The use of oxidation as a tool to rearrange molecules to less lipophilic products has the added benefits of unmasking other vulnerable groups and making the products simpler to conjugate. There are several CYP-mediated oxidations that have this effect on molecules.
Dealkylations
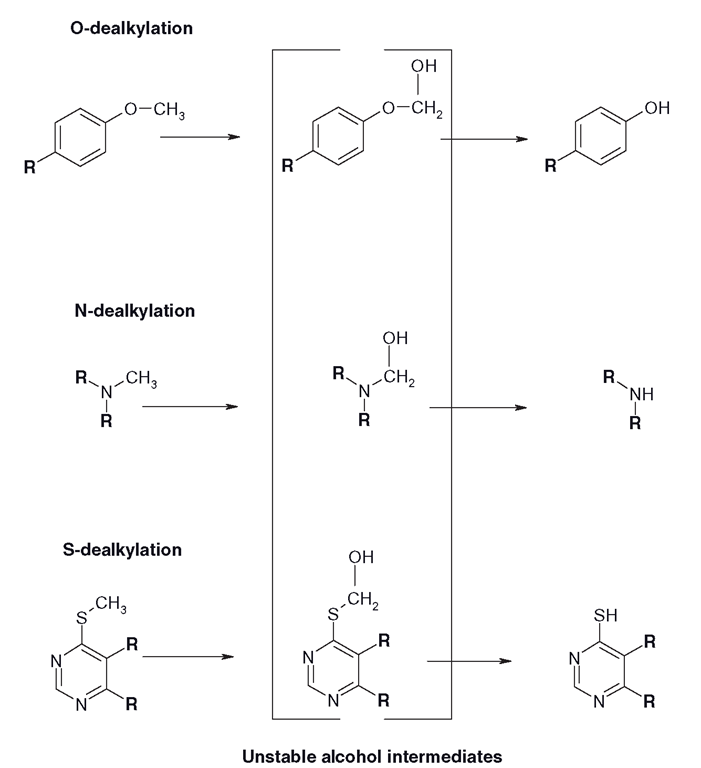
Alkyl groups, especially bulky ones, are very lipophilic and often are attached to drugs through ‘ hetero’ atoms, i.e. nitrogens, oxygens and sulphurs. From the perspective of biotransformation, it makes sense to remove the alkyl group, leaving the hetero group vulnerable for conjugation with glucuronides or sulphates (Figure 3.15’ . The quickest way to remove the alkyl group is to oxidize it to an alcohol. This should be a win-win situation, whether the product is stable or unstable, the alcohol (called a carbinolamine in the case of N-dealkylation) is usually unstable and splits off, forming an aldehyde. This reveals a less lipophilic heteroatom ‘handle’ for conjugation. If the alcohol is stable, then the drug is still more hydrophilic than it was and that might also be a target for subsequent conjugation. With substituted aromatic compounds it is easier for the CYP to oxidize an alkyl substituent group than the ring. Another result of dealkylation can be the splitting of a large lipophilic molecule into two smaller more hydrophilic ones (Figure 3.16).
Figure 3.15 Rearrangement reactions caused by the CYP-mediated oxidation of an alkyl group leading to the formation of a more water-soluble product, which is also more vulnerable to Phase II. The ‘waste products’ of the reactions are usually small aldehydes or ketones
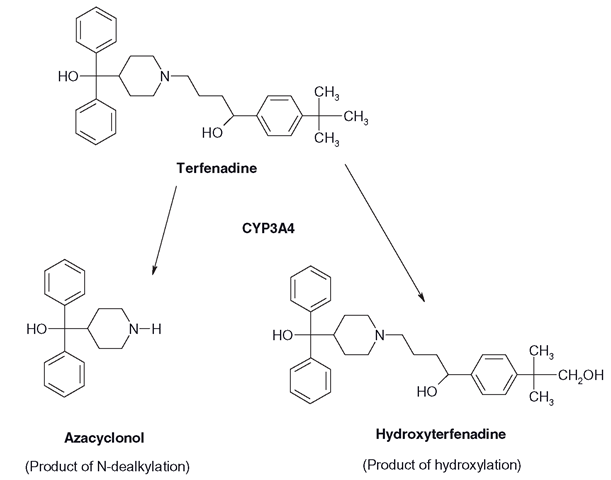
There are many examples of drugs that undergo this type of dealkylation. Imipramine, the TCA, is demethylated to form desmethyl imipramine, which also has pharmacological potency and is usually known as desipramine. The removal of one methyl group may not make much difference to the lipophilicity of a large molecule, although it may change its pharmacological effects. More than one alkyl group may have to be removed to make the compound appreciably less lipophilic. On the other hand, the N-dealkylation reaction of the antihistamine terfenadine has a much more dramatic effect (Figure 3.16). The oxidation of the alkyl group adjacent to the nitrogen causes an unstable alcohol to be formed, which splits away, taking half the molecule with it. Virtually the same reaction of alkyl oxidation at the other end of the parent molecule results in a stable alcohol that is then oxidized to a carboxy derivative (fexofenadine) which is not metabolized further and is less toxic than the parent drug.
