Chronic Treatments with Hcy-Thiolactone are Harmful
As predicted by the Hcy-thiolactone hypothesis [36] [37a, 37b], chronic treatments of animals with Hcy-thiolactone cause pathophysiological changes similar to those observed in human genetic hyperhomocysteinemia. For example, Hcy-thiolactone infusions in baboons [95] or Hcy-thiolactone-supplemented diet in rats [96] produce atherosclerosis. Treatments with Hcy-thiolactone cause developmental abnormalities in chick embryos [97], including optic lens dislocation [98], a characteristic diagnostic feature present in the CBS-deficient human patients [16; 17; 18]. However, rabbits, which have the highest levels of serum Hcy-thiolactonase/PON1, and thus efficiently detoxify Hcy-thiolactone [57; 58; 71], are resistant to detrimental effects of Hcy-thiolactone infusions [99; 100].
Immunogenic Properties of Hcy-Thiolactone- Modified Proteins
Hcy-thiolactone-mediated incorporation of Hcy into protein (Eq. 2) can impact cellular physiology through many routes. Protein modification by Hcy-thiolactone can disrupt protein folding, and create altered proteins with newly acquired interactions, or can lead to induction of autoimmune responses. During the folding process, proteins form their globular native states in a manner determined by their primary amino acid sequence [101; 102]. Thus, small changes in amino acid sequence caused by Hcy incorporation have the potential to create misfolded protein aggregates. Indeed, N-Hcy-proteins have a propensity to form protein aggregates [38]. Furthermore, the appearance of misfolded/aggregated proteins in the endoplasmic reticulum (ER) activates an unfolded protein response (UPR) signaling pathway, that, when overwhelmed, leads to cell death via apoptosis. Protein aggregates are known to be inherently toxic [103]. The toxicity of N-Hcy-LDL, which in contrast to native LDL, has the propensity to aggregate [92] and induces cell death in cultured human endothelial cells [40], is consistent with this concept. These pathways can be induced in cultured human endothelial cells and in mice by elevating Hcy [104; 105; 106; 107], which also elevates Hcy-thiolactone [49; 51]. Moreover, treatments with Hcy-thiolactone induce ER stress and UPR in retinal epithelial cells [108], as well as apoptotic death in cultured human vascular endothelial cells [39; 41]. In this scenario the formation of N-Hcy-proteins leads to the UPR and induction of the apoptotic pathway. Proteolytic degradation of N-Hcy-proteins can generate potentially antigenic peptides, which can be displayed on cell surface and induce adaptive immune response.
Atherosclerosis is now widely recognized as a chronic inflammatory disease that involves innate and adaptive immunity [7; 10; 11]. That inflammation is important is supported by studies showing that increased plasma concentration of markers of inflammation, such as C-reactive protein, interleukin-1, serum amyloid A, and soluble adhesion molecules are independent predictors of vascular events [9]. Autoantibodies against modified LDL were found to be elevated in vascular disease patients in some, but not all studies [12; 13]. Lipid peroxidation is thought to play a central role in the initiation of both cellular and humoral responses. Reactive aldehydes resulting from phospholipid peroxidation, such as malondialdehyde, 4-hydroxynonenal, and 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine can modify lysine residues in LDL and in other proteins. The resulting oxidized lipids-protein adducts, e.g., malondialdehyde-LDL, carry neo-self epitopes which are recognized by specific innate and adaptive immune responses. As will be discussed in the following sections of this topic, protein N-homocysteinylation by Hcy-thiolactone [35; 36] also appears to play an important role.
