Introduction
The process by which enzyme induction occurs has three main requirements:
• The hepatocyte must detect the presence of particular potentially persistent lipophilic drugs and/or toxins and correctly sense their concentration.
• The process of detection is translated into an increase in the capability of the appropriate metabolic system or systems within the cell, which will clear the drug and/or toxin as efficiently as possible.
• The complete (detection and action) system should be dynamic and reversible, so it is sensitive to further changes in drug concentration.
It is apparent that the main inducible CYPs, CYP1A1/1A2, CYP2C8/9 and CYP3A4, employ broadly similar systems to regulate their ability to respond to increases in drug concentration. Indeed, this commonality is borne out by observations that agents that don’t induce CYP3A4, don’I induce CYPs 2C8/9 and 2C19 either. The exception to this rule seems to be CYP2E1, which appears to have a unique system of induction. On a cellular level, we know that the type of induction mechanism involved with a given CYP is closely related to a combination of endogenous as well as xenobiotic-responsive functions. Indeed, the networks of various nuclear and cytoplasmic receptor systems described below can ‘cross-talk’, in that different receptors may modulate each other and even activate the same gene. This multiple system approach is used in aircraft to maximize safety and in the cell, to reduce the possibility of potentially disruptive chemicals remaining in the body unchallenged. Indeed, the various receptor systems which govern CYP expression act in concert with other aspects of biotransformation relevant to the inducing chemical. These include stimulating the proliferation of the SER to provide space for the CYPs to be anchored, as well as the production of sufficient quantities of REDOX partners to fuel the CYPs. The expression of conjugation and Phase III transporters is also up-regulated .This all translates into relatively rapid and profound effects on drug clearance. Clinically,rifampicin or a potent St John’s Wort extract can cause other drugs to fall out of their therapeutic windows in a couple of days. With anticonvulsants the process may take a few days longer and be complete in most patients in around three to five weeks. There is, however, a huge variation in particular CYPs and in individual clinical responses.
CYPs 1A1/1A2 and 1B1 induction
The AhR system
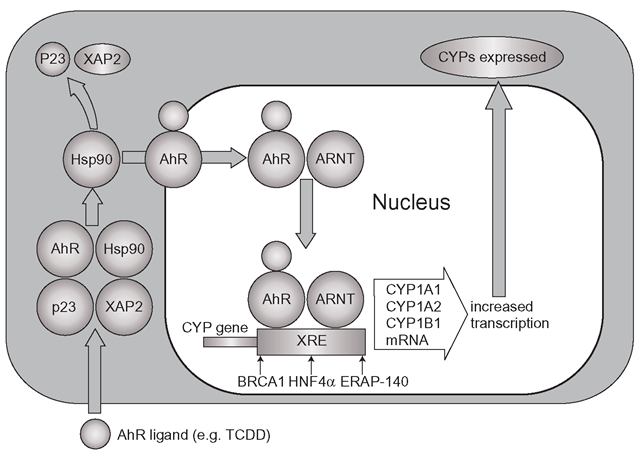
Although enzyme induction has been known clinically since the 1960s, it was not until the 1970s that the cellular basis of the process was unravelled by studying the effects of dioxin. In the cytoplasm of most cells a receptor complex can be found which consists of four components (Figure 4.1): the aryl hydrocarbon receptor, or ‘AhR’, heat-shock protein (Hsp90), co-chaperone p23 and an immunophilin called XAP2. Many receptors are complexed with several ‘chaperone’-like molecules mainly to ensure they keep a certain shape so they can bind their substrate, rather like polystyrene packing is used to help fragile articles survive postal systems. TCDD binds to the AhR complex which then migrates towards the nucleus. The AhR/TCDD complex then breaks away and it alone enters the nucleus and heterodimerizes (two different proteins form a complex) with the nuclear protein ARNT (aryl hydrocarbon nuclear receptor translocator) and this new complex binds to specific DNA sequences upstream of the CYP1A1/1A2 and 1B1 genes, which are termed xenobiotic-responsive elements (XRE) or sometimes DREs (drug or dioxin-responsive elements). Several other nuclear proteins are also required before CYP transcription and translation are fully engaged, such as oestrogen receptor associated protein (ERAP-140) and it is apparent that BRCA1 may also be important at this stage.
