloxidases, and their abundance in non-sclerotizing cuticle suggest that their role is to take part in defense against wounding and microorganisms, and not to be involved in sclerotization.
Laccases
Laccase-type phenoloxidases have been reported to be present in various dipteran larval cuticles shortly before and during puparium formation, such as larval cuticles of Drosophila virilis (Yamazaki, 1969), D. melanogaster (Sugumaran et al., 1992), Calliphora vicina (Barrett and Andersen, 1981), Sarcophaga bullata (Barrett, 1987a), and L. cuprina (Barrett, 1987b), and laccases have also been described from pupal cuticles of B. mori (Yamazaki, 1972) and M. sexta (Thomas et al., 1989), as well as from adult cuticle of the locust S. gregaria (Andersen, 1978). Insect laccases are structurally related to laccases of plant and fungal origin, and, in contrast to the insect o-diphenoloxidases, the laccase gene products contain a typical signal peptide sequence, indicating that the enzymes are secreted into the extracellular space.
Laccase activity appears in larval cuticles of D. virilis (Yamazaki, 1969) and L. cuprina (Binnington and Barrett, 1988) shortly before pupariation, and in both species the enzyme activity decreases gradually as puparial sclero-tization progresses. Laccase activity was demonstrated in pharate cuticle of adult locusts, S. gregaria, a few days before ecdysis, and it remained at high levels for at least 2 weeks after ecdysis. Activity has also been demonstrated in nymphal exuviae, indicating that the locust laccase is not inactivated by sclerotization (S.O. Andersen, unpublished data).
Insect laccases are active towards a broad spectrum of o- and p-diphenols: NBAD and NADA are among the best o-diphenolic substrates tested, and methyl-hydro-quinone is the best p-diphenolic substrate. Insect laccases are not inhibited by such compounds as thiourea, phen-ylthiourea, and sodium diethyldithiocarbamate, which are effective inhibitors of o-diphenoloxidases, but they are inhibited by carbon monoxide and low concentrations of fluorides, cyanides, and azides (Yamazaki, 1972; Andersen, 1978; Barrett, 1987a; Barrett and Andersen,1981). The laccases are not affected by treatments which will inactivate many other enzymes; the S. gregaria laccase remains active after blocking available amino and phenolic groups by dinitrophenylation or dansylation, and it survives temperatures up to about 70°C, but it is inactivated by treatment with tetranitromethane, which nitrates tyro-sine residues (Andersen, 1979b). The laccases appear to be firmly linked to the cuticular structure; typically they cannot be extracted by conventional protein extractants, but are readily extracted after limited tryptic digestion of the yet-unhardened cuticle (Yamazaki, 1972; Andersen, 1978). The enzyme was obtained from C. vicina larval cuticle by prolonged extraction at pH 8 without addition of any protease, but as latent protease activity is present in the cuticle, the release of laccase from the cuticular residue may be due to proteolysis (Barrett and Andersen, 1981). The enzyme is not released by tryptic digestion of already sclerotized cuticle.
The ultrastructural localization of laccase activity has been studied in the L. cuprina larval cuticle (Binnington and Barrett, 1988), where enzyme activity was observed in the inner epicuticle of late third instar larvae (about to pupariate), but not in epicuticle of younger larvae. The laccase activity in L. cuprina larval cuticle could be demonstrated without prior activation, in contrast to the cuticular o-diphenoloxidases, indicating that the laccase is not deposited as an inactive proenzyme in this insect, and neither is an inactive proenzyme likely to be present in pharate locust cuticle since enzyme activity was demonstrated without any activating treatment. Both full-length and amino-terminally truncated recombinant forms of M. sexta cuticular laccase were expressed and purified, and both forms had activities towards NADA and NBAD similar to that of the enzyme purified from pharate pupal cuticle, indicating that the M. sexta enzyme is not produced as an inactive proenzyme (Dittmer et al., 2009). A pro-laccase has been purified and partially characterized from cuticle of newly pupated pupae of B. mori (Ashida and Yamazaki, 1990; Yatsu and Asano, 2009). The inactive pro-laccase could be activated by treatment with various proteolytic enzymes, and the substrate specificities of the laccase variants obtained depended upon the protease used for activation.
Definitive proof that a cuticular laccase is responsible for cuticular sclerotization was obtained by demonstrating that inhibition of the laccase 2 gene product by means of RNA interference could prevent cuticular sclerotization in the beetle T. castaneum (Arakane et al., 2005). RNA interference inhibition of the laccase 1 gene product as well as the two o-diphenoloxidases in T. castaneum had no influence on cuticular sclerotization. Involvement of the laccase 2 gene in sclerotization has also been demonstrated in the beetle Monochamus alternatus (Niu et al., 2008), in the mosquito Culexpipiens (Pan et al., 2009), in M. sexta (Dittmer et al., 2009) and in B. mori (Yatsu and Asano, 2009), confirming that the diphenoloxidase responsible for cuticular sclerotization is laccase 2.
