Circulating Anti-Inflammatory Cytokines
Interleukin-4 and IL-10 are mainly secreted by Th2 lymphocytes and monocytes / macrophages and have anti-inflammatory properties [203], possibly by providing a negative feedback mechanism to limit the production of pro-inflammatory cytokines following cerebral ischaemia and HI. Previously, IL-10 has been shown to inhibit monocyte / macrophage synthesis of IL-6 and TNF-a by blocking gene transcription and down regulating the release of ICAM-1 and MMP-9 [45; 185].
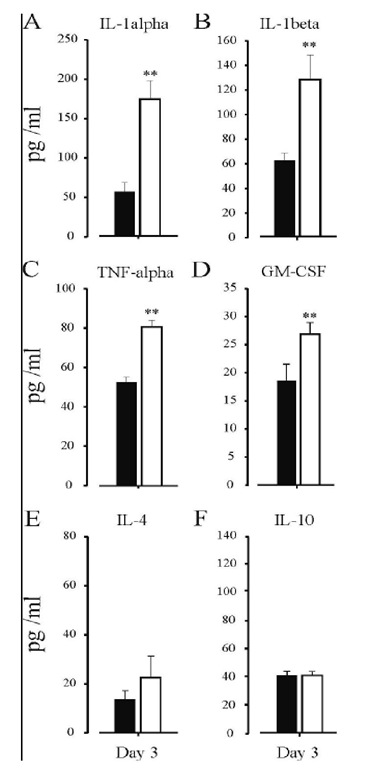
Figure 3. The effects of non-intervention control (black) and HI + saline (white) on circulating IL-1a (Panel A), IL-1P (Panel B), TNF-a (Panel C) GM-CSF (Panel D), IL-4 (Panel E) and IL-10 levels (Panel F) were assessed from plasma collected on day 3 post-HI. An increase in circulating levels of the pro-inflammatory cytokines, IL-1a, IL-1P, TNF-a, and GM-CSF, were seen following HI + saline treatment 3-days post-insult. No Change was seen in circulating levels of the anti-inflammatory cytokines, IL-4 and IL-10 following HI + saline treatment. ** = _p<0.01, versus non-intervention control.
In human stroke patients, increased levels of IL-10 have been seen in both the CSF and plasma, with levels peaking between 3 and 7-days post-stroke onset [158; 201]. Patients with acute cerebral ischaemia have increased IL-10 secreting monocytes compared with non-stroke controls [156]. However, unlike IL-10, no differences in the levels of IL-4 were detected in patients with or without neurological deterioration [213]. Pelidou and co-workers also failed to detect any differences in IL-4 secreting monocytes in ischaemic stroke patients compared to non-stroke controls [156]. These results suggest that IL-4, despite any inhibitory effects on pro-inflammatory cytokines, is less important than IL-10 in the acute period (12-24 hours) of cerebral ischaemia. Recently, Vila et al, reported that lower levels of the anti-inflammatory cytokine IL-10 and not IL-4 is associated with the onset of neurological deterioration in ischaemic stroke patients [213]. In this study, lower plasma levels of IL-10 were detected within 24 hours following stroke onset and were correlated with early deterioration in neurological symptoms.
The exact role that anti-inflammatory cytokines play in HI-mediated injury, therefore, is still to be fully established. IL-4 and IL-10 were not detected 12 hours post-HI in the rat [209]. Additional studies have also shown that, IL-10 levels were not significantly different from control groups in both asphyxiated newborns [175] and HI animals [12]. Furthermore, Clarkson and colleagues reported that there were no changes in either IL-4 or IL10 plasma levels 3-days post HI ([31] see Figure 3). These findings imply that anti-inflammatory cytokines, such as IL-4 and IL-10 do not contribute to the initial inflammatory response following HI. However, exogenous administration of IL-10 (i.v.) prevented the damage seen after endotoxin administration post-HI, suggesting that IL-10 has a therapeutic effect [60].
