Hcy-Thiolactone is Synthesized by Methionyl-tRNA Synthetase in Human Cells
As discussed above, the biosynthesis of Hcy-thiolactone via the Hcy editing pathway has been originally discovered in microorganisms, such as Escherichia coli [73] and the yeast Saccharomyces cerevisiae [52]. The first indication that Hcy-thiolactone is a significant component of Hcy metabolism in mammals, including humans, came with the discovery that Hcy-thiolactone is synthesized by cultured mammalian cells, such as human cervical carcinoma (HeLa), mouse adenocarcinoma (RAG), and Chinese hamster ovary (CHO) [55]. We also demonstrated that a temperature-sensitive MetRS mutant of CHO cells fails to synthesize Hcy-thiolactone at the non-permissive temperature, which indicates that MetRS is involved in Hcy-thiolactone formation in CHO cells [55].
Subsequent work has shown that human diploid fibroblasts in which Hcy metabolism has been deregulated by mutations in the CBS gene produced more Hcy-thiolactone than wild type fibroblasts [50]. Furthermore, supplementation of CBS-deficient and wild type human fibroblasts, and human breast cancer (HTB-132) cells with the anti-folate drug aminopterin, which prevents remethylation of Hcy to methionine by methionine synthase, greatly enhances Hcy-thiolactone synthesis. In general, human cancer cells produce more Hcy-thiolactone than normal cells [50; 55; 69].
Further experiments with cultured human umbilical vein vascular endothelial cells (HUVEC) suggest that Hcy-thiolactone synthesis is important in human vascular tissues [51]. These experiments have shown that in the presence of physiological concentrations of Hcy, methionine, and folic acid, HUVEC efficiently metabolize Hcy to Hcy-thiolactone. The extent of Hcy-thiolactone synthesis in human endothelial cells is directly proportional to Hcy, and inversely proportional to methionine, concentrations, consistent with the involvement of MetRS.
Figure 4. Editing in trans: The formation of methionyl thioesters catalyzed by MetRS.
Although folates are utilized in Hcy metabolism and DNA synthesis, it appears that folic acid limitation predominantly impacts Hcy metabolism, but not DNA metabolism, in endothelial cells. For example, physiological levels of folic acid (26 nM) present in the M199 media used in our studies are insufficient for transmethylation of Hcy to methionine and, as a result, Hcy is mostly converted to Hcy-thiolactone, while very little methionine is synthesized in these cells [51]. However, these levels of folic acid support endothelial cells growth when methionine is also present, which means that they are sufficient for DNA synthesis. Supplementation of endothelial cell cultures with folic acid redirects Hcy to the transmethylation pathway, which results in lower synthesis of Hcy-thiolactone and greater synthesis of methionine. The synthesis of Hcy-thiolactone in endothelial cell cultures is also inhibited by the supplementation with high-density lipoprotein (HDL) [51], which carries PON1 protein exhibiting Hcy-thiolactone hydrolyzing activity [57; 58; 59; 60].
Hcy-Thiolactone is Elevated in Hyperhomocysteinemic Humans and Mice
The findings that cultured human cells, including vascular endothelial cells, have the ability to metabolize Hcy to Hcy-thiolactone suggest that Hcy-thiolactone is likely to be synthesized in vivo in humans and animals. With the recent developments of highly selective and sensitive HPLC-based assays [53; 67], the demonstration of the in vivo relevance of Hcy-thiolactone became possible. In particular, the Hcy-thiolactone hypothesis [36] predicts that
Hcy-thiolactone will be elevated under conditions predisposing to vascular disease, such as hyperhomocysteinemia. As described in the following sections, this prediction has recently been confirmed in vivo in humans and mice.
