Abstract
Cerebral hypoxia-ischaemia (HI) results in a multi-faceted complex cascade of events causing cell death and neurological dawmage to the central nervous system. Furthermore, cerebral ischaemia results in cardiovascular complications that can further confound the prognostic outcome of patients. This topic addresses the cardiovascular changes that occur subsequent to an ischaemic insult, regulation of the insular cortex, changes in the autonomic nervous system and the role of various circulating cytokines (both pro-inflammatory and anti-inflammatory) and chemokines. In addition, markers of oxidative stress and cardiac enzyme release following an ischaemic insult are also discussed. Given that lack of treatment options available, the use of beta-blockers and pre- and post-conditioning paradigms as possible treatment option to prevent the occurrence of secondary cardiac abnormalities in addition to CNS injuries have also been addressed.
Keywords: autonomic nervous system, inflammation, oxidative stress, cardiac enzymes.
Abbreviations
Cardiovascular Abnormalities Following Cerebral Complications
Cardiovascular abnormalities are a well-known predisposing factor for cerebral ischaemia. However, the associated effects of an ischaemic stroke on cardiac function are not as well recognised. The first findings of cardiovascular complications secondary to an ischaemic episode were reported over half a century ago. Since these reports, a relatively small body of literature has shown the deleterious effects of cerebral ischaemia on the cardiovascular system in both clinical and experimental settings.
Cerebral Ischaemia
During the process of acute cerebral ischaemia, cardiovascular abnormalities such as elevated blood pressure (BP), arrhythmias and ischaemic cardiac damage are evident and can hinder the final prognostic clinical outcome [131; 144; 146; 198]. Even though cardiovascular abnormalities are known to occur as a consequence of cerebral ischaemia, the underlying pathophysiological mechanisms have not been fully elucidated and characterised. Cardiovascular complications secondary to an ischaemic event were first observed by Byer et al., and Burch et al., who noted previously undiagnosed electrocardiogram (ECG) abnormalities such as upright T waves and prolonged Q-T intervals [20; 21]. Since this finding others have found clinically and experimentally arrhythmias, increased BP, increased plasma catecholamine levels, increased serum cardiac enzyme levels, decreased heart rate variability and increased rates of cardiovascular-related sudden death commonly associated with cerebral ischaemia [134; 137; 140; 141; 184; 215]. Myocardial dysfunction has been shown to occur during the acute stages of either ischaemic or haemorrhagic strokes, contributing to increased morbidity and mortality [73].
It has been estimated that as many as 27% of mortalities in stroke patients, occur as a result of cardiac failure where no prior history of cardiovascular complications has been reported [184]. In addition, 15-40% of stroke patients experience ECG changes following cerebral ischaemia [146]. Furthermore, Orlandi et al., found that 76.2% of patients suffering from right hemispheric cerebral ischaemia developed arrhythmias within 24 hours of the initial insult [149]. Within 20 minutes of the initial insult, 75% of all stroke patients experience fluctuations in BP, with 30% still remaining hypertensive after 1 week [19; 215]. The development of arrhythmias, including paroxysmal supraventricular tachycardia, atrial flutter and ventricular fibrillation, are associated with an increased (1.5-3.0 fold) mortality in patients with cerebral ischaemia compared to those who do not develop any form of arrhythmia [173]. Assessment of heart rate dynamics revealed that long-term abnormalities can be used as a predictor of stroke-related mortality [122]. In addition, reduced baroreceptor reflex sensitivity and prolonged hypertension worsen the prognosis of stroke morbidity and mortality [44; 169]. Post-mortem analysis of otherwise healthy patients who perish from an ischaemic stroke, illustrates myocardial necrosis (myocytolysis), myofibrillar degeneration and subendocardial congestion [38]. Furthermore, cerebral ischaemia-induced myocardial damage is characterised pathologically by scattered foci of microlesions [68; 103]. Reducing the incidence of cardiovascular complications subsequent to cerebral ischaemia would clearly result in better prognosis and a long-term improvement in the quality of life.
Hypoxia-Ischaemia
In addition to neuronal damage as a consequence of hypoxia-ischaemia (HI), multi-organ dysfunction (MOD) has also been documented [81; 180]. However, unlike the brain where extensive work has been carried out to elucidate the underlying mechanisms of damage, little is know about the cardiovascular response following HI-induced injury. One mechanism that may cause the MOD associated with HI, is a process that is similar to the diving reflex. The diving reflex re-distributes blood flow away from the periphery and splanchnic area, to increase delivery to vital organs such as the heart, adrenals glands and brain, to protect these organ against ischaemic-injury [90]. However, prolonged redistribution of blood flow and diminished oxygen and nutrient supply associated with the hypoxia, will ultimately causes damage to these vital organs.
