Introduction
Alongside the CYPs, other cellular systems can accomplish oxidations of endogenous molecules and these systems are usually widespread in many tissues. Of these systems, the flavin monooxygenases (FMOs) have not received anything like the attention directed at CYPs, although realization of their significance in drug clearance is growing rapidly. Originally known as ‘Ziegler’s enzyme’, after its discoverer, the FMOs are actually the second most prolific oxidizers of drugs and xenobiotics after the CYPs. In humans, the five functional forms of FMO (designated 1-5) are coded for by separate genes found on chromosome 1. The nomenclature for FMOs is based around amino acid sequence commonality as with the CYPs. Individual isoforms are regarded as belonging to the same family if they possess greater than 82 per cent amino acid sequence homology. Of the five FMOs, FMO-3 is the most important in human liver and it is thought to be expressed at nearly 60 per cent of the level of CYP3A4, which gives some idea of its potential contribution to drug oxidation. FMO-5 is also found in the liver in similar proportions to FMO-3, but FMOs-1, 2 and 4 are not usually found in the adult liver. FMO-3 is not expressed in neonates at all and it seems that FMO-1 takes its place. FMO-1 is found mainly in the adult kidney and intestine, whilst FMO-3 is virtually absent in the kidney. A lack of selective experimental substrates for FMO-4 and 5 have made it difficult to study them, although they are not believed to contribute to xenobiotic clearance in man. FMOs are subject to genetic polymorphisms, and they are linked with the condition trimethylaminuria (TMAU), otherwise known as fish-odour syndrome.
Structure
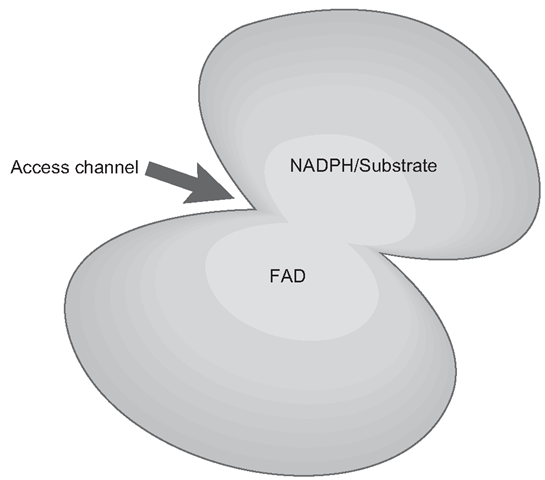
The main features of these enzymes are pretty well conserved and are virtually ‘standard issue’ across the animal kingdom. FMOs have some structural resemblance to oxidore-ductases (PORs) and a yeast FMO has been crystallized and through similar techniques used with CYPs, the sequence of its operations can be worked out through a series of ‘freeze-frames’ where FMO can be ‘observed’ binding its co-factor and substrate. The main structure of the enzyme could be described as clam-shaped, with a small ‘top shell’ and a larger bottom , shell , , The access channel is obviously between the two , shells , (Figure 3.6) . On the floor of the bottom ‘shell , there is a depression to which FAD is anchored firmly. On the top ‘shell’, there is a binding site which will fairly loosely hold NADPH which is the main co-factor. The NADPH is bound in the orientation where the adenine portion faces the upper shell, whilst the nicotinamide portion is aimed at the flavin of FAD.
Figure 3.6 Generic structure of a Flavin monooxygenase (FMO)
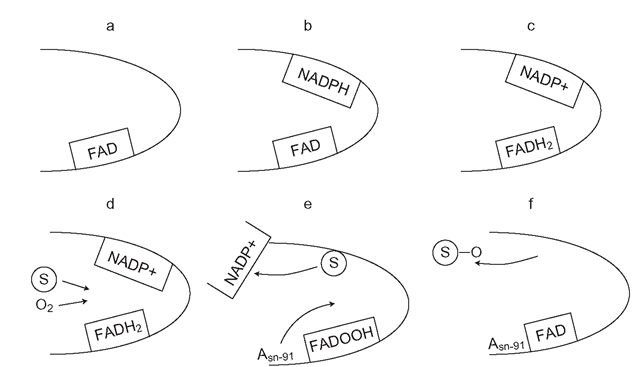
Figure 3.7 Process of Flavin monooxygenase catalysis: a-c outlines the first step or priming of the enzyme. The second step (d-’ involves oxygen binding and substrate (S) displacement of NADPH, followed by reaction of oxygen and substrate and release of the oxide
Mechanism of catalysis
FMOs basically use the co-factor NADPH as an electron donor and FAD as the built in electron carrier, which is similar to POR. The first step or ‘priming’ of FMO, involves NADPH transferring a hydride ion to the FAD, forming a FADH2 and NADP+ complex (Figure 3.7) . It is believed that this is probably the ’resting ’ state of the enzyme. The presence of a nucleophilic (electron rich) substrate seems to initiate step two, which involves FADH2 reacting with molecular oxygen (O2) to form FADOOH, which is called a hydroperoxyflavin. The substrate then displaces the NADP+ and is held surrounded by a sort of ‘j acket’ of water molecules. This means that it does not actually make contact with the enzyme. A crucial amino acid on the lower ‘shell’ of the FMO, called asparagine-91 (Asn-91) is instrumental in catalyzing the reaction of the two oxygen molecules held as FADOOH with the nucleophilic portion of the substrate, a nitrogen lone pair of electrons. This leads to one oxygen molecule reacting with the substrate forming an oxide, whilst the other forms water. The enzyme is eventually regenerated to FAD and then it ‘reloads’ itself with more NADPH ready to oxidize another substrate molecule.