W-Hcy-Proteins are Immunogenic
By generating structurally altered proteins, the modification by Hcy-thiolactone, like other chemical modifications, such as glycation, acetylation, methylation, ethylation, carbamylation [7], can render proteins particularly immunogenic. Indeed, intradermal inoculations of rabbits with N-Hcy-LDL induces the synthesis of anti-N-Hcy-LDL antibodies in these animals [109]. Furthermore, immunization of rabbits with Hcy-thiolactone-modified keyhole limpet hemocyanin (KHL) leads to generation of antibodies that bind to N-Hcy-LDL [89; 110]. Of considerable interest are the observations that antisera from such immunizations bound not only to the N-Hcy-LDL but to a variety of other human proteins on which the N-linked Hcy epitope was present, such as N-Hcy-albumin, N-Hcy-hemoglobin, N-Hcy-transferrin, N-Hcy-antitrypsin, but not to native unmodified proteins. Ns-Hcy-Na-acetyl-Lys, but not Ns-acetyl-Na-Hcy-Lys, prevented the rabbit antibodies from binding to human N-Hcy-hemoglobin. This shows that the rabbit IgG specifically recognizes Hcy linked by isopeptide bond to s-amino group of protein lysine residue; Hcy linked by peptide bond to a-amino group is not recognized. The rabbit antibodies bind short peptides containing the Ns-Hcy-Lys epitop. Hcy, Hcy-thiolactone, lysine, or unmodified lysine derivatives not are bound by the rabbit anti-N-Hcy-protein antibodies [110]. Furthermore, pre-immune rabbit serum exhibits significant titers of autoantibodies against N-Hcy-albumin [H. Jakubowski unpublished], which suggests that endogenous N-Hcy-proteins present in rabbit blood [61] are autoantigenic. Taken together, these data suggest that autoantibodies, once formed in vivo in response to N-Hcy-LDL would be capable of binding to endogenous N-Hcy-proteins.
An Auto-Immune Response to W-Hcy-Proteins in Humans
To determine whether N-Hcy-proteins are autoimmunogenic in humans, human sera were assayed for the presence of antibodies binding to N-Hcy-hemoglobin as an antigen. We found that each human serum tested showed some titer of IgG [110; 111; 112] and IgM (J. Perla-Kajan, T. Twardowski, H. Jakubowski, unpublished data) auto-antibodies against N-Hcy-hemoglobin or N-Hcy-albumin.
The plasma levels of anti-N-Hcy-protein autoantibodies [89; 110; 111; 112] and protein N-linked Hcy [45; 61; 82; 83] vary considerably among individuals and are strongly correlated with plasma Hcy, but not with Cys or Met [110]. Such correlations can be explained by the Hcy-thiolactone hypothesis [36]: elevation in Hcy leads to inadvertent elevation in Hcy-thiolactone, observed ex vivo in human fibroblasts [50] and endothelial cells [51; 53], and in vivo in humans [49; 53; 54; 67] and mice [36; 49]. Hcy-Thiolactone mediates Hcy incorporation into proteins and the formation of neo-self antigens, Ns-Hcy-Lys-protein (Eq. 1). Raising levels of neo-self Ns-Hcy-Lys epitopes on proteins trigger an autoimmune response. The presence of IgM and IgG autoantibodies against N-Hcy-proteins in human blood [35; 36] suggest that Hcy incorporation into proteins triggers both innate and an adaptive immune response in humans.
Antigen Specificity of the Human Anti-W-Hcy-Proteins Autoantibodies
The anti-N-Hcy-protein IgG autoantibodies specifically recognize an Ns-Hcy-Lys epitope on N-homocysteinylated human proteins, such as N-Hcy-hemoglobin, N-Hcy-albumin, N-Hcy-transferrin, and N-Hcy-antitrypsin. The thiol group of N-linked Hcy is important for binding and proteins containing the Ns-Hcy-Lys epitope with its thiol blocked by the thiol reagent iodoacetamide are not bound by these autoantibaodies. Small molecules, such as Ns-Hcy-Lys, Ns-Hcy-Na-acetyl-Lys, and Ns-Hcy-Na-acetyl-LysAla are also bound by these autoantibodies, as demonstrated by their effective competition for autoantibody binding to antigen-coated microtiter plate wells [110]. High specificity of these autoantibodies is further demonstrated by our finding that Ns-acetyl-Na-Hcy-Lys, in which Hcy is attached to the a-amino group of lysine instead of the s-amino group, did not compete with the human IgG binding. Lysine, LysAla, Na-acetyl-Lys, Hcy or Hcy-thiolactone also did not compete with the human IgG binding. Taken together, these data suggest that human IgG specifically recognizes Ns-Hcy-Lys epitope on an Ns-Hcy-Lys-protein and that the antigen specificity of the human anti-N-Hcy-protein autoantibodies is essentially identical to the specificity of the rabbit anti-N-Hcy-protein antibodies generated by inoculations with N-Hcy-LDL or N-Hcy-KLH [110].