Figure 3.16 Metabolism of terfenadine: essentially the same oxidation reaction applied in two different areas of the molecule leads to vastly different effects on the structure
N-dealkylation mechanisms
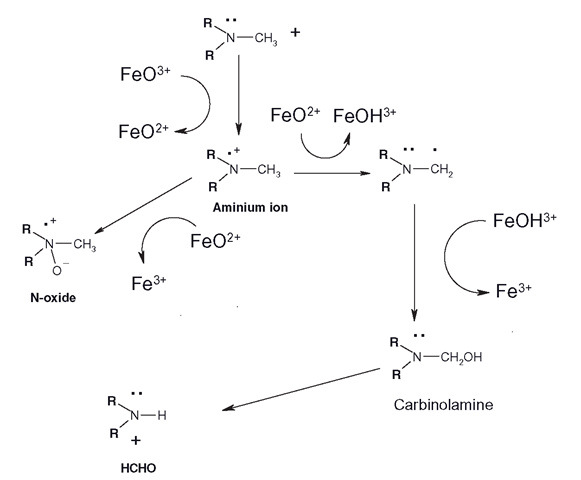
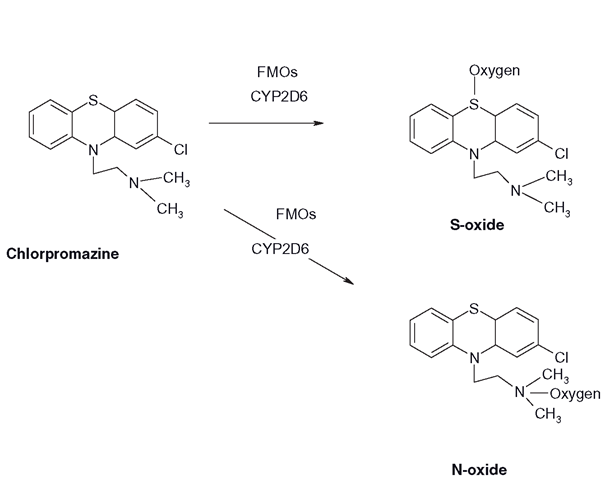
N-dealkylation is only part of the picture of the metabolism of how CYPs can oxidize heteroatoms. Before N-dealkylation occurs, CYPs have the option of oxidizing the substituted nitrogen itself to form an N-oxide (Figure 3.17). If N-oxide formation does not occur, then N-dealkylation can proceed. Again, this is a ‘win-win’ process, as N-oxides are more water-soluble than the parent drug. Generally, FMOs are credited with the vast majority of N-oxidations, but it has become apparent that CYPs can also accomplish them. Whether N-oxide formation or dealkylation occurs is dependent on factors such as the surrounding groups on the molecule and the CYP itself. The mechanism of N-oxidation and N-dealkylation is now believed to differ slightly from the majority of CYP-mediated hydrogen abstractions/oxygen rebound reactions. It begins with the CYP perferryl complex abstracting one of the nitrogen’s lone pair of electrons (Figure 3.17), forming an aminium ion (N’ . Once this has been created, either the oxygen reacts with the N’ giving the N-oxide, or the perferryl complex can abstract a hydrogen atom from one of the adjacent carbons forming a carbon radical. The reaction then proceeds as with most CYP oxidations, where the hydroxyl group bounces off the haem iron to react with the carbon radical to make the (usually unstable) alcohol, or carbinolamine. Chlorpromazine and the TCAs can undergo N-oxidation or N-dealkylations, as well as sulphoxide formation (Figure 3.18)and as mentioned in section 3.8 ’ flavin monooxygenases (FMOs) can accomplish these reactions also.
Figure 3.17 Pathways of CYP-mediated N-oxidation and N-dealkylation
Figure 3.18 Sulphoxide and N-oxide formation with chlorpromazine, by either CYPs or FMOs
Deaminations
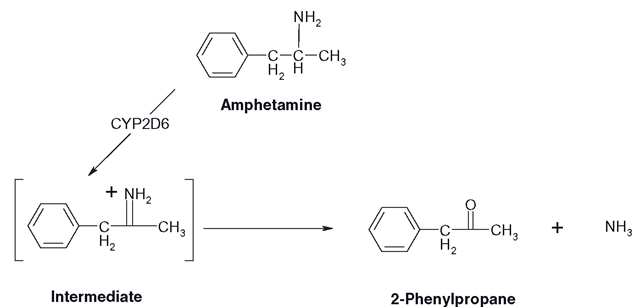
Amine groups in drugs can be primary, secondary or tertiary. Primary amines can be removed completely thorough conversion of the carbon—nitrogen single to a double bond, where the nitrogen loses an electron. Via a hydrogen atom from water, ammonia is formed with a ketone product. This is one of the fates of amphetamine (Figure 3.19’ . More amphetamine metabolism can be found.
Dehalogenations
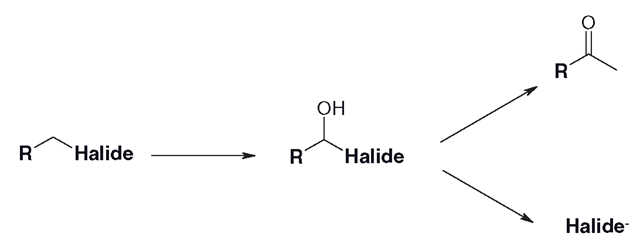
Using the same basic tool, oxidation to an alcohol, it is possible for CYPs to remove halogens (chloride, bromide or fluoride) from molecules, forming a ketone and a halogen ion. A number of volatile general anaesthetics are subject to this route of metabolism. The adjacent carbon to the halide is oxidized to a short-lived alcohol, which causes the movement of electrons towards the halogen, which dissociates (Figure 3.20).
Figure 3.19 Oxidative deamination of amphetamine
Figure 3.20 Removal of halides through an unstable alcohol intermediate
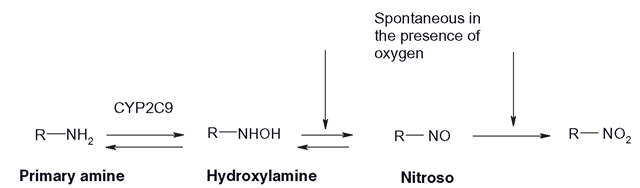
Figure 3.21 Primary amine oxidation