Release of Oxidative Stress Markers
Anti-oxidant defence mechanisms play an important role in the maintenance of cellular function and survival; aberrations in oxidative capacity have been implicated in conditions of ageing, inflammation and ischaemic reperfusion (IR) injury [53; 92]. The production of free radicals has been recognised to occur following cerebral ischaemia and known anti-oxidants have been shown to offer neuroprotection against free radical-mediated injury [32; 84; 195].
Markers of oxidative stress have also been found in the periphery including increased plasma 8-isoprostane (8-epiPGF2a) levels [171]. Plasma 8-epiPGF2a is a reliable marker of in vivo oxidative damage and is produced as a stable by-product following the non-enzymatic oxidation of tissue phospholipids by oxygen radicals [164]. Plasma malondialdehyde, which is another marker of oxidative damage, has also been found to be elevated for a period of 48 hours in patients following ischaemic stroke [182].
In stark contrast, decreased intrinsic anti-oxidant enzyme levels (such as superoxide dismutase (SOD)) have been found 48 hours following the onset of cerebral ischaemia. Specifically, lower serum and erythrocyte SOD and glutathione peroxidase (GPx) activities have been shown to occur in stroke patients [27; 192]. In addition, plasma levels of non-enzymatic anti-oxidants, a-tocopherol, ascorbic acid and uric acid are also decreased 24 hours post-ischaemia with all returning to control levels after 1 week with the exception of ascorbic acid and SOD [27]. A poor serum anti-oxidant profile following cerebral ischaemia has been shown to be associated with potentiation of neurological degeneration [114]. It is, therefore, possible that free radical production during and / or following cerebral ischaemia, whether derived centrally or peripherally, may account for some of the damage seen remote to the initial site of infarction.
Neural Regulation of the Heart
The ability for the brain to regulate cardiac function has been well established with many central nervous system (CNS) regions and mechanisms being implicated. The anatomical locations that are involved in the neural regulation of cardiac function, extends from the spinal cord to the cortex. Within the cortex, the insular cortex has received the most recent attention and will be the man focus of this section. In addition, changes in the cardiovascular autonomic system, which is comprised of a complex network of interconnections throughout the neural axis, will also be discussed.
Involvement of the Insular Cortex
Early reports suggested elderly patients who had suffered ischaemic lesions to the right hemisphere were at greatest risk of developing cardiovascular complications [74; 76]. In addition, patients with hemispheric lesions as opposed to lesions of the brainstem are more likely to develop arrhythmias [194]. The clinical symptoms of cardiovascular abnormalities following cerebral ischaemia are indicative of sympathetic hyper-function and / or parasympathetic hypo-function [13]. Indeed, cardiovascular abnormalities similar to those seen following cerebral ischaemia may be induced by intra-cerebral or systemic catecholamine infusion [26].
The right insular cortex has now been identified as representing the cortical site, which when subjected to ischaemia both experimentally and clinically, induces cardiovascular complications [127; 190]. The insular cortex lies within the region which receives its blood supply from the middle cerebral artery and is involved in the regulation of autonomic control of the heart [233]. Prolonged stimulation of this region produces ECG and myocardial impairment similar to that seen following cerebral ischaemia [145]. In addition, it has been demonstrated that the insular cortex has a role in cardiac chronotropic organization, with stimulation of the rostral posterior insular cortex in chronically anaesthetised rats resulting in tachycardia while stimulation of the caudal posterior insular cortex producing bradycardia [145]. An ischaemic lesion to the right insular cortex also induces specific patterns of neurochemical change. Assessment 5-days post-insult has revealed increased staining for tyrosine hydroxylase, an enzyme involved in catecholamine synthesis, and neuropeptide Y, a neurotransmitter that potentiates the post-synaptic effects of noradrenaline [5]. It is also believed that disinhibition of the insular cortex caused by excitotoxicity triggers an increased sympathetic output and cardiac complications ensue [28].
Involvement of the Autonomic Nervous System
Irregularities in the autonomic nervous system (ANS) can be associated with changes in both the sympathetic and parasympathetic systems [13; 102]. Changes in the ANS are reflected by increases in plasma and urinary catecholamine [134] and corticosteroid levels [143]. In addition, fluctuations in the ANS have also been associated with variable heart rate patterns [101; 137].
From animal and human studies, it is well established that anatomic and functional asymmetries exist in autonomic cardiac innervation. The parasympathetic and sympathetic nerves to the heart have parallel courses [85], with the right side mainly innervating the sinoatrial node (with antagonistic influences on its chronotropic function), while the left side mainly innervates the atrioventricular node and the ventricles. The nerves on the left side (left vagal branch) influence atrioventricular conduction, ventricular fibrillation threshold, QT timing and ST segments of ECG’s [80; 85; 132; 178; 179]. This led to the development of clinical corrective measures such as left-sided stellectomy used for the treatment of malignant ventricular arrhythmias with a long QT syndrome [132; 178]. Clinical observations have also suggested an association between right hemispheric lesions and the development of paroxysmal supraventricular tachycardia [111].
Since central autonomic pathways to the heart probably descend uncrossed [4], one should expect a corresponding asymmetry in the CNS control over cardiovascular function. This is supported by animal studies of experimental stroke showing that, right hemispheric lesions induce more pronounced sympathetic effects than lesions in the left hemisphere [24; 74]. It is established that CNS lesions in humans may induce ECG changes [147], cardiac arrhythmias [18], and disturbed cardiovascular reflexes [102], but whether lesions in the left as opposed to the right side of the brain have different consequences for autonomic heart rate control in humans is less well known. The involvement of the insular cortex and changes in the ANS have both been implicated in functional cardiac changes following an ischaemic-insult. Whether these changes and more importantly changes that occur on the right hemisphere compared to the left hemisphere are capable of producing lasting effects by themselves, is not known. It is most likely, however, that these changes work in synergism with other mechanistic changes that occur.