Anti-W-Hcy-Protein Autoantibodies are Associated with Stroke
Innate and adaptive immune responses directed against modified LDL are known to modulate the progression of vascular disease and increased plasma levels of markers of these responses are independent predictors of coronary events [6]. Although plasma levels of autoantibodies against oxidized or glycated LDL are often associated with vascular disease [12; 13], the role of anti-N-Hcy-protein auto-antibodies was unknown. In a case-control study [110], we examined the relation between anti-N-Hcy-protein auto-antibodies and stroke.
Our cohorts of 54 stroke patients (63.4 years old) and 74 healthy controls (66.3 years old) did not differ with respect to triglycerides, total cholesterol and LDL cholesterol levels, whereas HDL cholesterol was lower in stroke patients than in controls. We found significant differences in levels of anti-N-Hcy-protein IgG autoantibodies between the group of 39 male patients with stroke and the group of 29 healthy subjects. Male stroke patients had higher serum anti-N-Hcy-protein IgG levels than healthy controls [110]. Male stroke patients had also higher plasma tHcy than controls, consistent with earlier studies. Thus, both plasma tHcy and anti-N-Hcy-protein IgGs are associated with stroke in male subjects. Plasma levels of tHcy and anti-N-Hcy-protein IgG autoantibody in a group of 17 female stroke patients were similar to corresponding levels in a group of 45 female controls, suggesting that stroke in the female patients may have been caused by factors other than elevated Hcy or cholesterol. Furthermore, we found no differences in plasma cysteine or methionine concentrations between stroke patients and controls both for males and females. Thus, the high levels of anti-N-Hcy-protein autoantibodies in male stroke patients reflect high Hcy levels in these patients.
Anti-W-Hcy-Protein Autoantibodies are Associated with CAD
To test a concept that anti-N-Hcy-protein autoantibodies are an important feature of atherosclerosis, we examined the relation between anti-N-Hcy-protein autoantibodies and CAD in male subjects [111]. Our cohort of 88 male patients (45 years old) with angiographically documented CAD had significantly higher plasma levels of triglycerides, total cholesterol and LDL cholesterol, and lower levels of HDL cholesterol, compared to a cohort of 100 healthy male controls (43.5 years old). Significant differences in mean levels of anti-N-Hcy-protein IgG autoantibodies were found between a group of CAD male patients and a group of age-matched controls. Male CAD patients had 47 % higher serum levels of anti-N-Hcy-protein IgG autoantibodies than healthy controls. Levels of anti-N-Hcy-protein IgG were not associated with traditional risk factors. However, there was a weak positive correlation between the autoantibodies and plasma tHcy. Male CAD patients had also higher levels of plasma tHcy than controls, consistent with earlier studies. Thus, the higher levels of anti-N-Hcy-protein autoantibodies that are present in CAD male patients, like in stroke patients, reflect the higher levels of Hcy in these patients.
