Insulin Resistance
Cellular insulin resistance may presage frank diabetes by a decade or more and requires compensatory increases in plasma insulin levels to maintain glucose homeostasis in the face of impaired cellular insulin action, principally in skeletal muscle and liver [46]. Systemic hyperinsulinemia may accentuate cellular insulin action in insulin responsive tissues, such as the myocardium, that do not manifest cellular insulin resistance. In this regard, the mitogenic actions of insulin on myocardium during chronic systemic hyperinsulinemia bear directly the commonly observed finding of cardiac hypertrophy in diabetic cardiomyopathy [18].
Myocardial changes seen in insulin-resistant individuals could be caused by the impaired ATP synthesis noted in these patients, despite reduced oxygen delivery or increased workload. Insulin resistance results in decreased myocardial glucose uptake and oxidation, increased fatty acid oxidation, and altered myocyte gene expression [38].The slow rate of glucose transport across the sarcolemma into the myocardium restricts the glucose usage in the hearts of patients with insulin resistance [38,47]. Excessive myocardial fatty acid uptake could enhance insulin resistance, promote cell dysfunction and trigger myocyte apoptosis, resulting in myocardial dysfunction [48].
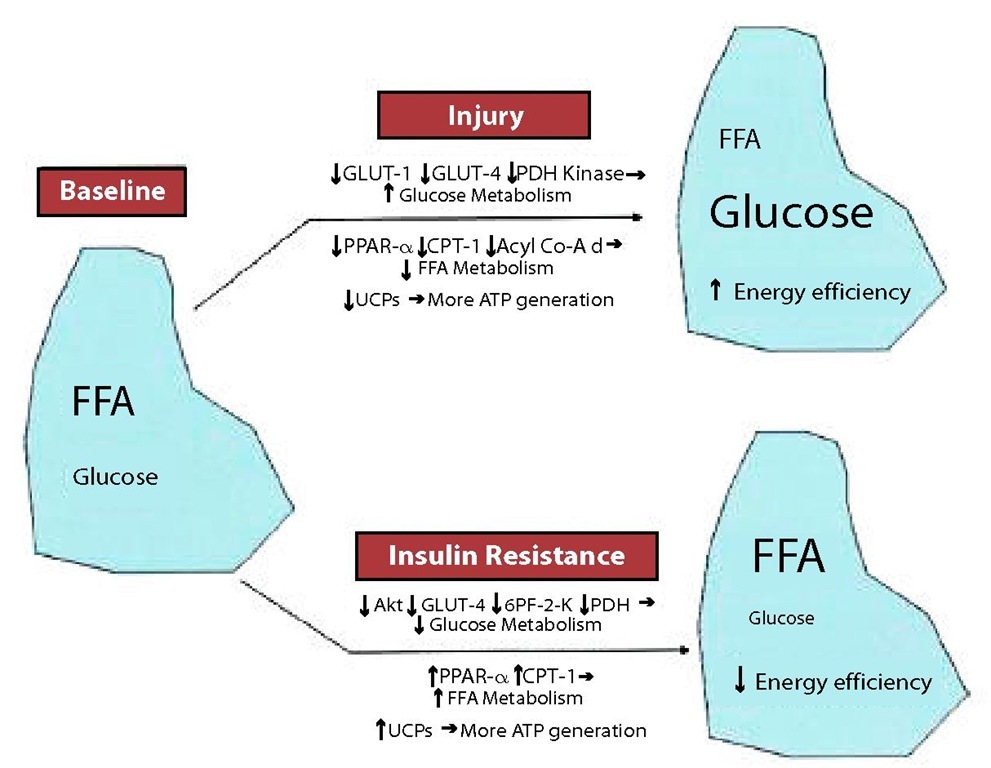
The normal adaptive response by an injured/failing heart involves a complex series of enzymatic shifts and up-/downregulation of transcription factors, ultimately resulting in increased glucose metabolism and decreased FFA metabolism to maximize efficiency [25,49,50]. In contrast, FFA metabolism is decreased, with decreased expression of the peroxisome proliferator-activated receptor (PPAR)-/retinoid X receptor complex and 2 enzymes critical to FFA metabolism, carnitine palmitoyl transferase-1 and medium-chain acyl-coenzyme A dehydrogenase [50,51,52]. These adaptive responses of the heart are inhibited in the setting of insulin resistance (Fig. 3). Although the initial myocardial metabolic switch in heart failure is down-regulation of FFA metabolism, the opposite occurs (up-regulation of FFA metabolism) in the setting of insulin resistance [53,54]. This increased reliance on FFA metabolism leads to increased oxygen consumption, decreased cardiac efficiency, and the potential for lipotoxicity [55,56]. Insulin resistance at its most fundamental level inhibits uptake and metabolism of glucose. It is likely this effect— preventing the heart from using its adaptive energy response to an insult—which contributes to heart failure and the vicious cycle of neurohormonal activation, serving to potentiate the myocardial dysfunction and further increasing energy requirements [25,51,57,58].