The beneficial effects of IL-10 may also stem from anti-inflammatory actions. That is, IL-10 has been shown to regulate soluble apoptotic proteins, such as sFas/APO-1 and sbcl-2, detected in CSF of human patients following cerebral ischaemia [199]. In addition, IL-10 has been shown to modulate neuronal vulnerability to excitotoxic ischaemic injury [70], as well as inhibit inducible nitric oxide synthase (iNOS; [72], which is a key enzyme involved in propagating pro-inflammatory pathways. Finally, animals deficient in the IL-10 gene exhibited larger infarcts; increased neutrophil infiltration; and increased levels of TNF-a, ICAM-1, MMP-2, MMP-9 and iNOS compared to their wild type controls [45; 70; 72; 185]. Evidence suggests that IL-10 rather than IL-4 is the important anti-inflammatory cytokine in ameliorating ischaemic-induced injury. The only drawback, however, is that peak levels occur either 3 days post cerebral ischaemia or not at all following HI, which is possibly too late to afford any significant protection against circulating pro-inflammatory cytokines, which are elevated within the first 48 hours.
Inflammatory Mediated Myocardial Damage
Recent evidence has shown that the pro-inflammatory cytokines, TNF-a and IL-1P synergistically impair human myocardial function through a mechanism associated with sphingosine [23]. Sphingosine is rapidly produced as a result of sphingomyelin hydrolysis by sphingomyelinases that results in the formation of the ceramide intermediate when cardiac myocytes are exposed to TNF-a [124]. In rat myocyte cultures, the ceramidase inhibitor, N-oleoyl ethanolamine, has been shown to inhibit the production of sphingosine and reverse the ionotropic effects associated with TNF-a [148]. TNF-a and IL-6 have been shown to attenuate myocardial contractility directly (which is reversible) via the immediate reductions in systolic cytosolic [Ca2+] associated with alterations in sarcoplasmic reticulum function [236]. In addition, TNF-a has been shown to also decrease myocardial contractility indirectly through nitric oxide-dependent attenuation of myofilament Ca2+ sensitivity [64].
Alternatively, TNF-a has been shown to provoke negative ionotropic effects in myocytes partially through the neutral sphingomyelinase pathway. This was shown within minutes following cardiomyocyte injury, where TNF-a decreased systolic function by alterations in Ca2+-induced Ca2+-release from the sarcoplasmic reticulum and also by disruption of the L-type calcium channels [109]. In this, the binding of TNF-a to the TNF-receptor type 1 (TNFR1) leads to the release of the sphingolipid metabolite, which is a stress-induced second messenger, via sphingomyelin degeneration. Oral and co-workers reported that production of sphingosine correlates directly with the imbalance in Ca2+ homeostasis, while blockade of sphingosine production negatively regulates TNF-a-induced contractile dysfunction [148]. This is due to the fact that sphingosine has been shown to decrease Ca2+ transients via the blockade of the ryanodine receptor, which impedes the Ca2+-induced Ca2+-release from the sarcoplasmic reticulum [125]. The exact mechanisms by which TNF-a exerts its pathophysiological effects are as yet not fully understood. It is suggested that TNF-a triggers the apoptotic pathway [108], which is possibly linked to the myocyte membrane TNFR1 and TNFR2 sites [126]. This is associated with the so called "death domain" that is found in the TNFRI, and suggests that TNF-a acting via the TNFRI site could mediate myocardial cell death via apoptosis [211].
Parasympathetic Nervous System and Inflammation
It has been well established that autonomic dysfunction is a strong correlate of morbidity and mortality resulting from cardiovascular disease, and recent work in humans has shown a correlation between abnormal heart rate variability and elevated levels of inflammatory cytokines such IL-6 and CRP [10]. However, the exact involvement of the ANS and inflammation are still being investigated. The vagus nerve has been shown to innervate the cardiovascular system in addition to other visceral organs such as the liver, spleen, and gut. Recent work by Tracey and colleagues demonstrated that by injecting lipopolysaccharides (LPS) into animals undergoing vagus nerve stimulation, resulted in a marked decrease in macrophage-mediated release of inflammatory cytokines (TNF-a, IL-1P, IL-18, and IL-6) and decreased incidence of death without affecting the release of the anti-inflammatory cytokine IL-10 [17], and these results were reversed following transsection of the vagal nerve. These results indicated that stimulation of the vagus nerve may play a functional role in regulating an anti-inflammatory response [17].