An age-adjusted risk for CAD related to seropositivity for anti-N-Hcy-protein IgG autoantibodies is 9.87 (95% CI 4.50-21.59, p<10-5). In multivariate logistic regression analysis, seropositivity to anti-N-Hcy-protein IgG autoantibodies (OR, 14.82; 95% CI, 4.47 to 49.19; p=0.00002), smoking (OR, 8.84; 95% CI, 2.46 to 31.72; p=0.001), hypertension (OR, 43.45; 95% I, 7.91 to 238.7; p=0.0001), and HDL cholesterol (OR, 0.015; 95% CI, 0.002 to 0.098; p=0.00002 for each unit increase) were independent predictors of early CAD in men <50 years old (%2=26.17, p<10-5 for the increment in goodness of fit as compared to a three variable model employing smoking, HDL cholesterol, and hypertension). These analyses show that elevated levels of anti-Ns-Hcy-Lys-protein autoantibodies significantly contribute to the risk of CAD in male patients.
Anti-W-Hcy-Protein Autoantibodies are Associated with Uremia
As discussed above, the levels of N-Hcy-protein are elevated in uremic patients on hemodialysis [86; 87]. These finding suggests that an autoimmune response against N-Hcy-protein might also be enhanced in these patients. This possibility was examined in a group of 43 patients (58.8 years old) who were on maintenance hemodialysis for an average of 50 months and an age and sex matched group of 31 apparently healthy individuals [113]. Significantly higher levels of anti-N-Hcy-protein IgG autoantibodies were found in the hemodiallysis patients, compared with controls. Like in our previous studies [110], the levels of anti-N-Hcy-protein IgG autoantibodies were strongly correlated with plasma total Hcy, both in hemodialysis patients and in controls. Among the hemodialysis patients, a subgroup of survivors of myocardial infarction (n=14) had significantly higher levels of anti-N-Hcy-protein IgG autoantibodies than a subgroup of hemodialysis patients without a history of CAD (n=29) [113]. Taken together, these data suggest that an autoimmune response against N-Hcy-proteins contributes to the development of CAD in hemodialysis patients.
Hyperhomocysteinemia, W-Hcy-Protein, and an Innate Immune Response
We also found that the levels of anti-N-Hcy-protein autoantibodies are weakly, but significantly, correlated with plasma CRP levels (r=0.24, p=0.002) [111]. This finding suggests that N-Hcy-protein can also elicit an innate immune response. Many investigators, but not all [114; 115; 116], have linked Hcy to immune responses. For example, a weak, but significant, association between plasma total Hcy and CRP was observed in the Framingham Heart Study [117] and in the Physician’s Health Study [118]. Holven et al. reported that in humans hyperhomocysteinemia is associated with increased levels of both CRP and interleukin-6 [119]. A similar positive association between Hcy and interleukin-6 was reported in patients with diabetic nephropathy [120]. Importantly, in the Holven et al. study, elevated level of interleukin-6 is observed in hyperhomocysteinemic individuals in the absence of hypercholesterolemia. Plasma total Hcy was positively associated with soluble tumor necrosis factor receptor in the Nurses’ Health Study [121]. A positive correlation is observed between plasma tHcy and neopterin (a marker of Th1 type immune response) in Parkinson’s disease patients [122]. Elevated Hcy is associated with elevated monocyte chemotactic protein-1 and increased expression of vascular adhesion molecules in humans [123; 124] and rats [125; 126; 127; 128]. Plasma Hcy is a determinant of TNF-a in hypertensive patients [129]. Furthermore, in mice dietary hyperhomocysteinemia is known to trigger atherosclerosis and enhance vascular inflammation, manifested by increased activation of NF-kB in the aorta and kidney, enhanced expression of VCAM-1 and RAGE in the aorta and TNF-a in plasma [14].
How Hcy can trigger these innate inflammatory responses is unknown, However, given that hyperhomocysteinemia causes elevation of Hcy-thiolactone and N-Hcy-protein levels in humans and mice [49], these responses are likely to be caused by N-Hcy-protein, particularly by N-Hcy-LDL. Consistent with this suggestion are the observations that N-Hcy-LDL is highly immunogenic [109], is present in human blood [61], and is taken up by macrophages faster than unmodified LDL [92]. Further studies are needed to elucidate the mechanism of Hcy-induced innate immune responses.