Importantly, cardiac dysfunction precedes the development of systemic hyperglycemia, implying that the altered cellular metabolism rather than systemic hyperglycemia is responsible for the cardiac dysfunction [59]. Treatment of insulin resistance in these models (with troglitazone, metformin, or exercise) prevents myocardial dysfunction, but therapy aimed at hyperglycemia itself without treating insulin resistance (sulfonylureas) showed no effect [60,61]. The prognostic impact of insulin resistance is independent of other variables, including peak oxygen consumption (VO2max) and left ventricular ejection fraction (LVEF), implying that insulin resistance is pathogenic rather than simply a marker for worsened heart failure [25,62].
Figure 3. MYOCARDIAL ENERGY METABOLISM IN RESPONSE TO INJURY AND INSULIN RESISTANCE. FFA: free fatty acids; Acyl Co-A d: medium-chain acyl-coenzyme A dehydrogenase; CPT: carnitine palmitoyl transferase; GLUT: glucose transporter; PDH: pyruvate dehydrogenase; PPAR: peroxisome proliferator-activated receptor; UCP: uncoupling protein; 6PF-2K: 6-phosphofructo-2-kinase.
Hyperglycemia
The mechanism whereby hyperglycemia mediates tissue injury through the generation of reactive oxygen species has been elucidated largely through the work of the Brownlee and colleagues. Hyperglycemia leads to increased glucose oxidation and mitochondrial generation of superoxide [18]. Taken together, these data provide mechanistic evidence linking hyperglycemia to altered expression and function of both the ryanodine receptor (RyR) and sarco(endo)plasmic reticulum Ca2-ATPase (SERCA2) that may contribute to decreased systolic and diastolic function. Hyperglycemia-induced oxidative stress also activates poly(ADP-ribose) polymerase-1 (PARP) [63]. The activation of the PARP regulates several cellular reactions like repair of DNA, gene expression and cellular overlife. The effects of PARP include increase in the formation of advanced glycosilation end products (AGE’s), through the diversion of the route of degradation of the glucose. Meantime, the excessive activation of the PARP can begin several cellular processes and cause cellular damage. In addition, hyperglycemia contributes to altered cardiac structure through posttranslational modification of the extracellular matrix [18]. The response to hypoglycemic therapy further confirms the correlation of myocardial functional and structural changes with glycemic control. Taken together, hyperglycemia, through multiple pathways, causes cardiac cellular and functional changes, possibly contributing to the development of cardiomyopathy [26].
Figure 4. EFFECTS OF HYPERGLICEMIA ON THE DIABETIC CARDIOMYOPATHY. RAAS: renin-angiotensin-aldosterone system.
Abnormalities in the Regulation of Calcium Homeostasis
There are changes in the level of the cardiomyocyte, which are not solely attributable to impaired coronary blood flow or interstitial fibrosis, including altered functional activity of ion channels and pumps and changes in gene expression of regulatory and modulatory proteins of Excitation-Contraction (E-C) coupling. The cellular defects associated with E-C coupling manifest as prolonged action potentials, slowed cytosolic Ca2+ fluxes and slowed myocyte shortening and lengthening [2,64,65].
