1. Introduction
It is clear that the phenotype of an organism cannot be completely described by its genotype alone. The phenotypic differences between humans, or between individuals of other species, appear to be greater than can be explained by the frequency of single nucleotide polymorphisms (SNPs) even when the environment is “controlled”. In some cases, we know that the variation in phenotype can be ascribed to epigenetic rather than genetic differences. Epigenetic changes involve modifications to the DNA, such as methylation of cytosine residues, and modifications to the chromatin, such as acetylation and methylation of histone proteins. One of the underlying aspects of epigenetic control of gene expression is that it is stochastic, that is, there is a certain probability of a particular epigenetic state being established at a particular locus, but this is not necessarily 100%, and has profound implications on phenotype.
2. Variegation
For centuries, variegated appearances have been observed in plants and mammals. Variegated leaves and brindle coat colors in dogs are good examples. In humans, the iris often has a flecked appearance. Variegation can be defined as the differential expression of a particular gene among cells of the same cell type. The more recent finding of variegation in the expression of transgenes has provided a tractable experimental system for studying the molecular basis of this phenomenon in mammals (Mintz and Bradl, 1991; for review, Martin and Whitelaw, 1996). For reasons that remain unclear, transgenes appear to be particularly sensitive to variegation, and, at least in these cases, differential expression of the foreign DNA element is the result of differential transcriptional activity. The differential transcriptional activity correlates with epigenetic modifications at the transgene locus. In general, expressing cells are found to display hypomethylation of cytosine residues and an open chromatin state at the transgene promoter, while nonexpressing or silent cells are found to be hypermethylated at CpG dinucleotides, with a closed chromatin state (Allen et al., 1990; Festenstein et al., 1996; Garrick et al., 1996; Weichman and Chaillet, 1997; Sutherland etal., 2000; Kearns etal., 2000). This chromatin compaction is reminiscent of what had already been reported as the molecular basis of a phenomenon called position effect variegation (PEV) in Drosophila (Henikoff, 1990), although Drosophila lacks CpG methylation.
3. Variable expressivity
Variable expressivity, where a range of expression states is observed among litter-mates, is another interesting feature of some transgenes. Variable expressivity, also called incomplete penetrance, is a term frequently used by clinicians to describe the feature of diseases where despite several family members being carriers of the disease, only some actually go on to present with symptoms. In these cases, the variable expressivity of the disease is rationalized by differences in quantitative trait loci (QTLs) between family members. However, variable expressivity at transgenes made in genetically identical, inbred strains of mice cannot be so simply explained. Originally, this was reported for transgenes made in mixed genetic backgrounds (Allen et al., 1990; Dobie et al., 1996), or closed colonies (Sutherland et al., 2000; Kearns et al., 2000), but these results have also been confirmed in inbred strains, where the individuals are genetically identical (Weichman and Chaillet, 1997). In these cases, transgene expression can range from very high, to variegated, to completely silenced, an unexpected event in isogenic individuals.
4. Subtle parent-of-origin effects
Furthermore, the extent to which a transgene is expressed can be dependent on the parent-of-origin of the transgene. This is often a subtle parent-of-origin effect, which differs from classic parental imprinting (Maggert and Golic, 2002; Preis etal., 2003). Classic parental imprinting refers to a situation in which an allele is active when inherited from one parent and inactive when inherited from the other. Subtle parent-of-origin effects refer to situations in which the probability of expression shifts depending on whether the allele has been inherited from the mother or the father. For example, the amount of variegation at the transgene may vary by approximately 20%, depending on parental origin (Preis etal., 2003). If the transgene displays variable expressivity, that is, a range of expression states between individuals, then the parental origin can shift the proportion of individuals in each class (Allen et al., 1990; Kearns et al., 2000). This shift is a probabilistic event, rather than a complete switch in expression, as seen in parentally imprinted alleles.
Recent work on Drosophila P transposon insertions has shown that 22 of 23 insertions into the Drosophila Y chromosome display subtle parent-of-origin effects (Maggert and Golic, 2002). In this case, the phenotypic assay is eye color, where the amount of variegation between the red and white pigment varies according to the parental origin of the Y chromosome. For some insertion sites, the transgene is expressed more following maternal transmission, and at other sites the reverse is observed. This result implies that large regions of the Y chromosome in Drosophila are subject to parent-of-origin effects (Maggert and Golic, 2002). In mammals, it is also possible that large portions of the genome are subtly imprinted since small effects could easily have been overlooked.
It is now clear that variegation, variable expressivity, and subtle parent-of-origin effects are not peculiar to transgenes but are seen in endogenous alleles in mice, plants, and Drosophila. To date, the reported alleles that display these effects produce visual phenotypes. It is still unclear how common such alleles are, and whether they exist in humans. In plants and mammals, these alleles are now termed metastable epialleles (Brink, 1960; Matzke etal., 1994; Hollick etal., 1995 and Rakyan et al., 2002).