Figure 4.1 Basic mechanism of CYP1A1 and 1A2 induction: the AhR receptor binds the inducer alongside Hsp90, but only the AhR receptor and the inducer cross into the nucleus to meet ARNT and together they bind to DNA xenobiotic response elements (XRE). Co-activators which induce expression of the CYP isoforms include BRCA1, ERAP-140 and HNF4a
BRCA1 (breast cancer 1 early onset) is a tumour suppressor gene which has myriad functions and is found on chromosome 17. Defects in this gene are associated with the early onset of breast cancer, mainly because the BRCA1 is vital in DNA repair. It is thought that healthy BRCA1 may be part of the process that ensures that CYP1A1/1A2/1B1 induction efficiently responds to challenge from xenobiotics like PAHs and TCDD. Defects in the gene do affect the response to TCDD in experimental breast cancer lines and it is possible that in addition to a lack of DNA repair in mutated BRCA1 individuals, there is also insufficient detoxification of potential carcinogens which would normally be oxidized and cleared. This is yet to be established clinically, although it is conceivable that with carcinogens that require CYP-mediated activation, defective BRCA1 could disable CYP induction and actually reduce toxic metabolite formation.
CYP1 family induction is an exceedingly sensitive system, as it responds powerfully to single figure nanomolar concentrations of TCDD. Interestingly, although CYPs 1A1/2 and 1B1 are in the same family, it appears that their expression can be controlled differentially through a complex interplay between the various co-activators such as ERAP, BRCA and the response elements on different areas of the respective isoform genes.
It is thought that a healthy AhR system obeys the law of mass action, so providing there are enough AhR receptors in the cell cytoplasm, then the more TCDD-like inducer molecules that appear in the cell, then more TCDD/AhR complexes will migrate to the nucleus and bind to the DREs. This will in turn increase CYP expression. At rest, this system operates in an analogous way to an idling engine, with a low level of ‘revolutions’ connected to a ‘throttle’, or the AhR receptor. A sudden influx of PAHs or related chemicals ‘floors’ the throttle, leading to increased CYP synthesis, which is capable of eventually clearing (by CYP-mediated oxidation) large amounts of the toxins from the cell. Release the ‘throttle’, the system subsides, saving energy and raw materials. It is rather ironic that in contrast to rats, humans barely metabolize TCDD itself and as mentioned,this toxin is breathtakingly persistent in man. Its clearance can be accelerated in cases of human poisoning by the consumption of foodstuffs which include the fat substitute Olestra, which will speed up the TCDD elimination half-life from seven to ten to ‘only’ one to two years.
The AhR system-endogenous function
The AhR/ARNT system is found in all tissues and is involved with the development of organs such as the liver, as well as the immune system. Indeed, part of the toxicity of polycyclic aromatics is linked with their disruption of AhR/ARNT controlled cellular processes. The AhR and ARNT receptor system is multifunctional, as TCDD induces other enzyme systems as well as CYP1A1/1A2 and CYP1B1, such as glutathione-S-transferase, aldehyde dehydrogenase and NADPH reductase (known as NADPH oxidoreductase, or NQO1). Therefore, it is easy to see that exposure to compounds which resemble TCDD will have a significant impact on the concentrations of many other endogenous molecules which are cleared by these enzyme systems. ARNT itself participates in many other cellular responses to hypoxia and hypoglycaemia through HIF proteins. There is some evidence that overactivity of the AhR system, unrelated to xenobiotics, is actually carcinogenic in itself, particularly in the lung and the pancreas. Indeed, the high level of CYP1B1 expressed in lung cancers is now thought to be a consequence of AhR overactivity, rather than an inductive effect of carcinogens, partly as considerable numbers of non-smokers develop lung cancer. This is probably not the case for CYPs 1A1 and CYP1A2 expression, which appears to be directly linked with exposure to cigarette-linked xenobiotics.
The AhR system-control and toxicological significance
As with all induction effects, CYP1 induction begins within hours of exposure to the toxin or drug, but it may take from several days to weeks before CYP expression is maximally induced. As well as TCDD, it is thought that PAHs and heterocyclic amines induce CYP1 isoforms in this way, as does the anti-ulcer agent omeprazole. Essentially, the ‘default mode’ for hepatic expression of CYPs 1A1/2 and CYP1B1 is in the ‘off’ or very low level position, as the liver does not normally encounter planar aromatics such as TCDD in serious quantities. Even after induction, the CYP1 family is subject to further post transcriptional regulation and there is also evidence that the CYP1A1/2 system can be switched off by other agents, such as the ’ orphan ’ (currently function unknown) nuclear receptor, the short heterodimer partner (SHP). This receptor can bind to a number of nuclear receptors such as RXR (retinoic acid X receptor) and several other receptors which control thyroid and oestrogen levels. SHP can also block the response of CYP1A1 to TCDD, by directly blocking ARNT. There are also likely to be a number of compounds that bind to SHP and regulate CYP1A1 activity. Interestingly, several flavonoids can also block the AhR system, preventing CYP1 induction and thus protecting cells from the consequences of reactive species formation. In the future, the AhR induction system could well be successfully therapeutically targeted to intercept the development of early stage lung tumours.