The oxidation process is a single step transfer of two electrons, rather than the CYP system of two successive single electron oxidations. Unlike many CYPs, which do not initiate their catalytic cycle unless more than one substrate molecule binds, FMOs are ready primed as the FADH2 and NADP+ complex can oxidize immediately; in fact, aside from nitrogen, they specialize in the oxidation of other nucleophiles, like sulphur and phosphorus. These groups are found in various drugs and xenobiotics and the respective oxides formed by the FMOs are more water soluble than the parent drugs. FMOs can also metabolize tertiary amines to form N-oxides in drugs such as TCAs, as well as morphine, methadone and pethidine (meperidine). They also can form the 1 and 3-N-oxide metabolites of the 2, 4, diaminopyrimidine antiparasitics (trimethoprim and pyrimethamine), as well as many other sulphoxide metabolites such as that of cimetidine. There is relatively little known to date as to details of substrate preferences for the different FMOs, although FMO-3 oxidizes generally smaller nucleophilic heteroatoms than FMO-1 which prefers drugs with bulkier side- chains, forming N- oxides of chlorpromazine, imipramine and orphenadrine. FMO-2 is sporadically expressed man and when it is expressed, it oxidizes sulphur, rather than nitrogen containing agents. There is no evidence so far that FMOs can be inhibited as drastically as CYPs and any relatively mild inhibition that might occur is more likely to be competitive (nitrogen/sulphur containing nucleophiles) rather than mechanistic inhibition. If inhibition occurs in vivo, it is thought it may be related to the influence of the combination of dietary factors and particular polymorphic variants of FMOs 1, 2 and 3.
Variation and expression
FMOs display some interesting differences and similarities in their expression and operation in comparison with CYPs. Like CYPs, their metabolites are usually more hydrophilic than the parent, although unlike CYPs, they are less likely to produce reactive species and their metabolites tend to be low in toxicity, such as the various N-oxides. Our knowledge of the regulation of FMOs is much less detailed than with CYPs, but their expression can be regulated hormonally, which accounts for the marked switch from predominant expression of hepatic FMO-1 to 3, which appears to be accelerated by the birth process.
As FMOs are considered by many to have evolved as a detoxification system, it seems inexplicable that FMO expression does not appear to be capable of responding to xenobiotic challenge over, say days or weeks, which is the hallmark of the complex and highly efficient CYP enzyme induction system.It is apparent that the variation in FMO expression is mostly genetic and it has been suggested that separate human populations may have evolved to express particular variants of these isoforms which detoxify flora and fauna of specific geographical regions. Clearly this theory suggests that individuals would be at significant risk if they strayed to a different area where potential local toxins could not be cleared by their FMO expression profile.
FMOs in drug development
Clinically, FMOs do represent a potentially attractive prospect in drug development. As mentioned above, they are not easily inhibited and their general lack of induction response makes their expression stable and not subject to unexpected and potentially dangerous changes induced by diet, alcohol/drug consumption or other small-molecule induced stimuli. From a practical standpoint, it is also useful that FMO-3 is expressed in quantities capable of clearing drugs in its own right. Additionally, if a new drug was to be cleared by a combination of FMOs and CYPs, the introduction of a potent CYP inhibitor to a clinical regime would not completely shut down all drug clearance, so preventing accumulation-mediated toxicity, as the FMO would continue to eliminate the drug. This has been demonstrated already to some extent by the family of gastroprokinetic agents, cisapride, mosapride and itopride. Poor gastric motility leads to accumulation of stomach acid, which causes pain and inflammation of gastro-oesphageal tissues. These drugs relieve these symptoms by improving gastric motility, although not without side-effects. In the presence of a CYP3A4 inhibitor cisapride can accumulate and extend the cardiac QT interval potentially lethally. The drug has been withdrawn in most countries, although apparently it remains highly effective for constipation in cats. In contrast, itopride, is cleared by FMO-3, thus making the drug potentially ‘,diot proof’, that is, it would be significantly safer and easier to use in complex real- world clinical regimens.
However, in common with CYPs, it is apparent that there is a very wide variation in the expression of FMOs between racial groups and individuals. There are also a large number of genetic polymorphisms for these enzymes and the clearance of some drugs, such as ranitidine, is related to FMO-3 expression in particular ethnic groups such as Koreans. Hence, the variation in expression of FMOs may complicate or even ultimately frustrate the strategy of designing drugs which are cleared exclusively by FMOs.