Inflammatory Mediated Response
For a chronic immune response to be initiated post-inflammation, antigen is processed and presented to lymphocytes, which is achieved via antigen-presenting cells (APCs; [34]. In the periphery, APCs are primarily either dendritic cells in the skin or monocytes in the circulation.
C-reactive protein (CRP) is an acute phase protein that is produced in the liver in response to a number of pro-inflammatory cytokines such as, IL-1P, IL-6 and TNF-a [152]. Previously, elevated levels of CRP have been associated with an increased risk of developing cardiovascular abnormalities in apparently healthy patients [168]. In addition, CRP has been used as a predictor of long-term cardiovascular events and an independent prognostic factor of poor outcome following stroke and has been shown to be significantly elevated for a period of 5 days following cerebral ischaemia [46; 171]. In addition, Smith et al., showed a significant correlation between both IL-6 and CRP and the severity of cerebral ischaemia [189]. CRP is also known to activate the complement system, of which components of this important self-defence immune system are involved in chemotaxis and opsonisation, and have been found to be elevated following cerebral ischaemia [154]. However, the complement pathway, if inappropriately activated can cause tissue injury due to activation of inflammatory pathways. Pedersen et al. reported a significant systemic increase in the terminal SC5b-9 complement complex that was preceded by an increase in CRP, and suggested that CRP may activate the complement system and subsequent activation of inflammation after stroke [154].
Neopterin is a guanosine triphosphate (GTP) metabolite, though its physiological function is not yet fully defined. Production of neopterin is thought to be only in monocytes and macrophages and has, therefore, been considered as a specific marker for the activation of these cells [117]. Previously, increased levels of neopterin have been shown to occur in patients with carotid atherosclerosis [226]. In addition, Grau et al., reported an increase in neopterin following cerebral ischaemia with peak levels seen on days 3 and 7 post-insult [67]. The production of monocytes and macrophages have been shown to have the ability to amplify thrombogenic events, by expressing tissue factor (TF; [47]. In addition, the expression of TF has been shown to induce thrombus formation on ruptured atherosclerotic plaques and has also been shown to play a role in MI and ischaemic stroke [205]. Tissue factor is a cellular receptor and cofactor for plasma factor VII(a), which has been shown to initiate the coagulation protease cascade that results in the stimulation of thrombin and fibrin [167]. Furthermore, it has been shown that TF-dependent coagulation activity is increased in the presence of arachidonic acid stimulated cyclo-oxygenase-1 metabolites prostaglandin (PG)G2 and thromboxane (TX)A2 and inhibited when in the presence of PGE2 [22]. Clear evidence has accumulated that demonstrates the catastrophic effects of circulating mediators. The expression of TF attributed to circulating monocytes and macrophages could account for the pathological presentations of myocytolysis and myofibrillar degeneration. However, further work is required in order to establish the exact role that TF maybe playing during the process of cardiac damage and also the role that pro- and anti-inflammatory cytokines are having.
Circulating Pro-Inflammatory Cytokines
Cerebral ischaemia evokes an inflammatory response that is characterised by the activation and release of cytokines, chemokines (chemotactic cytokines), endothelial-leukocyte adhesion molecules and proteolytic enzymes that contribute to tissue injury [51]. Production of pro-inflammatory cytokines and chemokines have been detected in experimental models of cerebral ischaemia and HI as well as human patients following acute ischaemic stroke or hypoxia-ischaemia encephalopathy (HIE; [34; 35; 51] see table 1). In addition, increased levels of pro-inflammatory cytokines are correlated with greater infarct size and poorer clinical outcome in patients following ischaemic injury [51; 200; 212].