Possible Roles of Anti-W-Hcy-Protein Autoantibodies in Atherosclerosis
Our findings that anti-N-Hcy-protein autoantibodies are elevated in stroke and CAD patients suggest that an autoimmune response against N-Hcy-proteins is an important feature of atherosclerosis [35]. In general, antibodies protect against exogenous pathogens and endogenous altered neo-self molecules to maintain homeostasis by neutralization and clearance. Like autoantibodies against oxidatively modified LDL [7], the anti-N-Hcy-protein autoantibodies can be beneficial or deleterious. For example, the clearing of N-Hcy-protein proteins from circulation by the autoantibodies would be beneficial. On the other hand, binding of the anti-N-Hcy-protein autoantibodies to N-Hcy-proteins [35; 89] in tissues may contribute to the deleterious effects of hyperhomocysteinemia on many organs [16; 17; 18]. For instance, if the neo-self Ns-Hcy-Lys epitopes were present on endothelial cell membrane proteins, anti-N-Hcy-protein autoantibodies would form antigen-antibody complexes on the surface of the vascular wall. Endothelial cells coated with anti-N-Hcy-protein autoantibodies would be taken up by the macrophage via the Fc receptor, resulting in injury to the vascular surface. Under chronic exposures to excess Hcy, the neo-self epitopes Ns-Hcy-Lys, which initiate the injury, are formed continuously, and the repeating attempts to repair the damaged vascular wall would lead to an atherosclerotic lesion [35; 36].
Hcy-Lowering Therapy and Anti-W-Hcy-Protein Autoantibodies
If anti-N-Hcy-protein autoantibodies reflect plasma tHcy levels and arise through the mechanisms postulated by the Hcy-thiolactone hypothesis [36], then lowering plasma tHcy by folic acid supplementation should also lower plasma levels of anti-N-Hcy-protein autoantibodies. This prediction was tested in groups of hyperhomocysteinemic (plasma tHcy>15 ^M) male patients (n=12) with angiographically documented CAD and healthy men (n=20) [112]. At baseline, the two groups did not differ with respect to age, tHcy, folate, lipid profile, and CRP. As in our two previous studies [110; 111], the baseline levels of anti-N-Hcy-protein autoantibodies were significantly higher in CAD patients than in healthy subjects and plasma tHcy was positively correlated with anti-N-Hcy-protein autoantibodies in both groups (r=0.77 to 0.85, p<0.0001 to 0.002) [112]. Furthermore, folate levels measured prior to folic acid supplementation correlated negatively with anti-N-Hcy-protein autoantibodies in healthy subjects (r=-0.58, p0.008) and in CAD patients (r=-0.9, p<0.0001).
Folic acid supplementation for 3 months or 6 months resulted in significant lowering of plasma tHcy (by 30%) and increased plasma folate levels (by 230%) in our CAD patients and controls, consistent with other Hcy-lowering studies [27; 28; 29; 30]. In healthy subjects, plasma levels of anti-N-Hcy-protein autoantibodies fell significantly (p<0.001) following 3 months (by 38%), and remained at a lower level at 6 months (by 48%), of folic acid supplementation. However, in CAD patients, surprisingly, plasma levels of anti-N-Hcy-protein autoantibodies fell by only 8.5-12% at 3 or 6 months of folic acid supplementation, but this effect was not significant [112]. The effects of Hcy-lowering therapy on anti-N-Hcy-protein autoantibodies suggest that the neo-self N-Hcy-protein antigens respond relatively quickly to changes in Hcy levels and can be cleared in healthy subjects. In contrast, the neo- self N-Hcy-protein antigens appear to persist in CAD patients and not to respond to Hcy lowering therapy. Interestingly, in another study the levels of anti-N-Hcy-protein IgG autoantibodies were found to be similar in groups of uremic patients on hemodialysis who were taking (n=37) or not taking (n=6) folic acid supplementation [113]. These findings suggest that the immune activation caused by protein N-homocysteinylation in uremia and in CAD patients cannot be easily reversed.