Cuticular Peroxidases
Several routes for the oxidation of catechols to o-quinones can be advantageous for an insect, especially when the different routes are regulated independently, and sclero-tization by means of peroxidase activity could be such an alternative route. Peroxidase activity has been demonstrated by histochemical methods in proleg spines of Calpodes ethlius larvae (Locke, 1969), and in larval and pupal cuticle of Galleria mellonella and Protoformia ter-raenovae (Grossmuller and Messner, 1978; Messner and Janda, 1991; Messner and Kerstan, 1991), peroxidase activity can also be observed intracellularly in different cell types in insects. It is not known whether the cuticular per-oxidase activities are identical to the intracellular enzymes, as the cuticular activities have never been properly characterized. Proteins can be cross-linked by means of the per-oxidase system, and it has been suggested that the enzyme could be involved in cuticular sclerotization (Hasson and Sugumaran, 1987). A peroxidase is likely to be involved in the cross-linking of the rubber-like elastic cuticular protein resilin (Andersen, 1966; Coles, 1966). This cuticular protein is cross-linked by oxidative coupling of tyrosine residues during its extracellular deposition, and the tyrosine radicals needed for the coupling are likely to be formed by a peroxidase-catalyzed oxidation process. Peroxidases can also oxidize catechols to semiquinone radicals, two of which may readily dismutate to form an o-quinone and a catechol. The enzyme needs hydrogen peroxide as one of its substrates, and Candy (1979) reported that locust cuticle contains glucose oxidase activity, which oxidizes glucose to D-gluconate with concomitant production of hydrogen peroxide. It was suggested that the hydrogen peroxide produced may participate in sclerotization reactions. Candy (1979) also reported that other enzymes involved in hydrogen peroxide metabolism, such as per-oxidase, catalase, and superoxide dismutase, are present in locust cuticle.
Peroxidase activity in solid cuticle may be involved in the production of dityrosine cross-links, and in oxidizing catechols to quinones for sclerotization. Small amounts of dityrosine have been obtained from sclerotized locust cuticle (Andersen, 2004a), and dityrosine as well as bromi-nated dityrosines have been obtained from the hardened exocuticle of the crab Cancer pagurus (Welinder et al., 1976). When a protein from M. sexta pupal cuticle was treated with a fungal laccase, a product was formed that reacted with a monoclonal antibody against dityrosine (Suderman et al., 2010), suggesting that laccases can oxidize tyrosine to dityrosine without involvement of hydrogen peroxide. Laccase-catalyzed formation of dityrosine residues was also described by Mattinen et al. (2005). It is not known whether dityrosine formation plays any important roles in sclerotization of solid cuticles, but it appears to be important for stabilization of other structural materials in insects. The eggshells of D. melanogaster are stabilized by formation of dityrosine cross-links between the protein chains (Petri et al., 1976; Mindrinos et al., 1980), and the hardening of Aedes aegypti egg chorion includes both peroxidase-mediated protein cross-linking through dityrosine formation, and diphenoloxidase-catalyzed cho-rion melanization (Li et al., 1996). The hydrogen peroxide necessary for dityrosine formation in A. aegypti chorion is produced in an enzymatic process by which NADH is oxidized with concomitant reduction of molecular oxygen to hydrogen peroxide. The necessary supply of NADH for this process is provided by enzyme-catalyzed oxidation of malate coupled to reduction of NAD+ (Han et al., 2000a, 2000b). It is unknown whether a similar system for providing hydrogen peroxide is involved in sclerotization of some insect cuticles.
orfAo-Quinones and para-Quinone Methides
The three types of oxidases, o-diphenoloxidases, laccases, and peroxidases, can all oxidize NADA and NBAD to their respective o-quinones; when the oxidation process is catalyzed by laccases and peroxidases free radicals are produced, but this is not the case when oxidations are catalyzed by o-diphenoloxidases. The o-quinones are reactive compounds, which react spontaneously with nucleo-philic groups, and they can be enzymatically isomerized to p-quinone methides, which will also react with nucleo-philic compounds. It has been reported that the laccase in M. sexta pupal cuticle oxidizes o-diphenols to a mixture of o-quinones and p-quinone methides, indicating that a specific isomerase is not an absolute requirement for p-quinone methide formation (Thomas et al., 1989). An enzyme catalyzing o-quinone isomerization has been partially characterized from larval cuticle of H. cecropia (Andersen, 1989a) and S. bullata (Sugumaran, 1987; Saul and Sugumaran, 1988), both cuticles being of the soft, pliant type. The enzyme is also present in the hemolymph of S. bullata (Saul and Sugumaran, 1989a, 1990), where it participates in defense reactions. So far the enzyme has not been demonstrated in cuticles which are sclerotized in connection with ecdysis, and the available evidence is insufficient to decide whether the isomerase is involved in cuticular sclerotization or whether its function is restricted to defense purposes.