CYP1A1/2 and CYP1B1 induction is of high toxicological significance in the lung in non-ciliated ‘Clara’ cells, which are in the forefront of the detoxification of pollutants in inspired air. These cells have more than half their volume given over to SER and induction of CYP1A1/2 by PAHs in tobacco leads to the formation of reactive epoxides, which attack DNA, forming ’ adducts ’ or small PAH-related structures which are covalently bound to DNA and are strongly linked with lung carcinogenicity. It has been suggested that the high state of lung induction of CYP1A1 leads to an increased Clara cell exposure to reactive species of oxygen generated by 1A1 even when it is not metabolizing substrates. This is because CYPs 1A1 and 1A2 are thought to ‘leak’ reactive oxygen species and this -drip-drip – effect might make as great a contribution to DNA damage as the reactive aromatic metabolites.Other lung – specific toxins include CYP2E1- activated vinylidene chloride. The likelihood of the development of lung tumours in response to metabolism depends on the degree of CYP induction, the amount of carcinogenic species produced, their detoxification and the efficiency of DNA repair mechanisms.
CYP2B6 2C8/2C9/C19 and 3A4 Induction
The Nuclear Receptor System
Unlike the cytosolic AhR system described above, these CYPs are controlled by transcription factors which are usually (but not always) found in the nucleus, so they are termed nuclear receptors (NR). These receptors are the means by which a number of steroid hormones. vitamins, mineralocorticoids and glucocorticoids translate commands from other tissues to activate specific genes to express a variety of proteins as well as the CYPs.
The NRs are regulated by master controlling nuclear receptors, known as hepatocyte nuclear factors (HNFas). The most important identified so far is HNF4a that is continuously active and regulates so many processes, (organ development as well as lipid and insulin metabolism) that it is probably the key to normal liver function, although it is found in other tissues. HNF4a is so important that gene knockout studies have shown that animal embryos do not survive without it. It is likely that HNF4a directly or indirectly regulates all endobiotic and xenobiotic biotransformational processes.
NRs are fairly similar in structure, containing an NiIerminal DNA binding domain (DBD) and a C-terminal hormone/chemical-binding domain, rather like one of those international electric plug appliance adaptors. When the hormone or xenobiotic is absent, the C-terminal domain, sometimes called the ligand binding domain (LBD) is locked in the ‘off’ position by a co-repressor protein complex. The binding of the appropriate ligand to the LBD causes it to rearrange and release its co-repressor. The receptor then attracts and binds a co-activator complex. There are several co-activators, part of a series of proteins known as p160s, such as SRC-1 (steroid receptor co-activator 1) and the splendidly named GRIP1 (glucocortoid receptor interacting protein 1). Once the activated complex is formed, it seeks to bind a specific DNA hormone response element (HRE), also termed XRE, or xenobiotic response element.
Among all the various nuclear receptors, there is further subdivision, in that some NR receptors such as the thyroid and vitamin D receptors (TR & VDR), CAR (constitutive androstane receptor) and PXR (pregnane Xiieceptor) form complexes with RXR (the retinoic acid receptor) in order to bind HREs. The presence of HNF4a is then required to make all these binding processes productive and activate gene transcription. Of the NRs, CAR and PXR are the focus of particular attention, as they control the expression of the CYPs among other biotransformational systems. (Figures 4.2a and b and Figure 4.3).
CAR mediated control of CYP expression
CAR is also known under nuclear receptor classification as NR1I3 and it modulates basal biotransformational metabolism (among many other processes) and it is under the direct control of HNF4a as well as the glucocorticoid receptor. In common with the AhR receptor, it too is found in the cytoplasm, complexed with Hsp90, p23, an immunophilin and a protein called CCRP (CAR retention protein; Figure 4.2a). However there is a vital difference. To follow the idling engine analogy used above of AhR, CAR operates like the throttle is held half way down by itself, running the engine at half its capable number of revolutions. CAR does not need to be stimulated into action by agonists, it is already, as its name suggests, a ‘constitutive’ receptor, driving the expression and activity of CYPs and conjugation systems as well as transporter systems such as the OATPs and NTCP.The presence of inducers, such as steroid hormones and drugs (phenobarbitone, primidone, phenytoin and rifampicin) merely speeds up the process, forcing the ‘throttle’ towards the floor and there are also several steroid inhibitory factors which cause the throttle to be released, sometimes as far back as ‘idle speed ’ .