Human Genetic Hyperhomocysteinemia
It is well established that genetic deficiencies in the CBS or MTHFR gene lead to great elevation of plasma tHcy levels in humans and mice [16]. However, it was not known whether these genetic deficiencies affect Hcy-thiolactone levels. To answer this question we studied 14 patients with homocystinuria due to homozygous mutations in the CBS gene, 4 patients with hyperhomocysteinemia due to a homozygous mutation in the MTHFR gene, 6 unaffected siblings heterozygous for the MTHFR mutation, and 9 healthy unrelated subjects. We found that the CBS deficiency in humans leads to elevation of Hcy-thiolactone levels: mean plasma Hcy-thiolactone concentration in CBS deficient patients (14.4 nM) was 72-fold higher than in normal subjects [49]. This finding is consistent with my previous ex vivo observations that cultured human CBS-deficient fibroblasts synthesize more Hcy-thiolactone than normal fibroblasts [50].
We also found that 5-methyltetrahydofolate deficiency, caused by the MTHFR mutation leads to elevation of Hcy-thiolactone levels in humans: plasma Hcy-thiolactone in MTHFR-deficient patients (11.8 nM) was 24- or 59-fold higher than in MTHFR heterozygous or normal individuals, respectively [49]. This in vivo finding is consistent with our previous ex vivo observations that limiting availability of folic acid greatly enhances Hcy-thiolactone synthesis in human fibroblasts [50] and vascular endothelial cells [51]. It should be noted that, because MTHFR-deficient patients, like CBS-deficient patients, were on Hcy-lowering therapy, their Hcy-thiolactone concentrations represent minimal values. In one patient for whom samples were obtained before therapy, the therapy resulted in lowering plasma Hcy-thiolactone from 47.3 nM to 16.6 nM (tHcy was lowered from 208 ^M before therapy to 66.2 ^M after therapy) [49].
Mouse Dietary Hyperhomocysteinemia
Feeding a high methionine diet over extended periods of time is often used as a useful model of experimental hyperhomocysteinemia and atherosclerosis [14; 25; 26]. We found that plasma and urinary Hcy-thiolactone levels in mice fed a normal diet have a mean value of 3.7 nM and 140 nM, respectively [49]. We also found that a high methionine diet causes 3.7-fold and 25-fold increases in plasma and urinary Hcy-thiolactone, respectively, in mice. The distributions of Hcy-thiolactone between plasma and urine in mice fed a normal diet and humans are similar: much higher Hcy-thiolactone concentrations accumulate in urine than in plasma (urinary/plasma Hcy-thiolactone is 37 in mice [49] and 100 in humans [54]). This shows that urinary clearances of Hcy-thiolactone in mice and humans are similar, and that in mice, like in humans [54], >95% of the filtered Hcy-thiolactone is excreted in the urine. Furthermore, significantly higher urinary/plasma Hcy-thiolactone ratios are found in mice fed hyperhomocysteinemic diets than in the animals fed a normal diet. This finding suggests that urinary clearance of Hcy-thiolactone is much more efficient in hyperhomocysteinemic mice, compared to animals with normal tHcy levels.
Protein Lysine Residues are Targets for the Modification by Hcy-Thiolactone
Hcy-thiolactone is a novel Hcy metabolite, discovered in living organisms in the 1990′s. Thus, although its propensity to react with primary amino groups has been recognized shortly after its chemical synthesis in the 1930′s, the reactions of Hcy-thiolactone with proteins remained virtually unexplored, until the end of 1990′s [46].
The discovery that Hcy-thiolactone and proteins containing N-linked Hcy (W-Hcy-protein) are formed by cultured mammalian, including human, cells has led to a hypothesis that the chemical reactivity of Hcy-thiolactone may underlie the involvement of Hcy in the pathology of human vascular disease [50]. This in turn prompted detailed studies of the reactions of Hcy-thiolactone with proteins [35; 36; 38; 46; 50; 70; 71; 82].