Table 1. Comparison of cytokine and chemokine levels after hypoxia-ischaemia and ischaemic stroke in both animals and humans
|
Cytokine |
Chemokine |
||||
|
|
HI |
Stroke |
|
HI |
Stroke |
|
IL-1a |
 |
 |
MCP1 |
 |
 |
|
IL-1P |
 |
 |
MCP2 |
 |
 |
|
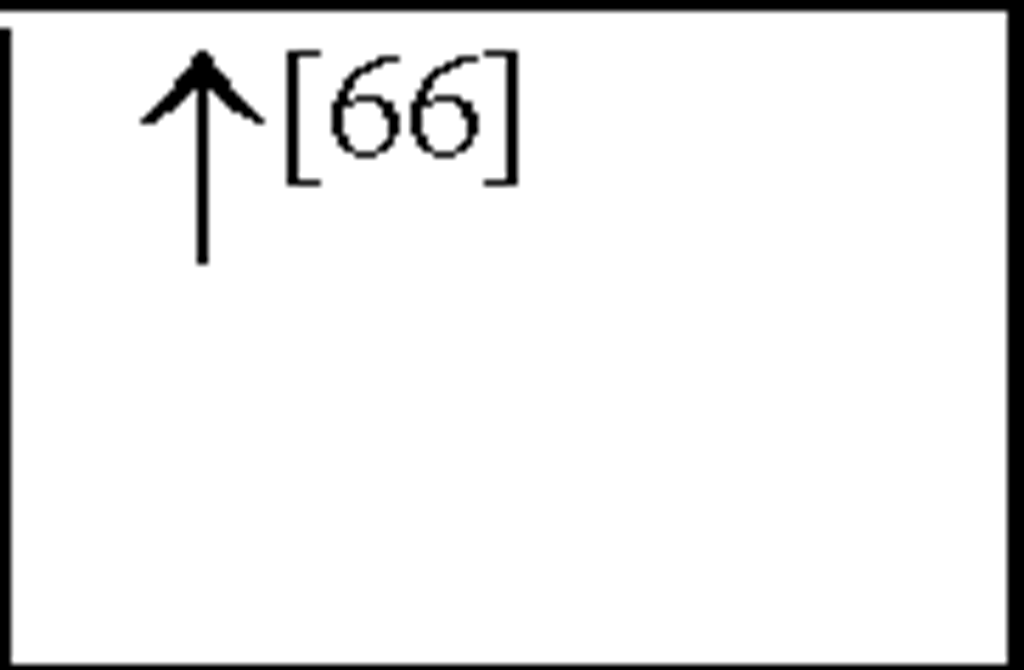
TNFa |
 |
 |
MCP3 |
 |
|
|
TNFP |
 |
MIP1a |
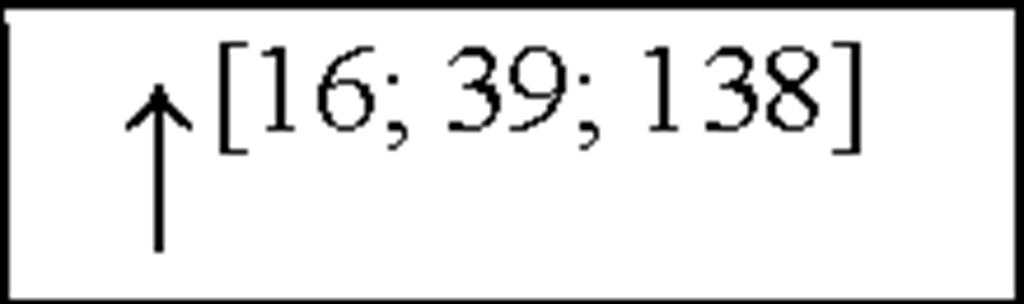 |
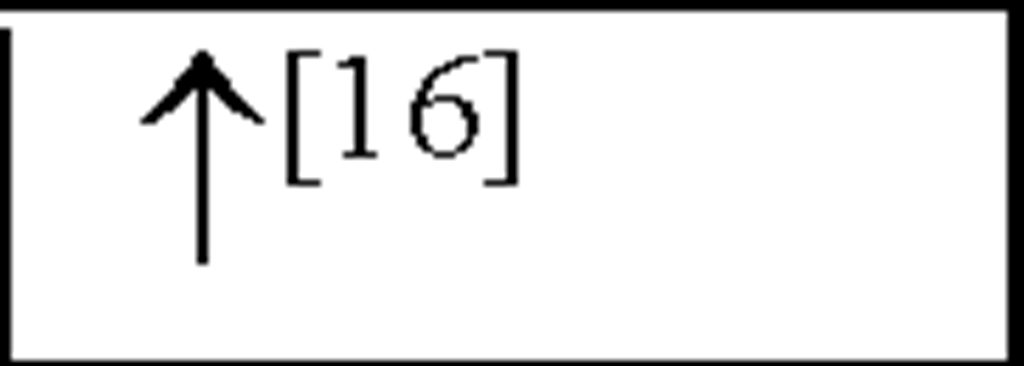 |
|
|
IL-2 |
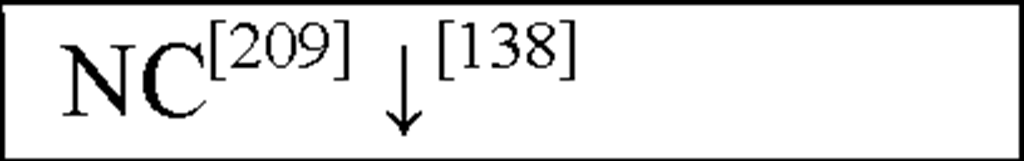 |
 |
MIP1P |
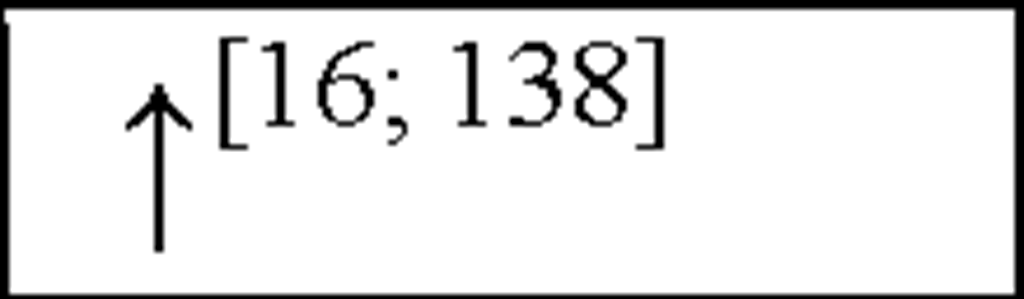 |
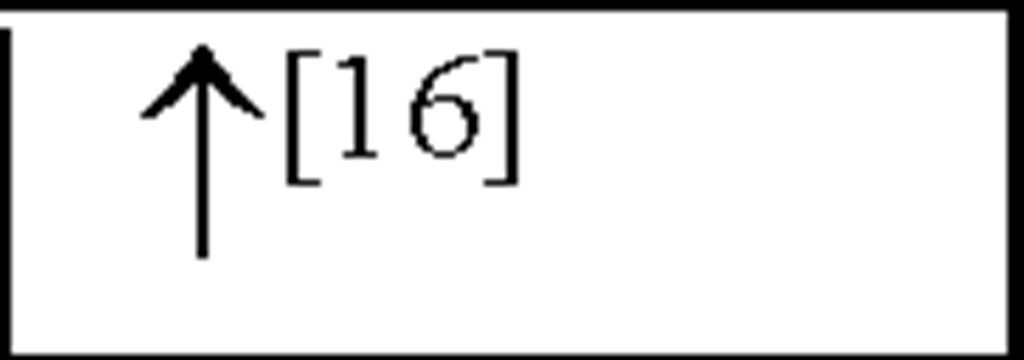 |
|
IL-3 |