Taken together, the effects of Hcy-lowering therapy on anti-N-Hcy-protein autoantibodies support the involvement of Hcy in the synthesis of these autoantibodies according to a mechanism postulated by the Hcy-thiolactone hypothesis [36] (Figure 5).
Furthermore, our findings that lowering plasma Hcy by folic acid supplementation lowers anti-N-Hcy-protein autoantibodies in control subjects, but not in patients with CAD, support the involvement of an autoimmune response in CAD [112]. These findings also suggest that, while primary Hcy-lowering intervention by vitamin supplementation is beneficial, secondary intervention may be ineffective, and may explain at least in part the failure of vitamin therapy to lower cardiovascular events in MI patients [29; 30].
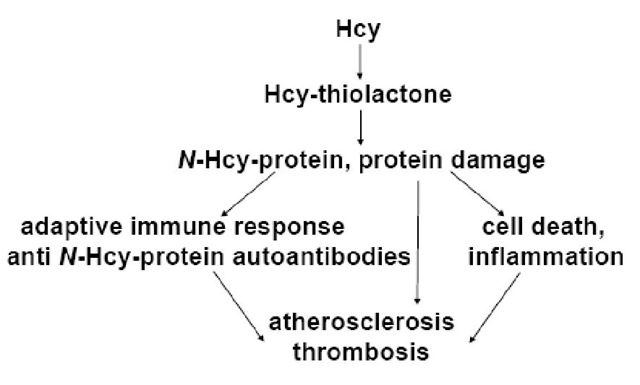
Figure 5. Hcy-thiolactone-mediated incorporation of Hcy into proteins leads to the induction of anti-N-Hcy-protein autoantibodies and is associated with atherosclerosis and thrombosis in humans.
Conclusion
Accumulating evidence suggests that elevated Hcy contributes to adaptive and innate immune responses in atherosclerosis in humans and experimental animals. In this topic, I have discussed the evidence supporting a concept that the incorporation of Hcy into protein via isopetide linkages, causes alterations in the protein’s structure and the formation of neo-self antigens that elicit anti-N-Hcy-protein autoantibodies, and emphasized their potential importance in vascular disease (Figure 5). Of many known natural Hcy metabolites, only the thioester Hcy-thiolactone can mediate the incorporation of Hcy into proteins via stable isopeptide bonds. Protein N-homocysteinylation creates altered proteins with newly acquired interactions, including immunogenic properties. Elevated levels of Hcy-thiolactone and protein N-linked Hcy are observed in genetic and dietary hyperhomocysteinemia in humans and mice. Levels of protein N-linked Hcy are also elevated in CAD patients. Protein N-homocysteinylation leads to the formation of neo-self protein N-linked Hcy epitopes, which cause an immune response in humans, manifested by the induction of anti-N-Hcy-protein autoantibodies. Levels of these autoantibodies correlate with plasma total Hcy, are elevated in stroke and CAD patients, and thus may play an important role in atherosclerosis. Primary Hcy-lowering vitamin therapy lowers the levels of anti-N-Hcy-protein autoantibodies in healthy subjects. In contrast, secondary vitamin intervention appears to be ineffective in reducing an autoimmune response: it lowers plasma tHcy, but not anti-N-Hcy-protein autoantibodies in CAD patients. These results support the Hcy-thiolactone hypothesis, which states that the metabolic conversion of Hcy to Hcy-thiolactone followed by the non-enzymatic protein modification by Hcy-thiolactone is an underlying mechanism that contributes to the pathophysiology of hyperhomocysteinemia. We are only beginning to understand pathophysiological consequences of N-Hcy-protein accumulation. Along with other aspects of protein N-homocysteinylation, identifying anti-N-Hcy-protein autoantibodies, and understanding their roles in health and disease are likely to yield an understanding of the basic mechanisms that evolved to deal with the consequences of Hcy-thiolactone formation.