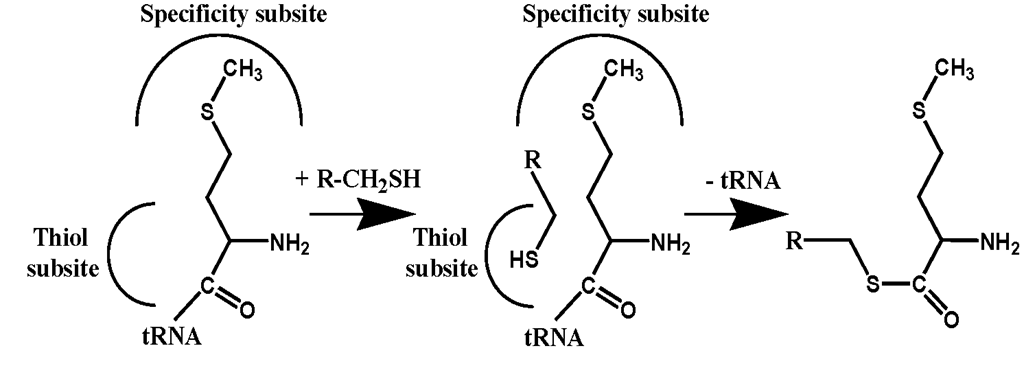
Initial studies have established that [35S]Hcy-thiolactone added to human or animal serum disappears with a half-life of is from 0.25-1.5 hours, depending on the source of serum. I found that the disappearance of Hcy-thiolactone in serum is due to two major reactions: the formation of an #-Hcy-protein adduct, in which Hcy is attached via an isopeptide bond to the s-amino group of a protein lysine residue (Equation 2) [38; 50], and enzymatic hydrolysis by serum paraoxonase/Hcy-thiolactonase to Hcy, which then forms a mixed protein-S-S-Hcy disulfide, mostly with the Cys34 of serum albumin (Figure 1) [38; 57; 58; 71; 82]. In the presence of [35S]Hcy-thiolactone, each individual human or rabbit serum protein becomes #-homocysteinylated in proportion to its abundance in serum [38].
Hcy-thiolactone has a propensity to modify amino groups of free amino acids, albeit less efficiently than free lysine [50]. However, only the side chain amino groups of lysine residues in proteins, but not any other amino acid residues, are modified by Hcy-thioalctone [38; 71; 82]. In particular, Hcy-thiolactone does not appreciably react with the side chains of arginine, histidine, serine, or thereonine. Moreover, the N-terminal a-amino group in human serum albumin, hemoglobin, cytochrome c, or fibrinogen does not appear to react with Hcy-thiolactone. Using proteomic approaches only internal lysine residues were identified as targets for Hcy-thiolactone modification [42; 83; 84].
Second order rate constants for reactions of Hcy-thiolactone with individual purified proteins indicate that #-homocysteinylation is relatively robust and goes to completion within a few hours at physiological conditions of pH and temperature. A major determinant of the reactivity of most proteins with Hcy-thiolactone is their lysine content. For proteins that vary in size from 104 to 698 amino acid residues there is a very good correlation (r = 0.97) between protein’s lysine content and its reactivity with Hcy-thiolactone. Larger proteins, such as fibrinogen (3588 amino acid residues) and low-density lipoproteins (LDL) (~5,000 amino acid residues), react with Hcy-thiolactone ~6-fold less efficiently than expected from their lysine contents. Of many lysine residues present in a protein only a few are predominant sites for the modification by Hcy-thiolactone, as has been shown for albumin [83], hemoglobin (R. Glowacki, H. Jakubowski, unpublished data), fibrinogen [42], and cytochrome c [84].
Protein W-Linked Hcy is a By-Product of Human Hcy Metabolism
Evidence from Tissue Culture Studies
The first indication that protein N-linked Hcy is likely to be an important component of Hcy metabolism in humans came from studies of Hcy-thiolactone metabolism in human tissue cultures [50]. Proteins from normal and CBS-deficient fibroblasts and breast cancer cells have been shown to contain small amounts of protein N-linked Hcy (0.4 to 2.4% relative to protein methionine). When metabolic conversion of Hcy to methionine was inhibited by the anti-folate drug aminopterin, the amounts of Hcy, Hcy-thiolactone, and protein N-linked Hcy increased [50].
