Nature of aromatics
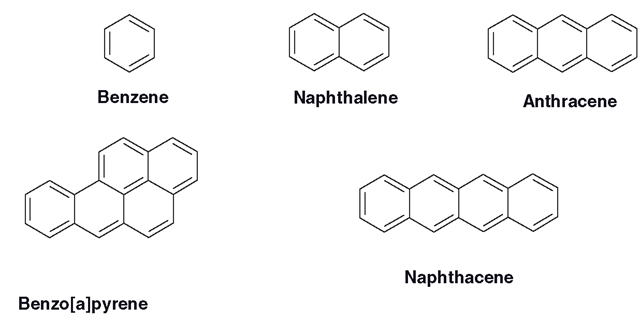
Large, highly lipophilic, planar and stable molecules with few, if any, vulnerable functional groups look to be a difficult proposition to metabolize (Figure 3.8). Indeed, if there are any aliphatic groups, or non-aromatic rings associated with an aromatic molecule, these will often be attacked rather than the aromatic group. The ring hydroxylation of amphetamines is the exception to this. Polycyclic hydrocarbons are not easy to clear and they are perceived by living systems as a potent threat. This is reflected in the elaborate expression system which modulates the non-constitutive isoform CYP1A1, which has evolved to deal with them, which is highly effective. These include molecules such as those shown in Figure 3.8. The simplest aromatic is benzene and this can be oxidized by CYP1A1 eventually to phenol, which is more reactive, but more water-soluble than benzene and vulnerable to sulphation and glucuronidation during conjugative metabolism.
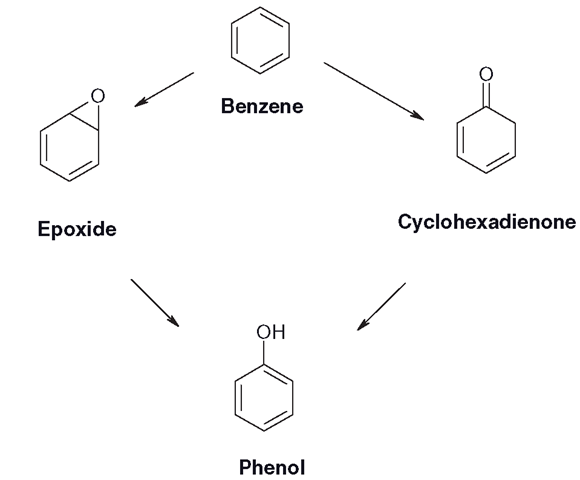
The oxidation of benzene
There are several intermediates formed during the oxidation of benzene (Figure 3.9). The two main routes are the cyclohexadienone and an epoxide; in the presence of water both stages will rearrange to form the phenol. During this process, the hydrogen atom close to the oxygen will sometimes be moved around on the ring, or even lost. This is known as the NIH shift.
Epoxidation is defined chemically as a reaction where an oxygen atom is joined to an unsaturated carbon to form a cyclic, three-membered ether. Epoxides are also known as arene oxides and vary enormously in their stability, which depends on the electron density of the double bond being oxidized: the higher the density, the more stable the epoxide. This means that epoxides of varying stability can be formed on the same molecule, due to differences in electron densities and this is most apparent in benzpyrene. The anticonvulsant carbamazepine forms a number of epoxides and the 10, 11 derivative is stable enough to be pharmacologically active, whilst bromobenzene 3,4 epoxide’s half-l ife in blood is less than 14 seconds. Generally, arene oxides form phenols or diols in the presence of water (as does carbamazepine 10, 11 epoxide), although the cytosolic enzyme epoxide hydrolase.
Figure 3.8 Some aromatic hydrocarbon molecules
Figure 3.9 Main pathways of benzene hydroxylation
The phenols and diols are usually substrates for sulphation or glucuronidation. Although the process of aromatic hydroxylation is difficult to achieve and the phenolic product is more hydrophilic, the structural features of the larger polycyclics mean that this process can lead to the formation of unstable carcinogenic reactive intermediates.