The development of ECG abnormalities and injuries to systemic organs in infants having suffered from acute birth asphyxia has been documented [160; 162]. These studies attributed the systemic organ injury to hypo-perfusion resulting from a decrease in cardiac output. In addition, it has been shown that as many as 78% of neonates sustain some degree of cardiovascular impairment following an hypoxic episode at birth, as indicated by elevated cardiac enzymes or requiring pressor/volume support [81]. In this report, Hankins et al. showed maximal cardiac impairments 5-days post-insult in humans [81]. In addition, Yang et al., have demonstrated injury to myocardial mitochondria following HI induced brain damage; associated with calcium overload and a concurrent decrease in mitochondrial complex IV activity [231]. Furthermore, maturation of the myocardial enzyme, lactate dehydrogenase (LDH) was also shown to be decreased following HI-induced brain damage in young rats [42].
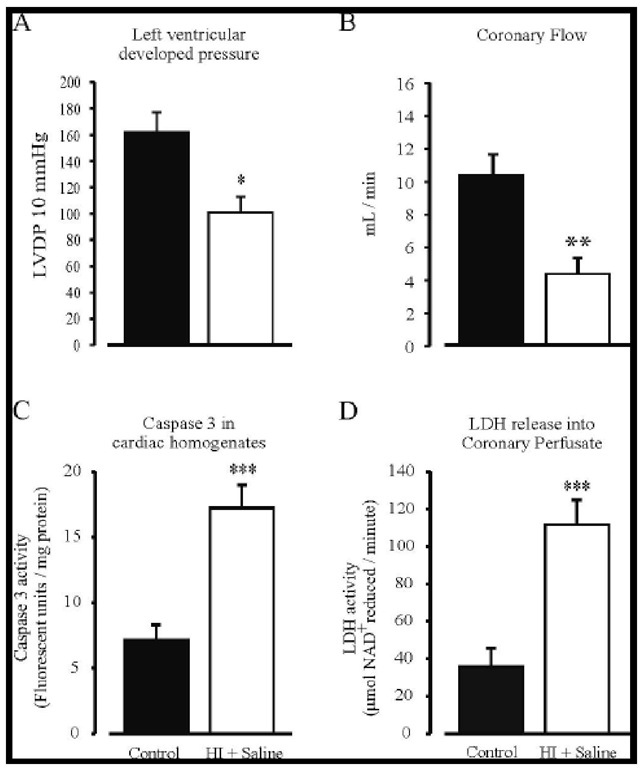
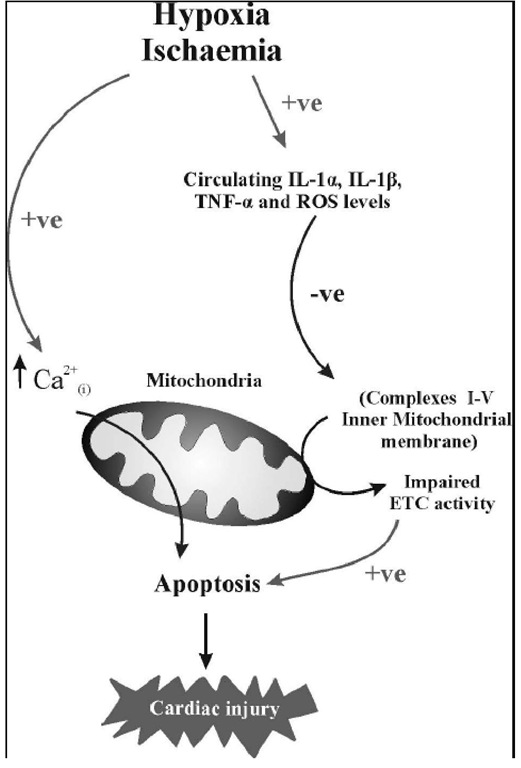
Recent work has shown that cardiac haemodynamics (left ventricular developed pressure (LVDP) and coronary flow (CF); see Figure 1A and B) are impaired following an HI-insult and are most pronounced 7-days post-HI (Clarkson, Kapoor, Harrison, Jackson and Sammut, unpublished data), which is consistent with Hankins findings in 2002 where maximal findings were seen 5-days post hypoxic insult in humans [81]. Furthermore, we show impaired mitochondrial enzyme kinetics, and increased cardiac caspase-3 activity coupled with increased LDH leakage into the Langendorff perfusate (see Figure 1C and D) 7-days post-HI. We also demonstrate that following an HI-induced insult, there is a significant increase in circulating interleukin (IL)-1P and tumour necrosis factor (TNF)-a levels (see below [31]). The increases in pro-inflammatory cytokine levels occur prior to the impairment in both cardiac haemodynamics and mitochondrial energetics. These findings of an HI-induced increase in cardiac caspase-3 activity driven either through an extrinsic pathway involving inflammation or through an intrinsic mitochondrial-channelled pathway, confirms the presence of myocardial apoptosis, which may consequently play an important role in cardiac damage following cerebral HI (see Figure 2).