Further experiments with cultured human umbilical vein endothelial cells provide evidence that the formation protein N-linked Hcy is likely to be important in human vascular tissues [51]. These experiments show that the formation of protein N-linked Hcy occurs concomitantly with the synthesis of Hcy-thiolactone in the presence of physiological concentrations of Hcy, methionine, and folic acid. Like the levels of Hcy-thiolactone, levels of protein N-linked Hcy are directly proportional to Hcy, and inversely proportional to methionine concentrations. Supplementation of endothelial cell cultures with folic acid inhibits the synthesis of extracellular and intracellular protein N-linked Hcy by facilitating the conversion of Hcy to methionine, thereby indirectly preventing synthesis of Hcy-thiolactone by methionyl-tRNA synthetase. The formation of extracellular, but not intracellular, protein N-linked Hcy in endothelial cell cultures is inhibited by supplementation with HDL [51], which carries an Hcy-thiolactone-hydrolyzing enzyme, paraoxonase 1 [57; 58; 59; 60].
The mode of Hcy incorporation into endothelial cell protein has been established by using Edman degradation, a classic protein chemistry procedure which releases from proteins amino acids having free a-amino group. About half of total Hcy incorporated into protein was found to be sensitive to Edman degradation [45; 51], suggesting that Hcy incorporation is due to reactions of Hcy-thiolactone with protein lysine residues (Equation 2) [38; 50]. The presence of a fraction of N-Hcy-protein that is resistant to Edman degradation suggests that translational, S-nitroso–Hcy-mediated, incorporation of Hcy into protein [65] also occurs in endothelial cell cultures.
Protein N-Linked Hcy is Present in Humans
To examine a possibility that N-Hcy-protein is relevant in vivo in the human body, I have developed a highly selective and sensitive HPLC-based methods for the determination of protein N-linked Hcy [61] 61a]. The initial sample workup removes free and disulfide-linked Hcy by extensive treatments with the reducing agent dithiothreitol. The method is based on a quantitative conversion of protein N-linked Hcy to Hcy-thiolactone, which is achieved by acid hydrolysis under reducing conditions (in the presence of dithiothreitol). Hcy-thiolactone is then purified and quantified by HPLC on a cation exchange column with multi-wavelength diode array UV detection, including A240 [61] or fluorescence detection after post-column derivatization with orthophtaldialdehyde [61a].
That protein N-linked Hcy is present in human plasma proteins was first described in 2000 [82]. Subsequent studies have shown that protein N-linked Hcy is present in serum albumin purified from various organisms, including human. Protein N-linked Hcy occurs in all purified individual human blood proteins examined so far [61]. The highest amounts of protein N-linked Hcy, 50 mol%, are present in human and equine ferritins [61a]. In human blood, 0.36-0.6 mol% of protein N-linked Hcy is present in human hemoglobin, serum albumin, and y-globulins, respectively. Other serum proteins, such as fibrinogen, LDL, HDL, transferrin, and antitrypsin contain from 0.04 to 0.1 % of protein N-linked Hcy. #-Hcy-hemoglobin, present in normal blood at a concentration of 12.7 ^M, constitutes a major Hcy pool in the human blood [61]. Interestingly, rodents have more N-linked Hcy in their blood proteins that humans [61a].
Although the levels of protein N-linked Hcy in individual human blood proteins correlate with the reactivity of these proteins toward Hcy-thiolactone [61], protein N-linked Hcy may also arise by S-nitroso-Hcy-mediated translational mechanism, in which Hcy substitutes a protein methionine residue [45]. However, the presence of protein N-linked Hcy in pig albumin [61], which does not contain methionine, strongly suggests that Hcy-thiolactone-mediated mechanism is responsible for Hcy incorporation.