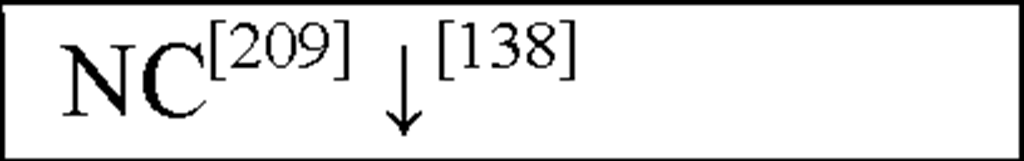 |
MIP2 |
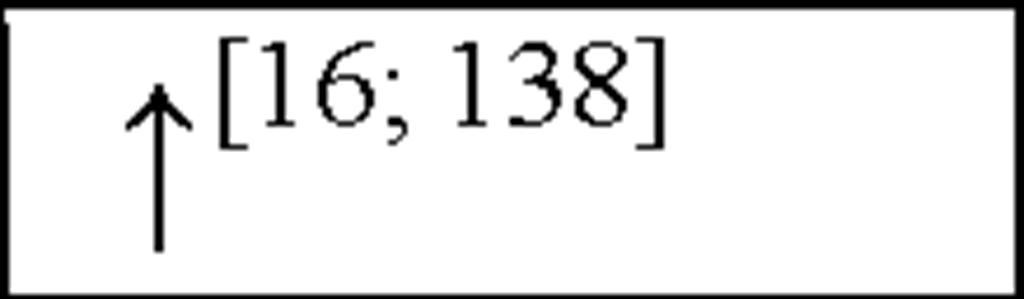 |
||
|
IL-4 |
 |
 |
RANTES |
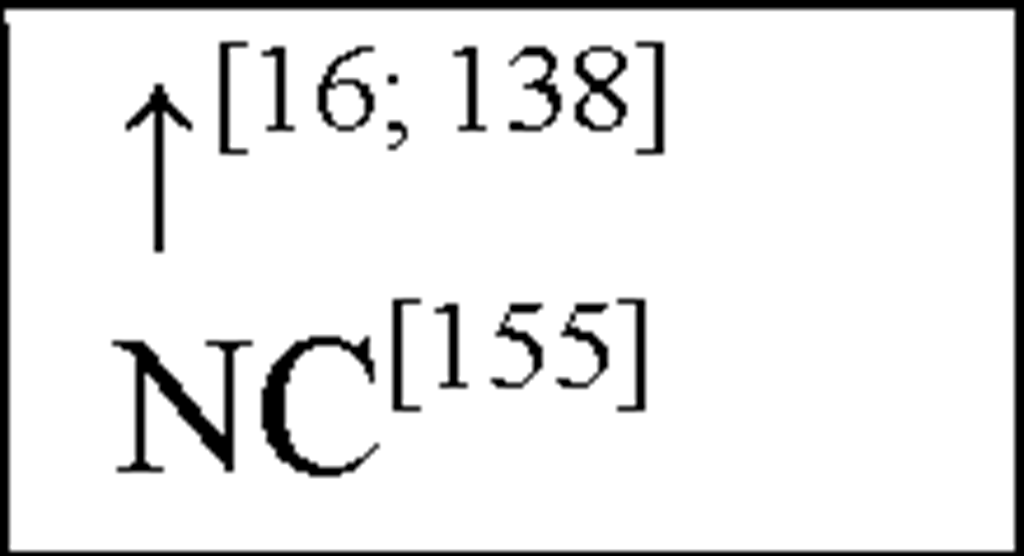 |
 |
|
IL-5 |
 |
 |
gro |
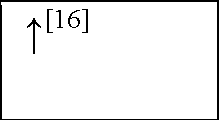 |
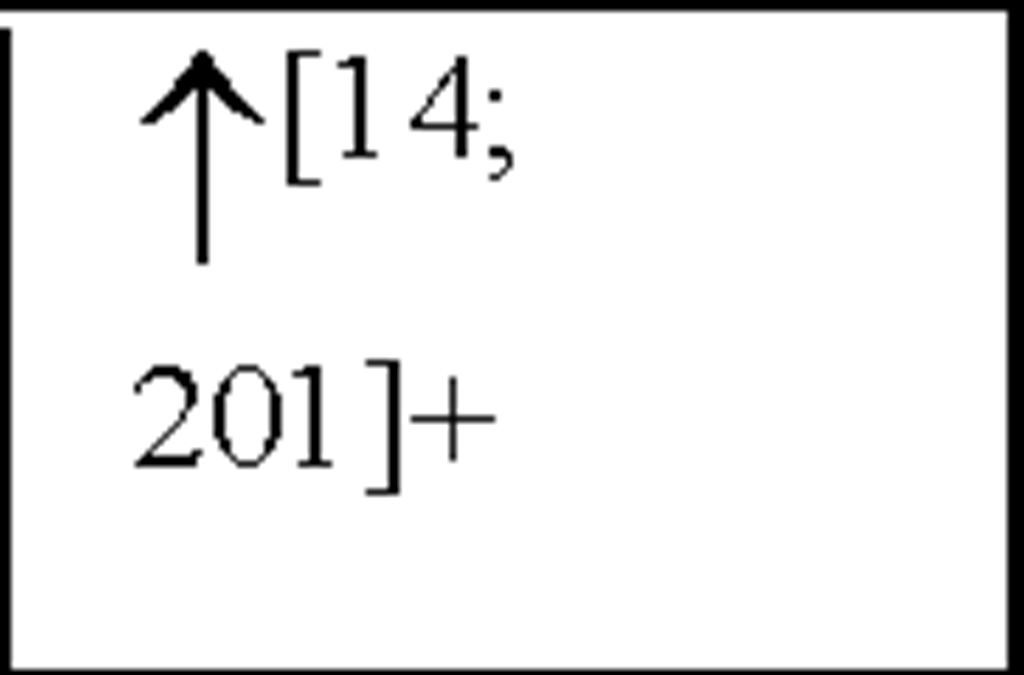 |
|
IL-6 |
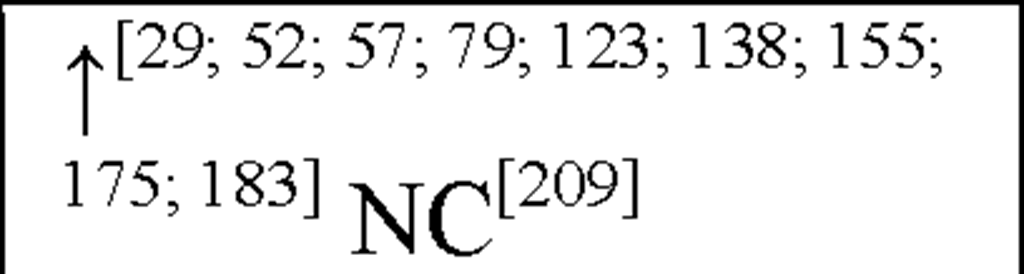 |
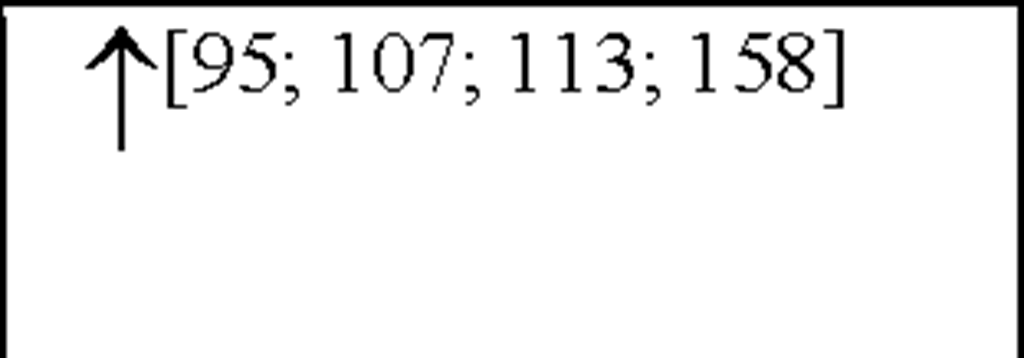 |
IL-8 |
 |
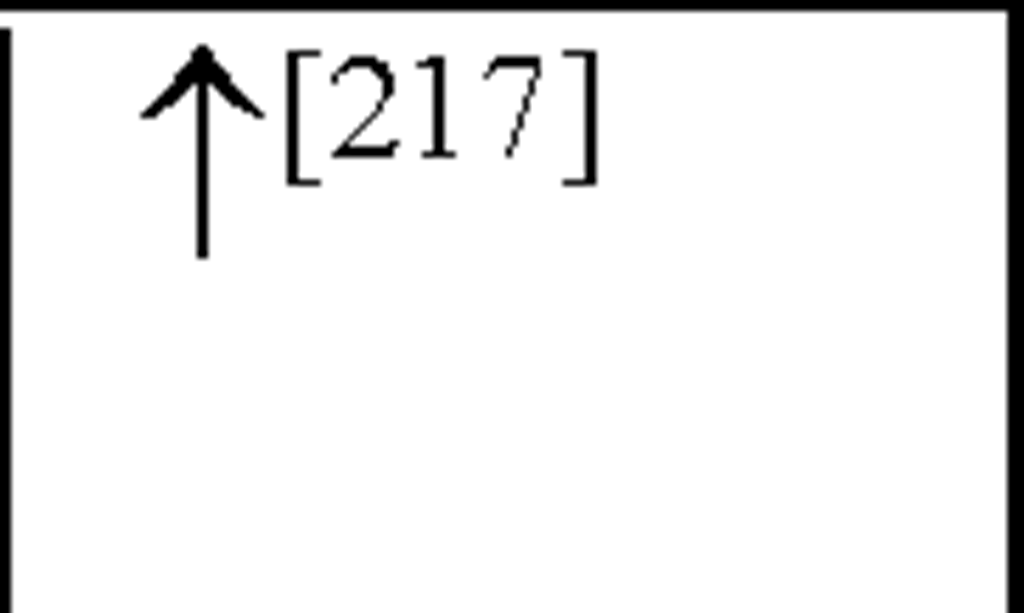 |
|
IL-7 |
 |
||||
|
IL-9 |
 |
||||
|
IL-10 |
 |
 |
|||
|
IL-11 |
 |
||||
|
IL-13 |
 |
||||
|
IL-18 |
 |
||||
|
TGFP |
 |
||||
|
IFNy |
 |
 |
|||
|
GM-CSF |
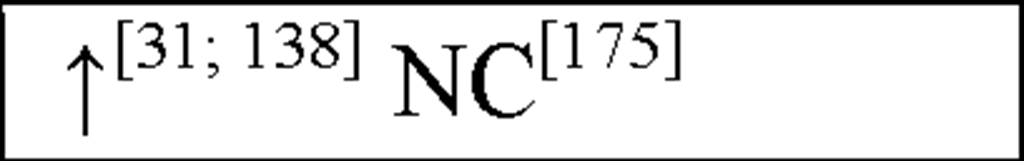 |