More recently, the mechanisms by which vagal nerve stimulation results in an antiinflammatory response have been described by Tracey and colleagues [153; 207; 216]. In nicotinic a7 subunit knockout mice, electrical stimulation of the vagal nerve no longer prevented release of inflammatory cytokines, indicating that the a7 subunit of nicotinic receptors plays an important role in regulating the vagal nerve-mediated anti-inflammatory response [153]. In addition, stimulation of a7 subunit knockout mice with LPS resulted in a greater release of inflammatory cytokines compared to wild-type mice [216]. Macrophages have been shown to express nicotinic (cholinergic) receptors comprising of five a7 subunits, which are thought to be involved in the cholinergic anti-inflammatory reflex [216]. Inhibition of the nicotinic receptors in primary human macrophages resulted in a marked dose- dependent reduction in high mobility group box 1 (HMGB1) inflammatory cytokines following stimulated with endotoxin (200 ng/mL) [216]. These results were unable to be inhibited with the muscarinic antagonist, atropine, however, the nicotinic antagonist, a-conotoxin, inhibited the action of acetylcholine on this receptor [216], indicating that acetylcholine inhibits HMGB1 release via the a7 nicotinic receptors.
Treatment Strategies
The cascade of events that occurs following neuronal injuries is complex and it is unlikely that any single intervention will prevent the entire cascade from being initiated. This damage not only resides within the hemisphere of damage but can also propagate to the contralateral hemisphere (see [31; 33; 35]. In addition to the neural component of damage, clear evidence as outlined above illustrates damage to the cardiovascular system. Therapies that target multiple steps in the cascade may limit neuronal injury more effectively than interventions with single modes of action. Outlined here are a couple of therapeutic interventions that have received growing attention over recent years, anaesthetic preconditioning and more recently post-conditioning and the use of beta-blockers.
Pre-Conditioning
Previous work has shown that exposing organs, such as the brain and heart to brief periods of sub-lethal ischaemia, initiates ischaemic tolerance via a pre-conditioning phenomenon [136; 206]. Two distinct patterns of ischaemic tolerance have been noted: the acute phase, whereby effects are seen within minutes and then subsequently disappear after 23 hours and the late phase, whereby effects develop over a period of several hours and can last up to several days or weeks [136; 206].
One of the pioneering publications in the field of pre-conditioning came in 1986 when Murry and colleagues reported that myocardial damage as a result of a coronary artery occlusion, is markedly ameliorated if the heart had prior exposure to brief periods of sub-lethal ischaemia [133]. Similarly, these pre-conditioning effects have also been observed in human subjects [234]. In addition, a recent report has also suggested that a transient ischaemic attack can induce ischaemic pre-conditioning within the brain [225]. The CNS has been highlighted as being the most vulnerable organ system in the body to an ischaemic insult. For instance, a brief disruption (5 minutes) to cerebral blood flow (CBF) has been shown to cause neuronal injury, while cardio-myocytes and kidney cells require 20-40 minutes of ischaemia to induce cellular damage [112].
Cerebral ischaemia-induced pre-conditioning was first reported by Kitagawa and co-workers using a model of global ischaemia, whereby 2 minutes of transient ischaemia provided significant protection against subsequent global ischaemia 24 hours after the initial insult [97; 98]. Since these findings, others have shown in a rat model of unilateral carotid artery ligation coupled with hypoxia, that hypoxic-pre-conditioning can induced significant protection to both striatal and hippocampal regions [15; 62].