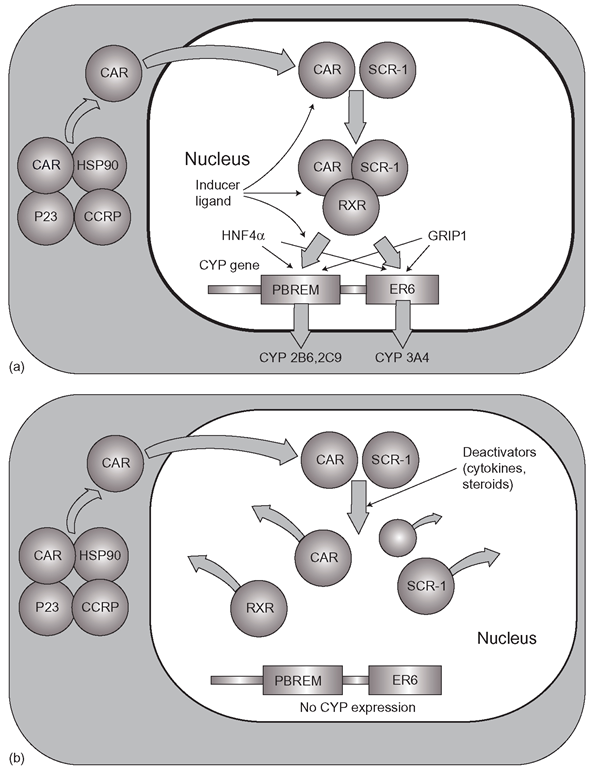
Figure 4.2 (a) Constitutive androstane receptor (CAR)-mediated control of CYP2 series and CYP3A4. CAR and SCR-1 bind the inducer ligand inside the nucleus, bind retinoic acid X receptor (RXR) and activate the CYP expression (b) Possible mechanism for the modulation of CAR-ligand activated CYP induction: a series of endogenous deactivators cause break up of the CAR/RXR/ SCR-1/ligand complex and induction is switched off
CAR operates the induction pathways through changes in its binding affinities for RXR, its co-activator (SRC-1, or steroid receptor co-activator-1) and DNA. The CAR/RXR complex is a heterodimer (like AhR and ARNT) and basic CAR function comprises the continuous recruitment of RXR by CAR followed by association with SRC- 1 into a complex. The CAR/RXR/SRC – 1 complex binds to DNA (Figure 4.2) at the PBREM (phenobarbitone-responsive enhancer module) in the CYP2B gene (particularly CYP2B6) and to the Everted Repeat 6 (ER6) element of the CYP3A4 and CYP2C9 genes. HNF4a binding to the CYP promoter sites then ensures protein synthesis will occur. Other coactivators like GRIP1 may also be involved. This is the ‘half-way down throttle’ stage. If inducers of CAR appear, they increase the stability of the rather – wobbly – CAR/RXR complex making it more rigid and thus promoting a better fit with SRC-1 followed by more productive PBREM binding, pushing the throttle towards the floor- proportionally to the quantity and potency of the inducer. Why some chemicals are very potent inducers and others less so is not entirely clear, as exactly how inducers interact with CAR is not fully understood. It is likely to involve the agent dephosphorylating the receptor rather than full binding of the inducer to CAR. The more the chemical stabilizes CAR throughout its recruitment and binding processing of its co-activators, the more potent the inducer. It some ways, the system is rather like gradually engaging the clutch in a car with manual transmission. There are several mechanisms for restricting or even switching off CAR operation, and some steroids such as progesterone and various androgens inhibit CAR, so ‘-ifting the foot off the throttle (Figure 4.2b). Variation in CAR expression is one of the main reasons why there is so much interindividual expression in biotransformation. Overall, CAR also regulates CYP2C8, CYP2C9 and CYP2C19 as well as CYP2A6 and UGT1A1, as well as various transporters.In the light of the emerging role of cytochrome b5 as an additional significant supplier of CYP reducing power, it would be surprising if CAR and HNF4a did not control the expression of this REDOX partner also.