 |
|||
|
M-CSF |
 |
||||
|
G-CSF |
 |
||||
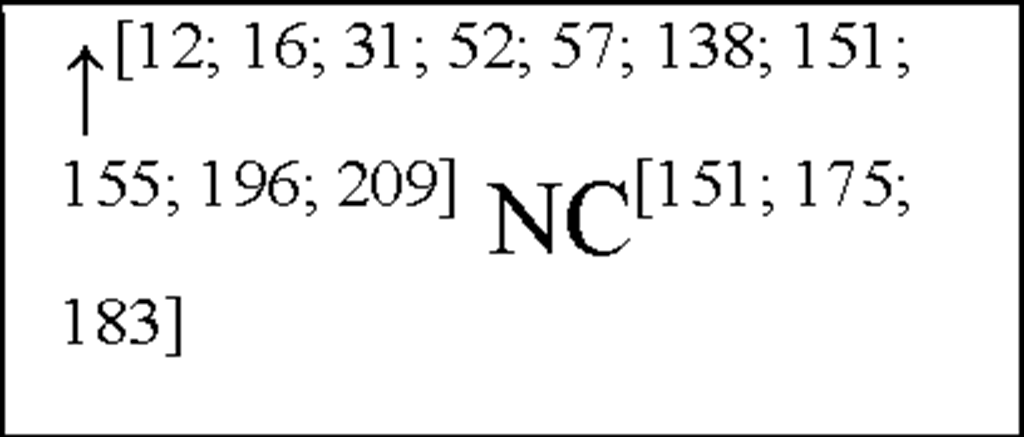
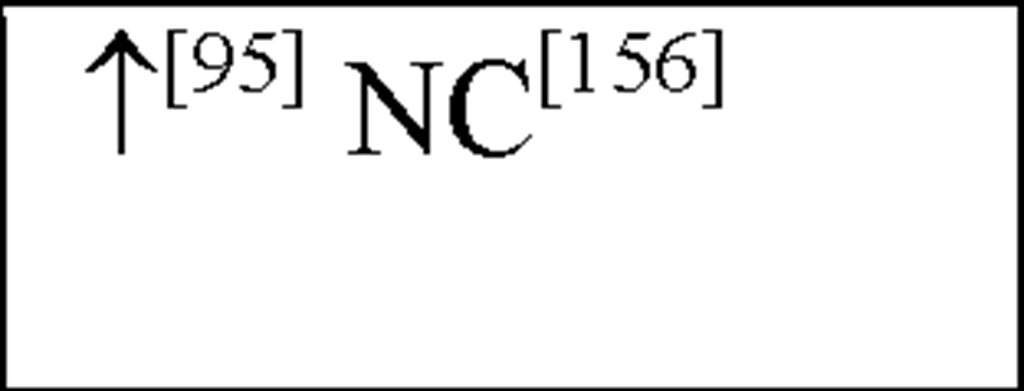
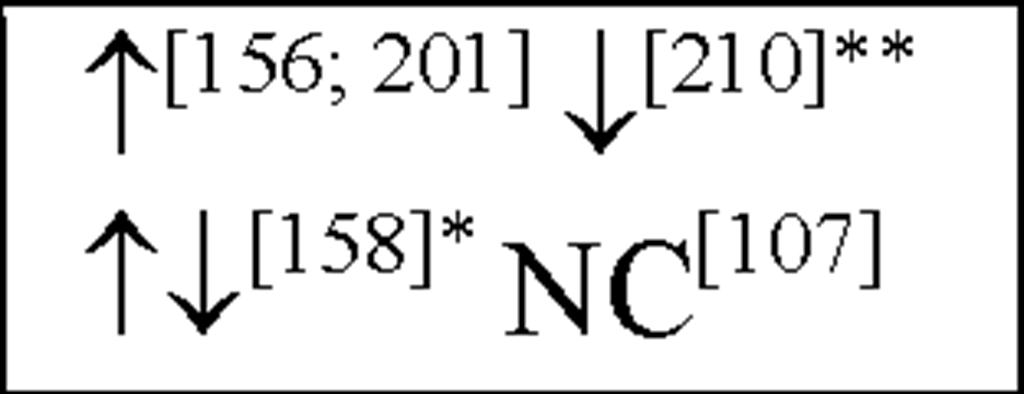
NC, no change in the level of that cytokine/chemokine in HI animals/humans compared to control animals/humans.![tmp17C-102_thumb[2] tmp17C-102_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmp17C102_thumb2_thumb.png) increase/decrease in the level of that cytokine/chemokine in HI animals/humans compared to control animals/humans. + indicates an increase in IL-8 levels in the ischemic lesion, but not significantly. * Indicates that the levels were subject to changes with time in comparison to controls. ** This study correlated the levels of IL-10 and the incidence of stroke. (adapted from references [34; 35]).
increase/decrease in the level of that cytokine/chemokine in HI animals/humans compared to control animals/humans. + indicates an increase in IL-8 levels in the ischemic lesion, but not significantly. * Indicates that the levels were subject to changes with time in comparison to controls. ** This study correlated the levels of IL-10 and the incidence of stroke. (adapted from references [34; 35]).