The various adducts obtained by incubating samples of insect cuticle with catechols and nucleophiles will be discussed below, but it seems reasonable to ask whether the two P-hydroxylated compounds, NANE and NBANE, present in various sclerotized cuticles and in hemolymph, are formed in the cuticle by reactions between water and the p-quinone methides of NADA and NBAD, respectively, or whether they are synthesized outside the cuticle — for instance in the epidermal cells — and transported to the cuticle. In the former case they will simply be by-products of the sclerotization process, and in the latter case they could be alternative sclerotization compounds.
Dehydro-NADA and Dehydro-NBAD
The p-quinone methide formed by isomerization of NADA-o-quinone can be further isomerized to a,P-dehydro-NADA (8), a NADA derivative carrying a double bond between the a- and P-carbon atoms of the side chain. The enzyme responsible for this isomerization has been called N-acetyldopamine quinone methide/1,2-dehydro-N-acetyldopamine tautomerase (Saul and Sugumaran, 1989b). The activity has been reported to be present in larval cuticle of S. bullata (Saul and Sugumaran, 1989b, 1989c) and D. melanogaster (Sugumaran et al., 1992). Small amounts of an enzyme activity catalyzing the isomerization of NBAD p-quinone methide to dehy-dro-NBAD has been demonstrated in extracts of C. vicina larval cuticle (Ricketts and Sugumaran, 1994). Several cuticles, which are sclerotized by mixtures of NADA and NBAD, will readily convert NADA to the dehydro-deriv-ative, while conversion of NBAD only occurs to a minor extent, if at all (Andersen, 1989b; Andersen et al., 1996).
The dehydro-NADA formed during cuticular sclero-tization can be oxidized by o-diphenoloxidases as well as laccases, and the resulting side-chain-unsaturated qui-nones may react spontaneously with other catechols to give substituted dihydroxyphenyl-dihydrobenzodioxines (13) (Andersen and Roepstorff, 1982; Sugumaran et al, 1988). The presence of various dihydroxyphenyl-dihydrobenzo-dioxine derivatives in naturally sclerotized cuticle indicates that oxidation products of dehydro-NADA tend to react with available catechols during sclerotization (Andersen and Roepstorff, 1981, 1982; Andersen, 1985), and that presence of dihydroxyphenyl-benzodioxine derivatives in sclerotized cuticles can be used as an indication for the presence of a dehydro-NADA forming activity.
The sclerotization of some types of cuticle is dominated by reactions involving the dehydro-NADA quinones, while sclerotization of other types of cuticle is dominated by reactions of NADA and NBAD o- and p-quinones with matrix proteins. The ability to form benzodioxine derivatives from NADA is much more pronounced in such cuticular types as locust femur, H. cecropia larval head capsule, and D. melanogaster puparia, than in locust mandibles, D. melanogaster larval cuticle, and T. molitor pupal cuticle (Andersen, 1989c, 1989d). The observed differences may be due to different amounts of the enzymes involved in the process, but it has been suggested that the cuticular diphenoloxidases, isomerases, and tautomerases occur together in large enzyme complexes, enabling one enzyme to deliver its products directly to the next enzyme for further processing (Andersen et al., 1996; Sugumaran, 1998). The evidence for such complexes is insufficient, but should not be disregarded.
Catechol Adducts Formed in vitro
In the original sclerotization scheme, suggested by Pryor (1940a, 1940b), o-quinones react preferably with E-amino groups from lysine residues, but both in vitro and in vivo studies have shown that the imidazole group in histidine residues is the preferred group for reactions with both o-quinones and p-quinone methides, and that other groups can also take part.