Anaesthetics and Pre-Conditioning
Early work in the 1960s showed clear evidence that general anaesthetics can offer tolerance against cerebral ischaemia that is induced during periods of temporary carotid occlusion [227]. This was further backed up in 1966 by Goldstein and colleagues who reported that pentobarbital can offer tolerance to cerebral anoxia [65]. Since these two early findings, a considerable amount of work has been carried out illustrating that intravenous and volatile anaesthetics can decrease the amount of neuronal injury during periods of insult. For instance, in a rat model of spinal cord injury, halothane, fentanyl/nitrous oxide and lidocaine all provided significant protection against injury [36]. Furthermore, halothane, sevoflurane and pentobarbital have all been shown to afford protection in a rat model of focal cerebral ischaemia [223; 224]. Clear evidence exists that demonstrates exposing adult rats to volatile anaesthetics (i.e. isoflurane or halothane), can trigger both acute and late phases of ischaemic tolerance within the brain [93; 241; 242].
Over the past decade, the volatile anaesthetics halothane, isoflurane, sevoflurane and desflurane have all been shown to provide significant protection against focal cerebral ischaemia and also provide significant improvement in neurological outcome [30; 49; 223; 228]. Both halothane and desflurane have been shown to afford significant neuroprotection following 2 hours intraluminal middle cerebral artery occlusion (MCAo) and 22 hours of reperfusion [78]. In this model of focal cerebral ischaemia, treatment with desflurane provided greater protection than halothane. The anaesthetic-induced neuroprotection with halothane has also been shown to be maintained even when the pericranial temperature is controlled [222].
Isoflurane has also been shown to prevent hippocampal neuronal injury in an in vitro model of cerebral ischaemia due to oxygen glucose derivation (OGD; [163]. In addition, 30 minutes of isoflurane pre-treatment provided significant protection against HI-induced neurodegeneration 24 hours later [239]. However, the protection afforded by isoflurane in vitro only delays and does not prevent neuronal damage in an MCAo model of focal cerebral ischaemia in vivo [94].
During periods of anaesthesia, auditory, visual and tactile stimuli reach the CNS, however, processing of this information is disturbed [8]. It is thus generally thought that anaesthetics preferentially act on the CNS. It is considered that ion channels, particularly gamma-aminobutyric acid (GABA)A receptors, are the most-likely target for anaesthetics within the CNS [59; 128]. However, understanding the exact mode of action of anaesthetics is plagued by the fact that most general anaesthetics act on numerous ion channels sites, with limited selectivity at a variety of lipophilic sites associated with neural membranes [30].
Over the past 50 years the noble gas, xenon, has been studied for its anaesthetic properties, which has revealing many salubrious qualities [41]. In addition to antagonising the N-methyl-D-aspartic acid (NMDA) receptor [58], xenon has been shown to have several advantages over many other volatile anaesthetics in use today. For instance, xenon has an extremely low blood/gas partition coefficient which allows for rapid a induction and emergence [135]. In addition, xenon has been shown to exert minimal effects on heart rate, mean arterial pressure and cardiac contractility [193], thus providing ideal haemodynamic stability.
In a series of in vitro studies xenon has been shown to reduced injury in a mouse neuronal-glial cell culture induced by either NMDA, glutamate, oxygen deprivation or OGD [229]. In addition, the neuroprotective effects of xenon have been assessed in in vivo models of acute neuronal injury involving administration of excitotoxins to rats [120; 161], cardiopulmonary bypass in rats [121], MCAo in mice [87], cardiac arrest in pigs [177], and HI in neonatal rats [119]. Furthermore xenon has also been shown to induce pre-conditioning in many organs, including both the brain and heart [165], which would make xenon an ideal candidate to offer protection against secondary cardiac damage in addition to affording significant neuroprotection.
Involvement of Inflammation in Pre-Conditioning
A growing body of evidence has accumulated over recent years, highlighting the immune system, and more importantly cytokines and chemokines as key mediators, not just in HI [16; 35; 159; 170], but also in other neurodegenerative disorders such as Alzheimer’s disease [34]. In addition, these mediators have been shown to be closely inter-related in a complex and often vicious positive feedback cycle, in that pro-inflammatory cytokines are known to induce reactive oxygen species (ROS) and vice versa [3; 56; 63].