Markers of inflammation are not confined to the ischaemic tissue and evidence now suggests that inflammatory responses, even though initiated within the CNS, are also present systemically. Several studies have found elevated levels of pro-inflammatory cytokines in the periphery following cerebral ischaemia [54; 171]. The potential cardiac consequences of the increased peripheral pro-inflammatory mediators are seldom recognised, but they may play a pivotal role in the development of secondary cardiac dysfunction subsequent to cerebral ischaemia and HI. Indeed, a robust inflammatory response following cerebral ischaemia is associated with a higher occurrence of subsequent cardiovascular events [46].
TNF-a, a potent inflammatory cytokine is produced by mononuclear leukocytes (primarily macrophages; [2] and is known to induce myocyte apoptosis, and is thought to be an important mediator in the pathogenesis of MI [37; 221]. In addition, serum and cerebrospinal fluid (CSF) levels of TNF-a have been shown to be elevated following cerebral ischaemia and are correlated with a deterioration in neurological outcome [48; 212].
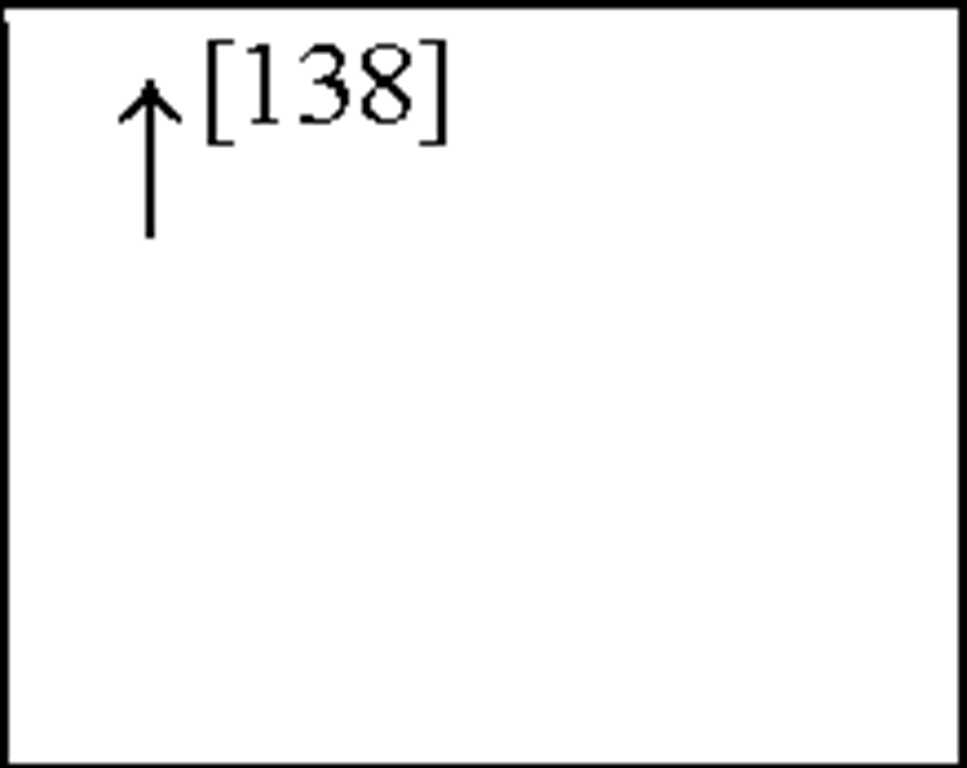
In human asphyxiated babies and newborn infants with HIE, CSF and plasma IL-1P and TNF-a levels were shown to be elevated within 48 hours [57; 151; 183]. In addition, asphyxiated babies that develop cerebral palsy (CP) or neurological deficits within 1 year have increased IL-ip and TNF-a levels within 48 hours after birth [57; 151]. In addition, following HI in neonatal rats, IL-1a, IL-ip and TNF-a have all been shown to be elevated 3-days post-insult ([31] see Figure 3) This suggests that both IL-ip and TNF-a play an important role in the acute stages of inflammation following HI, akin to their actions in peripheral inflammatory responses.
Interleukin-6 (IL-6) has both neurodegenerative and neuroprotective effects [61] and may hence play a dual role in pathologies of cerebral injury. The expression of IL-6 has been shown to occur as a result of other pro-inflammatory cytokines, in particular IL-1P and TNF-a [181]. Expression of IL-6 mRNA and bioactivity has been shown to increase following focal cerebral ischaemia in the rat [115]. Elevated CSF IL-6 levels in acute ischaemic stroke patients has been shown to correlate with the volume of infarction [200]. In patients with acute ischaemic stroke, some studies have reported an association between circulating IL-6 concentrations and brain infarct volume, stroke severity, or outcome up to 6 months [51; 158; 212]. Conversely, other studies have reported no association between serum IL-6 concentrations and infarct volume or stroke severity at 3 months [54; 200]. Previous studies have reported peak values of serum IL-6 within the first 10 days of stroke [51; 54; 158]. The question arises as to whether IL-6 might directly contribute to infarct pathogenesis, or is simply a marker of CNS or other inflammatory injury? In contrast, IL-6 has several proinflammatory effects [91; 208] which may contribute to the induction and evolution of early inflammatory injury in the brain and its vasculature. At the same time, IL-6 has been shown to have both neurotrophic [71] and anti-inflammatory properties [176] that may contribute to recovery following cerebral ischaemia.