Protein N-Linked Hcy is Elevated in Hyperhomocysteinemia and is Associated with Coronary Artery Disease (CAD) in Humans
The Hcy-thiolactone hypothesis [36] predicts that protein N-homocysteinylation will be elevated under conditions conducive to atherosclerosis, such as hyperhomocysteinemia. The verification of this prediction became possible with the development of sensitive chemical [61] and immunological assays [85] for protein N-linked Hcy in humans. Indeed, as predicted by the Hcy-thiolactone hypothesis, protein N-linked Hcy is elevated in subjects with genetic hyperhomocysteinemia [45; 61; 71; 82].
I found that human plasma contains from 0.1 to 13 ^M protein N-linked Hcy, which represents up to 25% of plasma total Hcy [61]. Plasma concentrations of protein N-linked Hcy correlate positively with tHcy, suggesting that plasma tHcy level is a determinant of protein N-linked Hcy level. Interestingly, in some subjects, plasma levels of protein N-linked Hcy are lower than expected from their tHcy content; this suggests that factors other than tHcy can affect plasma protein N-linked Hcy levels [61]. A likely candidate for a determinant of plasma protein N-linked Hcy levels, is Hcy-thiolactonase activity [57; 59; 60], which has been shown to affect the formation of protein N-linked Hcy in HUVEC cultures [51] and in human serum in vitro [58].
We found that plasma protein N-linked Hcy levels are significantly elevated in CBS- or MTHFR-deficient patients and that CBS-deficient patients have significantly elevated levels of pro-thrombotic N-Hcy-fibrinogen [130]. These findings provide an explanation for increased atherothrombosis observed in CBS-deficient patients. Furthermore, plasma protein N-linked Hcy is elevated 10-fold in mice fed a pro-atherogenic high-methionine diet [131]. Inactivation of Cbs, Mthfr, or the proton coupled folate transporter (Pcft) gene in mice results in 19- to 30-fold increase in plasma protein N-linked Hcy levels [131]. These finding provide evidence that protein N-linked Hcy is an important metabolite associated with Hcy pathophysiology in humans and mice.
Other investigators have studied protein N-homocysteinylation in uremic patients [86; 87] to explain a link between hyperhomocysteinemia and higher cardiovascular risk and mortality observed in these patients [88]. Significantly higher protein N-linked Hcy levels were found in hyperhomocysteinemic uremic patients on hemodialysis than in control subjects [86; 87]. Interestingly, protein N-linked Hcy comprises less tHcy in hemodialysis patients than in control subjects [86; 87]. Similarly, protein N-linked Hcy comprises less tHcy in patients with higher plasma tHcy (50-120 ^M) than in patients with lower plasma tHcy (5-40 ^M) [61]. The lower protein N-linked Hcy/tHcy ratios suggest that the Hcy-thiolactone clearance is more effective at higher tHcy levels. This suggestion is supported by a finding that in mice fed a hyperhomocysteinemic high Met or Hcy diet urinary/plasma Hcy-thiolactone is 7-fold or 4-fold higher, respectively, compared to mice fed a normal diet [49].
Hyperhomocysteinemia in CAD patients is linked with increased mortality in these patients [23]. In one clinical study which examined a relationship between Hcy and coronary heart disease, plasma protein N-linked Hcy levels, like tHcy levels, were significantly higher in coronary heart disease patients than in controls [85]. Furthermore, there was a weak but significant positive correlation between protein N-linked Hcy level and the number of diseased coronary arteries: the higher protein N-linked Hcy level the greater the number of afflicted arteries.