Previous studies have shown that following ischaemic pre-conditioning, TNF-a and IL-1P mRNA levels are increased as measured using real-time PCR [218; 219]. The peak levels of IL-1P expression following ischaemic pre-conditioning, however, was significantly less compared to the permanent occlusion of the MCA, 87 copies versus 546 [218]. In addition, others have shown that pre-treatment of rats with low doses of bacterial LPS induces cytokines and subsequently protects against later ischaemic injury [202]. Furthermore, IL-1P has also been shown afford significant protection following direct administration just prior to cerebral ischaemia, which was negated by co-administration of the endogenous IL-1 antagonist, IL-1ra [142].
The use of anaesthetics to interact with inflammatory pathways is not well characterised. The release of leukotriene B4 and IL-1 from activated human monocytes has been shown to be dose-dependently inhibited following treatment with lidocanine and bupivacaine in vitro [188]. In a study assessing acute hyperoxic lung injury in rabbits, pre-treatment with an intravenous lidocaine infusion at clinically relevant concentrations markedly decreased the release of IL-1P and TNF-a from the injured lung and also negated the influx and activation of neutrophils [197]. In addition, local anaesthetics such as lidocaine, bupivacaine and amethocaine have been shown to inhibit both the spontaneous and also the TNF-a-induced stimulation of IL-1P and IL-8 with lidocaine also stimulating the secretion of the antiinflammatory molecule IL-1ra [110].
Recent work has shown that clomethiazole (CMZ), in addition to modulating the GABA receptor offers protection via anti-inflammatory mechanisms [31; 82; 186; 187]. For example, CMZ has been shown to inhibit p38 mitogen-activated protein kinase, in turn attenuating the induction of the immediately early genes c-fos and c-jun in LPS-stimulated cortical glial cultures [186]. More recently, CMZ has been shown to inhibit the IL-1P-induced expression of glial c-fos and iNOS mRNA levels in vitro [187]. Likewise, in a model of experimental extracorporeal circulation, plasma concentrations of IL-6, IL-8 and TNF-a were reduced by CMZ [82]. Furthermore, CMZ-treatment decreased the HI-induced increase in iNOS activity in a model of HI [33]. Most recently, CMZ has also been shown to decrease the HI-induced increase in circulating pro-inflammatory mediators (IL-1a, IL-ip and TNF-a) and stimulate an increase in the anti-inflammatory cytokine IL-10, in turn providing significant protection to mitochondrial energetics both contralateral and ipsilateral to the occlusion [31]. Ample evidence now supports the view that CMZ has both GABAmimeting and anti-inflammatory properties, and these properties together provide neuroprotection. We have also more recently shown that CMZ is able to offer protection against secondary cardiac damage following HI (Clarkson, Kapoor, Harrison, Jackson and Sammut, unpublished data)
Post-Conditioning
Significant work has highlighted anaesthetic pre-conditioning as a means for offering protection to the CNS against injury. Over resent years the term post-conditioning, which has significant clinical benefits over pre-conditioning, has been coined and shown to afford significant protection that is similar to that seen with pre-conditioning paradigms [40; 214]. In 2003 Zhao and colleagues introduced ischaemic post-conditioning, which is defined by a series of intermittent ischaemic episodes during the reperfusion phase which they showed offered significant protection against myocardial injury following the ligation of the left anterior descending artery [240]. Since this study, ischaemic post-conditioning has been shown to involve the release of adenosine [96] and also the activation of ERK, production of nitric oxide and opening of mitochondrial adenosine-triphosphate sensitive potassium channels [232]. With the exception of one recent study showing that LPS, which is well-known to induce preconditioning, can decrease the recruitment of leukocytes post both cerebral and spinal cord injuries [43], all work to date has been carried out on the heart. Whether the same post-conditioning mechanisms that have been elucidated following injury to the heart are the same following cerebral HI, is yet to be examined.