In humans, CSF, plasma and serum IL-6 levels are up-regulated in asphyxiated newborns and HIE infants within 48-90 hours after birth [29; 57; 123; 175; 183]. Indeed a positive correlation between IL-6 levels and HIE severity / clinical outcome has been shown, suggesting that IL-6 is pro-inflammatory in this setting [123; 175].
Circulating Chemokines
The p-chemokines (which are primarily chemoattractants for mononuclear cells) consist of macrophage inflammatory protein (MIP)-ia, MIP-ip, monocyte chemoattractant protein (MCP)-1, MCP-2 and regulated on activation normal T cell expressed and secreted (RANTES). Recently, elevated levels of MCP-1 have been seen in the CSF 24 hours following ischaemic stroke, however, these levels did not correlate with the corresponding levels seen in the plasma [116]. Plasma concentrations of intracellular adhesion molecule-1 (ICAM-1) and MCP-1, have also been shown to be elevated following cerebral ischaemia [171].
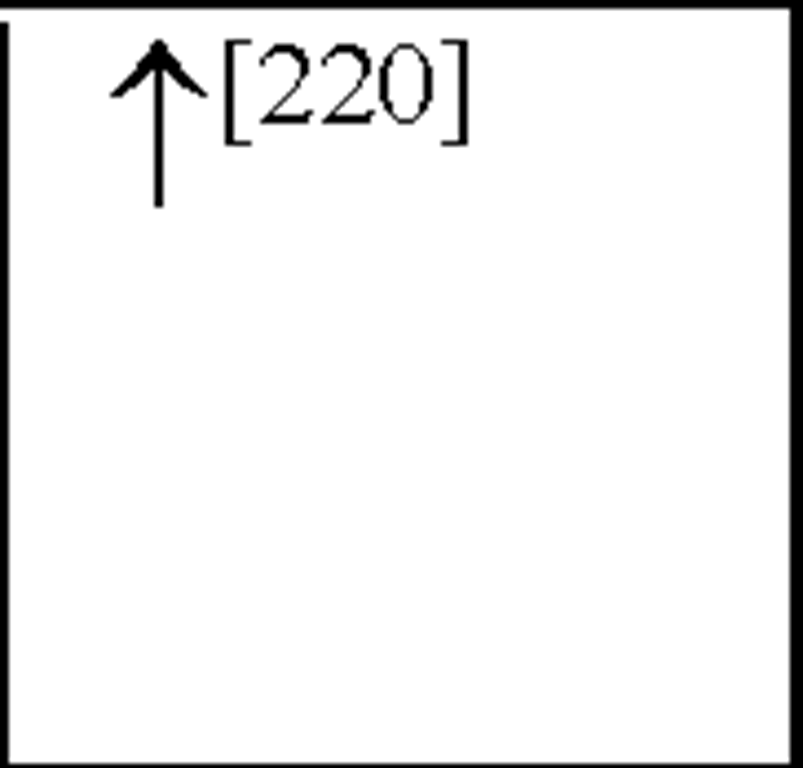
Interleukin-8 (the archetype chemokine) is a member of the a-chemokine family and plays a central role in neutrophil accumulation and activation. Grau et al., reported a significant increase in IL-8 plasma levels on day 1 following an ischaemic event, with levels still remaining elevated 3 and 7-days post-insult [67]. Circulating monocytes have been shown to be an important source of IL-8, which is a strong chemoattractant for polymorphonuclear neutrophils (PMNs) that can cause tissue damage by vessel plugging and release of oxygen-derived free radicals and proteinases [100]. In addition, IL-8 has also been shown to contribute to atherogenesis due to its mitogenic and chemoattractant effects on T lymphocytes and smooth muscle cells [204] and to plaque rupture by interference with matrix metalloproteinase-1 (MMP-1) expression [9; 130]. These results have been shown to be similar to results obtained from MI [1] as well as from stroke patients [105]. Recently, studies have shown both IL-8 and granulocyte macrophage-colony stimulating factor (GM-CSF) CSF levels are increased following cerebral ischaemia, peaking 2-days post-insult [201]. Furthermore, IL-8 levels have been shown to be higher in CSF than in plasma [105]. Studies have also shown an increased number of circulating IL-8 mRNA expressing PMNs (primarily neutrophils) after stroke and plasma IL-8 levels were correlated with the expression of IL-8 mRNA [104; 105].
In asphyxiated newborns (including those that develop CP), IL-8 concentrations in the CSF and serum were increased compared to control newborns and there was a positive correlation between the severity of HIE and the level of IL-8 [57; 175]. Therefore, IL-8 is crucial to the development of acute inflammation such that the neutrophil chemoattractant function of IL-8 may in fact be important to the initial development of HI-induced neuronal damage. These findings strengthen the hypothesis that monocytes and activated microglia are important contributors of increased IL-8 levels following cerebral ischaemia and cells in the peripheral circulation contribute to these increases. Recent work has also shown an increase in GM-CSF plasma levels following HI ([31] see Figure 3). In addition, clear evidence demonstrates that pro-inflammatory cytokines and chemokines present in the periphery may contribute to cardiovascular injury following cerebral ischaemia.