Using polyclonal rabbit anti-N-Hcy-protein IgG antibodies [89], we have demonstrated that N-Hcy-protein is present in human cardiac tissues [90]. For example, we observed positive immunohistochemical staining of myocardium and aorta samples from cardiac surgery patients. Control experiments have demonstrated that the staining was specific for N-Hcy-protein. No immunostaining was observed with rabbit preimmune IgG, with iodoacetamide-treated tissues (which destroys the Ne-Hcy-Lys epitope), or with the antibody pre-adsorbed with N-Hcy-albumin [90]. Further support for a role of N-Hcy-protein in atherogenesis is provided by our finding of increased immunohistochemical staining for N-Hcy-protein in aortic lesions from ApoE-/- mice with hyperhomocysteinemia induced by a high methionine diet, relative to the mice fed a control chow diet [90].
Modification by Hcy-Thiolactone Causes Protein Damage
In proteins that were studied thus far, usually a few lysine residues are predominant targets for the modification by Hcy-thiolactone. For example, Lys525 [83] is a predominant site of albumin N-homocysteinylation in vitro and in vivo. Four lysine residues of cytochrome c (Lys8 or 13, Lys 86 or 87, Lys 99, and Lys 100) are susceptible to N-homocysteinylation [84]. Twelve lysine residues of fibrinogen (7 in Aa chain, 2 in Bp chain and 3 in y chain) were found to be susceptible to the modification by Hcy-thiolactone [42]. Four lysine residues (Lys16, Lys56 in a chain and Lys59, Lys95 in p chain) are predominant sites of N-homocysteinylation in hemoglobin (H. Jakubowski, R. Glowacki, unpublished data).
The acylation of a basic s-amino group of a protein lysine residue (pK=10.5) by Hcy-thiolactone generates an Ns-Hcy-Lys residue containing a much less basic amino group (pK~7) and a free thiol group (Eq. 1). This substitution is expected to significantly alter protein structure and function. Indeed, hemoglobin, albumin [83], and cytochrome c [38] are sensitive to N-homocysteinylation; incorporation of one Hcy/mol protein induces gross structural alterations in these proteins. For instance, N-Hcy-cytochrome c becomes resistant to proteolytic degradation (by trypsin, chymotrypsin, and pronase) [84] and susceptible to aggregation due to intermolecular disulfide bond formation [38], which also interferes with the red-ox state of the heme iron by rendering it reduced [84]. N-Hcy-hemoglobin, in contrast to unmodified hemoglobin, is susceptible to further irreversible damage by oxidation. Of the two physiological forms of human albumin, albumin-Cys34-S-S-Cys (containing cysteine in a disulfide linkage with Cys34 of albumin) is modified by Hcy-thiolactone faster than mercaptoalbumin (containing a free thiol at Cys34). Hcy-thiolactone-modified and unmodified forms of albumin exhibit different susceptibilities to proteolytic degradation by trypsin, chymotrypsin, or elastase [83].
Other proteins are inactivated only by incorporation of multiple Hcy residues. For example, complete loss of enzymatic activity occurs after N-homocysteinylation of eight lysine residues in MetRS (33% of total lysine residues) or eleven lysine residues in trypsin (88% of total lysine residues) [38]. Furthermore, extensively N-homocysteinylated proteins, such as fibrinogen, transferin, globulins, myoglobin, RNase A, and trypsin are prone to multimerization and undergo gross structural changes that lead to their denaturation and precipitation [38]. Chicken egg lysozyme is also denatured by extensive N-homocysteinylation [91].
N-Hcy-LDL, in which 10% or 25% lysine residues have been modified (i. e., containing 36 and 89 mol Hcy/mol LDL), is taken up and degraded by human monocyte-derived macrophages significantly faster than native LDL [92]. However, N-Hcy-LDL containing eight molecules of Hcy/mol LDL is taken up and degraded by leukemic L2C guinea pig lymphocytes to the same extent as native LDL via the high affinity LDL-specific receptor pathway [93].
Hcy-thiolactone may also inactivate enzymes by other mechanisms. For example, lysine oxidase, an important enzyme responsible for post-translational collagen modification essential for the biogenesis of connective tissue matrices, is inactivated by micromolar concentrations of Hcy-thiolactone, which derivatizes the active site tyrosinequinone cofactor with a half-life of 4 min [94].