Oxidative stress caused by toxic molecules may play a critical role in subcellular remodeling and abnormalities of calcium handling that lead to subsequent diabetic cardiomyopathy. Alterations in regulatory proteins and contractile proteins, sarcoplasmic (endoplasmic) reticulum Ca2+-ATPase (SERCA2) and Na-Ca2 exchanger function may be important contributors to abnormal myocardial carbohydrate and lipid metabolism in diabetes. These changes likely result from accumulation of toxic molecules such as long-chain acylcarnitines, free radicals, and abnormal membrane lipid content19. Changes in gene expression that affect E-C coupling and cellular metabolism contribute to myocardial dysfunction in diabetic cardiomyopathy. At the cellular level, defective E-C coupling has been implicated as one of the root causes of the contractile dysfunction associated with diabetic cardiomyopathy. One of the most consistent and early changes seen in the hearts of individuals with diabetes is the prolongation of the ventricular action potential [4].
Cardiomyopathy in streptozotocin-induced type 1 diabetes is characterized by a decrease in the expression of SERCA2 [4,66], a change that is seen in most animal models of heart failure. In animal models of type 2 diabetes or insulin resistance, SERCA2 activity is also compromised, but a decrease in the expression of the protein is not always apparent [67]. The alteration in SERCA2 activity is most probably dependent on the severity and duration of diabetes. Impaired SERCA function has been consistently found to coincide with myocyte insulin resistance in animal and in vitro models of type 1 and 2 diabetes68,69. Furthermore, instigating insulin treatment in diabetic rats restored SERCA2a levels to normal, increased intracellular Ca2+ transient currents, and improved myocardial function following ischemia-reperfusion [70,71].
Besides, advanced glycosylation end products form irreversible cross-links within or between many proteins, such as SERCA2a, causing their inactivation and subsequently leading to abnormal cardiac relaxation and contractility [72,73].
Microvascular Disease
Diabetes is recognized by characteristic changes in microvascular architecture. These changes include abnormal capillary permeability, microaneurysm formation, subendothelial matrix deposition, and fibrosis surrounding arterioles. Coronary blood flow reserve in diabetic patients is reduced even in the absence of obstructive coronary artery disease and left ventricular hypertrophy [74]. Hyperglycemia also can lead to an enhanced synthesis of vasoconstrictor prostanoids by the endothelium and activation of protein kinase C. This vasoconstriction can promote myocardial hypertrophy, endothelial dysfunction, and ventricular hypertrophy [74]. Protein kinase C, an intracellular signaling molecule, is activated in diabetes and can lead to endothelial dysfunction by reducing the bioavailability of nitric oxide while increasing oxygen-derived free radical production. It also can enhance leukocyte adhesion, increase albumin permeability, and impair fibrinolysis [74,75]. Therefore, activation of this enzyme contributes significantly to the development of microvascular complications, as seen in diabetic neuropathy and nephropathy.
There can be correlation between diabetic cardiomyopathy and microangiopathy, due to the similarities between diabetes and idiopatic miocardiopathy in what concerns the coronary disease [76]. About 72% of normotense diabetic patients present in around 72 % of the diabetic patients without arterial high blood pressure were watched obvious disease of small pots, whereas in non-diabetics this finding was only 12 % [77]. Besides, abnormalities of the reserve of coronary flow have been solidly demonstrated in diabetic patients without epicardic coronary arterial disease. Perivascular and interstitial fibrosis and miocardic hypertrophy were also frequent finds in diabetics [78].
The capacity of the vascular bed to meet metabolic demands may be impaired by abnormal epicardial vessel tone and microvascular dysfunction [76,79]. Diabetics have impaired endothelium-dependent relaxation [80], a defect that may be related to inactivation of nitric oxide by advanced glycosylation end products and increased generation of free radicals [81]. The abnormal vasodilator response in diabetes extends to the coronary microcirculation [82]. Besides, microcirculatory dysfunction in diabetics may be due in part to downregulation of the expression of vascular endothelial growth factor (VEGF).
Even in patients with no known coronary artery disease, microvascular dysfunction and decreased coronary flow reserve can be present. Such findings have been demonstrated particularly in the insulin-resistant/diabetic cardiomyopathy population [83,84]. In the absence of resting flow abnormalities, this is less likely to be a cause of resting left ventricular dysfunction but could contribute to left ventricular dysfunction with stress or exercise. In addition, a mismatch between coronary blood flow and myocardial glucose uptake has been demonstrated [85].