A clear body of evidence has accumulated that illustrates cardiovascular abnormalities following an ischaemic-insult. However, the underlying mechanisms associated with this damage have not been fully elucidated. Outlined below are a few mechanisms that may contribute in part to the cardiac damage seen post-cerebral-insult.
Figure 1. The effects of non-intervention control (black) and HI + saline (white) on LVDP at 10 mmHg (Panel A), sinus coronary flow (Panel B), Caspase 3 activity (Panel C) and LDH leakage into coronary perfusate (Panel D) were assessed from hearts isolated 7-days post-HI. Panel A shows an impairment in LVDP at 10 mmHg following HI. Panel B shows a decrease in sinus coronary flow following an HI-insult. Panel C shows increased caspase 3 activity following an HI-insult. Panel D shows increased LDH leakage from hearts following HI + saline treatment. * = P<0.05, ** = P<0.01, *** = P<0.001 versus non-intervention control.
Release of Cardiac Enzymes
Membrane encapsulated cellular constituents are externalized after cellular damage, enabling assessment of tissue injury through analysis of tissue-specific enzyme levels within the circulation. A number of studies have shown that creatine-kinase-myocardial-band (CK-MB), a cardiac specific enzyme, is elevated in certain patients following cerebral ischaemia, subarachnoid haemorrhage and in patients with head trauma [50; 77; 141]. However, it should be noted that these changes are in the absence of any clinical evidence of underlying cardiovascular complications. Assessment of post-stroke serum levels has been proposed as an indicator of early ischaemia-mediated death [141].
Figure 2. Schematic diagram showing proposed mechanisms contributing cardiac damage following cerebral hypoxia-ischemia. -ve = negative effect; +ve = positive effect.
Compared to the abrupt rise and fall (24 hours) of CK-MB following myocardial infarction (MI), the temporal pattern of CK-MB elevation after stroke is gradual and sustained for a period of days, implying a prolonged state of cardiac deterioration [11; 166]. The myocytolysis accompanying cerebral ischaemia is believed to be the cause of raised CK-MB levels. Two early reports showed differential CK-MB enzyme expression patterns between stroke and non-stroke patients suffering acute MI [139; 141]. These reports demonstrated sharp increases in CK-MB levels within the first 2 days following an MI, however, in stroke patients, CK-MB enzyme levels exhibited a slow, yet gradual increase which did not peak until the 6th day. The authors described this process as reflecting a sub- acute process that is compatible with diffuse myocardial injury occurring during the first week following a stroke and mirrors the changes seen in catecholamine levels [139].
Troponin T, which is regarded as a more specific cardiac enzyme, was not elevated following cerebral ischaemia in one stroke study, suggesting CK-MB elevations following a stroke are not cardiac in origin [11]. However, Ay et al, may have failed to study an appropriate patient population when assessing secondary cardiac dysfunction following cerebral ischaemia. The inclusion in their cohort of relatively young patients with left hemispheric lesions and the absence of sympathetic activity does not parallel what is encountered in patients with cardiac complications following cerebral ischaemia. As stated previously, secondary cardiac dysfunction following cerebral ischaemia commonly results from right hemispheric insular cortex lesions in elderly patients. In a different study, James and co-workers established the importance of these parameters by demonstrating 17% of aged ischaemic stroke patients had raised troponin T levels 12-72 hours following the initial insult, and that this elevation was associated with a 3-fold increased likelihood of mortality [89]. In addition, Moller and colleagues reported a significant elevation in troponin T levels following asphyxiation in neonatal infants [129]. Furthermore, these same authors reported that asphyxiated infants who subsequently develop heart failure have higher troponin T levels than asphyxiated infants who do not develop heart failure. We suggest that cardiac specific Troponin T may indeed serve as a reliable indicator of stroke-induced myocardial damage.