Use of Beta-Blockers
Increased sympathetic activity has been found after acute stroke [25; 75; 134] and is associated with poor neurological prognosis [172]. Beta-blockers, which have an anti-sympathetic effect, are neuroprotective during cardiac surgery [6]. In an animal model of cerebral ischaemia, pre-treatment with the beta-blocker, carvedilol, decreased the infarct volume and neurological deficits by >40% [174]. Possible mechanisms for the neuroprotection include shifting the haemoglobin-oxygen dissociation curve to the right [157], decreasing TNF-a and IL-1P levels [174], offering membrane-stabilisation and antioxidant effect [7], blocking sodium and calcium channels [150], and inhibiting protein kinase C [191] and phosphatidate hydrolase [106].
Clear evidence now exists illustrating that carvedilol, which is classically known for its actions on hypertension and congestive heart failure, has multiple modes of action. Recent evidence shows that carvedilol is neuroprotective in both in vivo global [118] and focal [174] models of cerebral ischaemia. Following focal ischaemia, carvedilol was shown to significantly attenuate the ischaemia-induced increase in TNF-a and IL-1 P mRNA levels. Furthermore, the metabolites of carvedilol, SB 211475 and SB 209995, have been shown to be more potent than carvedilol for inhibiting lipid peroxidation in rat brain homogenates [55; 238]. Therefore, these metabolites may also be responsible for some of the neuroprotective effects associated with carvedilol treatment.
Carvedilol is also thought to exert its neuroprotection via antioxidant properties, as carvedilol offers significant protection in in vitro model of free radical mediated neuronal injury [118; 238]. In addition, carvedilol has been also shown to prevent apoptosis following myocardial ischaemia-reperfusion injury [237]. Based on these findings, clear evidence exists the highlights the use of carvedilol or possibly other beta-blockers as novel agents in preventing either cerebral ischaemia or HI-induced neural injury, as well as preventing the secondary cardiovascular events that occur subsequent to the neural insult.
Conclusion
Following a cerebrovascular accident, a multi-faceted cascade of events occurs leading to cell death and neurological impairments of the CNS. It is well known clinically that clipping off cerebral aneurysms, carotid endarterectomy and cardiopulmonary bypass present a high risk for transient focal cerebral ischaemia. And one of the most imminent predisposing factors of cerebral ischaemia is cardiovascular complications. However, as outlined in this review, considerable evidence exists that clearly highlights cardiovascular complications subsequent to cerebral ischaemia and HI. These cardiovascular impairments pose a clinical threat and can confound neurological outcome and survival. The heart is tightly regulated by the brain, and damage to the insular cortex and ANS, clearly result in irregularities in cardiac function. One of the major pathways of damage following cerebral ischaemia is inflammatory mediated damage that precipitates primarily from a peripheral origin. And clear evidence exists that illustrates that circulating inflammatory mediators are capable of directly inhibiting cardiovascular function resulting in damage.
At this point, current therapeutic strategies do no more than alleviate the symptomatic presentations and cardiovascular monitoring plays no role, other than to possibly minimize any further neurological damage. Resent evidence however, clearly illustrates that preconditioning paradigms, the use of beta-blockers and possibly post-conditioning paradigms offers significant neuroprotection that might also stem to preventing subsequent cardiac impairments. Even though anaesthetic pre-conditioning has been shown to be beneficial in preventing neuronal injury, only a few groups are actually addressing this subject matter. Given the complexity of anaesthetic-mediated pre-conditioning and or post-conditioning in regards to neuronal protection, clearly more work needs to be carried out in order to validate this mechanism as a possible treatment protocol. However, given the data collected to date highlighting the putative beneficial effects of anaesthetics in protecting the CNS from injury, this in it self should be enough reason to further explore this topic at hand. It is a clear possibility the controlled anaesthetic treatment may be used as a treatment not only for patients with acute ischaemic stroke and HIE.