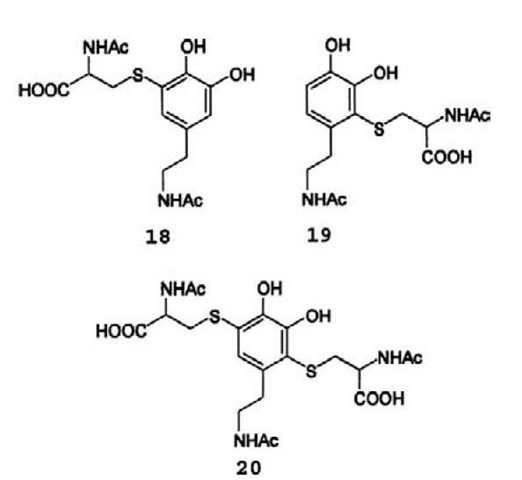
Incubation of NADA and N-acetylcysteine with blowfly (Sarcophaga bullata) larval cuticle resulted in formation of an adduct (18) where the sulfur atom in N-acetylcysteine is linked to the 5-position of the NADA moiety (Sugumaran et al., 1989b), indicating that SH-groups are good acceptors for the o-quinone of NADA. Electrochemical oxidation of dopamine in the presence of N-acetylcysteine gave a mixture of C-5 and C-2 (19) monoadducts together with a disubstituted product, 2,5-S,S’-di(N-acetylcysteinyl)dopamine (20) (Figure 4 Xu et al., 1996a; Huang et al., 1998;). Since the monoad-ducts are more readily oxidized to quinones than the parent catechol, a monoadduct formed between an oxidized catechol and a protein-linked cysteine will be more prone to be reoxidized to quinone than a free catechol, and after reoxidation it can react with a nucleophilic group in a neighboring protein chain to form a covalent crosslink between the proteins. Thus cysteine—catechol based cross-links are possible, but they are not likely to play an important role in cross-linking cuticular proteins since cysteine residues are rare in cuticular proteins. Perhaps the scarcity of cysteines in cuticular proteins is related to the readiness with which they react with o-quinones.
Figure 4 Structure of adducts formed between W-acetylcysteine and NADA.
When locust cuticle is incubated with NADA and benzenesul-finic acid, the oxidized NADA is trapped by adduct formation with the sulfinic acid, and only when all sulfinic acid has been consumed will o-quinones be available for reaction with cuticular proteins, and for isomerization and further metabolism to polymeric compounds (S.O. Andersen, unpublished data). If significant amounts of free SH-groups were present in the cuticular matrix proteins, that could in a similar way delay further metabolism of the o-quinones, and cause sub-optimal sclerotization.
Methionine residues can also react with o-quinones to form adducts, and such adducts have been suggested to take part in sclerotization (Gupta and Vithayathil, 1982; Sugumaran and Nelson, 1998), but so far no methionine-containing adducts have been reported from sclerotized cuticles.
Electrochemical oxidation of NADA in the presence of N-acetylhistidine gave mono-adducts where a nitrogen atom in the imidazole ring is linked to either the C-6 (21) or the C-2 ring position (22) in NADA (Figure 5), the C-6 position being the preferred position (Xu et al., 1996b). Electrochemical characterization of the C-6 and C-2 N-acetylhistidine-NADA adducts showed that both adducts are more reluctant than NADA itself to be oxidized (Xu et al., 1996b), indicating that formation of adducts involving two N-acetylhistidine residues linked to the same NADA residue is rather unlikely.
Using H. cecropia larval cuticle to oxidize a mixture of NADA and N-acetylhistidine resulted in a mixture of products, and both the C-6 ring position and the P-position of the side chain (23) (Figure 5) were involved in adduct formation (Andersen et al., 1991, 1992a). The formation of a side-chain adduct indicates that the P-position was activated, probably due to formation of NADA p-quinone methide, demonstrating that cuticular oxidation of catechols is more complex than electrochemical oxidation.
Incubation of larval cuticle of H. cecropia with NADA and compounds containing a free amino group, such as a-N-acetyllysine and P-alanine, resulted in formation of several products, and adducts were identified with the amino groups linked to either the 6-position of the ring in NADA (24) or to the P-position of the side chain (25) (Figure 6). The former adducts have a quinoid structure, indicating that the initially formed catecholic adducts are spontaneously oxidized by exposure to air. The adducts containing an amino acid linked to the P-position of the side chain were stable and not readily oxidized (Andersen et al., 1992b). A product obtained by prolonged incubation of cuticle with NADA and a-N-acetyllysine was identified as a 4-phenylphenoxasin-2-one (26) (Figure 6), a compound composed of three NADA residues joined to one a-N-acetyllysine residue (Peter et al., 1992).
The structure of one of the products formed during incubation of blowfly larval cuticle with NADA indicated that the o-quinone of NADA can react with water to form N-acetyl-3,4,6-trihydroxyphenylethylamine (6-hydroxy-NADA), which will couple oxidatively with another NADA residue to give the dimeric compound (27) (Figure 7 Andersen et al., 1992c;). Products indicating formation of 6-hydroxy-NADA have not yet been obtained from in vivo sclerotized cuticles, and in vitro formation is probably due to the large excess of water in the incubation medium, contrasting with the relatively low water content in sclerotizing cuticle (30-40% of the cuticular wet weight (Andersen, 1981)). The incubation of NADA with blowfly larval cuticle also resulted in formation of small amounts of a product (28) (Figure 7) consisting of two NADA-residues linked together via their 6-positions (Andersen et al., 1992c). A corresponding 2,6′-linked dimer of NADA was suggested as intermediate in formation of the above-mentioned 4-phenylphenoxasin-2-one (26) (Peter et al, 1992).