Clinical Picture and Symptoms
There are 2 important components in the clinical diagnosis of diabetic cardiomyopathy: the detection of myocardial abnormalities and the exclusion of other contributory causes of cardiomyopathy. An important challenge in the clinical diagnosis of diabetic cardiomyopathy has been the lack of any pathognomonic histological changes or imaging characteristics associated with the diagnosis [74].
The definitive diagnosis of diabetic cardiomyopathy is difficult to be established, principally because the signs, symptoms and finds of diagnostic examinations are unspecific. The diagnosis of diabetic cardiomyopathy currently rests on noninvasive imaging techniques that can demonstrate myocardial dysfunction across the spectra of clinical presentation [74]. Besides, the clinical picture and laboratorial what took the suspicion of diabetic cardiomyopathy can be resulting of pathologies very prevalent between the diabetic patients, like arterial high blood pressure, coronary disease and obesity. The clinical demonstration of the diabetic cardiomyopathy is usually characterized for dyspnea due to the pulmonary congestion resulting from the diastolic dysfunction of the left ventricle. Subsequently, with the advancement of the disease, compromising of the systolic performance can occur, aggravating the severity of heart failure. The signs and symptoms of right heart failure, as well as the clinical form of dilated cardiomyopathy with global heart failure, are not common in the diabetic cardiomyopathy [86]. It is important to emphasize that with our current knowledge, there is still no consensus in the precise imaging definition of diabetic cardiomyopathy, but evidence of hypertrophy or diastolic dysfunction is likely crucial to support a diagnosis of diabetic cardiomyopathy, but is not specific to it [74].
Diastolic function parameters in diabetic patients are analogous to those in animal studies. Left ventricular ejection time is often reduced, and the length of the pre-ejection period and the ratio of pre-ejection period to left ventricular ejection time are often increased. Diastolic inflow patterns are frequently abnormal, reflecting underlying abnormalities in relaxation and/or reduced myocardial compliance. Left ventricular diastolic dysfunction appears to be quite common in well-controlled type II diabetic patients without clinically detectable heart disease [19].
Studies that have examined both systolic and diastolic dysfunction in both type I and type II diabetes suggest that the latter is more susceptible to preclinical changes. The lack of an association between diabetes and LV diastolic dysfunction in young diabetic subjects (35 yr) may relate to the prevalence of type I diabetes [19,87]. Nonetheless, another comparison of both type I and type II adult diabetic patients also showed no significant difference in mean rate-corrected pre-ejection period, left ventricular ejection time, electromechanical systole, and pre-ejection period/left ventricular ejection time ratio compared with those of age- and sex-matched normal subjects [88]. The mechanism of protection of type I diabetic patients may relate to protective effects of insulin therapy and lack of insulin resistance. Indeed, animal data suggest correction of abnormal function with insulin therapy, with indices of cardiac performance significantly greater in insulin-treated rats when compared with control rats [19].
A number of studies in both animals and humans have shown structural changes in parallel with the functional changes of diabetic heart disease, in the absence of hypertension, coronary artery disease, or intraventricular conduction defects [89,90,91]. These results indicate LV fibrosis in the early stages of type II diabetes. In another study using modern stereological techniques to quantify changes in the morphology accompanying streptozotocin-induced diabetes, the results showed that the time to peak tension and relaxation of papillary muscles was prolonged, the heart weight to body weight ratio was increased, and the volume of extracellular components was increased 3-fold in diabetic rats. At the same time, this study also demonstrated that the volume, surface density, and total surface area of capillaries as well as volume fraction of myocyte mitochondria were reduced, and oxygen diffusion distance to myocyte mitochondria was increased in the diabetic animals [92].
Similar structural alterations have been described in diabetic hearts without significant epicardial coronary disease in humans. The most prominent histopathological finding in diabetic patients is fibrosis, which may be perivascular, interstitial, or both. As the disease progresses, there is increased myocyte loss and replacement fibrosis. Thus, the increased myocardial tissue reflectivity in diabetics may represent an early marker of diabetic cardiomyopathy [19].