Examples of murine metastable epialleles include agouti viable yellow (Avy) (Perry et al., 1994), agouti intracisternal A particle yellow (Aiapy) (Michaud et al., 1994), and agouti hypervariable yellow (Ahvy) (Argeson, 1996), also axin fused (axinFu) (Reed, 1937; Ruvinsky and Agulnik, 1990; Zeng et al., 1997), axial defects (Essien et al., 1990), disorganization (Hummel, 1959), and murine CDK5 activator binding protein IAP (mCABPIAP) (Druker, et al., 2004). It is interesting to note that Avy, Aiapy, Ahvy, axinFu, and mCABPIAP all arose as a result of the stable integration of a retrotransposon. In the case of the agouti alleles and axinFu, the variable expressivity arises as a direct result of variable activity at a cryptic promoter in the retrotransposon long terminal repeat (LTR), which reads out into the adjacent DNA (Michaud et al., 1994; Perry et al., 1994 and Argeson, 1996). It is possible that all endogenous metastable epialleles will turn out to be under the control of nearby retrotransposons. Given that over 9% of the human genome has been classified as retrotransposon-like (International Human Genome Sequencing Consortium, 2001), metastable epialleles may also be present in reasonable numbers in humans.
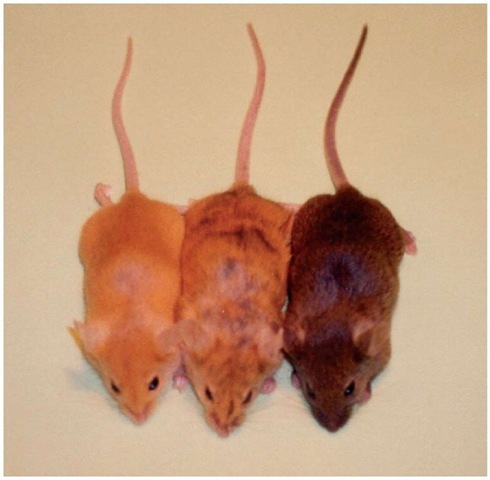
The Avy allele is perhaps the best-characterized metastable epiallele. In this case, the allele results from an intracisternal A particle (IAP) insertion, upstream of the agouti gene (Duhl etal., 1994). When the cryptic promoter in the IAP LTR is active, it causes constitutive expression of the agouti gene (Duhl etal., 1994). Agouti is a signaling molecule that causes a shift in pigment production from black to yellow. Normally, the agouti gene is under the control of hair-cycle-specific promoters, and is produced for only a short period in the hair growth cycle, to produce a subapical yellow band on an otherwise black hair. The resultant mouse appears brown (an agouti coat). However, when the agouti gene is constitutively expressed, a completely yellow coat results, along with other pleiotropic effects including obesity and diabetes. Sometimes, a genetically identical mouse will have an agouti-colored coat (termed pseudoagouti). In this case, the cryptic promoter is silent, and CpG-methylated, and the agouti gene undergoes normal spatiotemporal expression (Morgan et al., 1999). A spectrum of intermediate mottled phenotypes, where there is variegated Avy allele expression, is also observed (see Figure 1).
The Avy allele displays a subtle parent-of-origin effect (Morgan et al., 1999), as do many other endogenous murine metastable epialleles (Reed, 1937; Belyaev etal., 1981; Essien etal., 1990; Duhl etal., 1994; Argeson, 1996; Rakyan etal., 2003). In this case, a yellow female produces a greater proportion of yellow offspring than a yellow male. There is a 15% difference in the proportion of pseudoagouti mice produced from these reciprocal crosses (Morgan et al., 1999).
Figure 1 Genetically identical mice carrying the Avy allele display variegation and variable expressivity. The mouse on the left is termed yellow, the middle mouse is termed mottled, and the mouse to the right is termed pseudoagouti
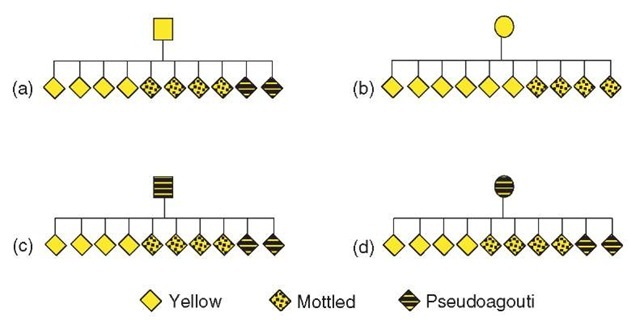
Again, this is a small, but significant shift in the spectrum of coat color phenotypes seen at the Avy allele, which is dependent on parental origin (see Figure 2).
5. Transgenerational epigenetic inheritance
An additional effect seen at some murine transgenes (Hadchouel et al., 1987; Allen etal., 1990; Kearns etal., 2000; Sutherland etal., 2000) and some metastable epialleles, including the Avy allele, is transgenerational epigenetic inheritance (Belyaev etal., 1981; Wolff, 1978; Morgan etal., 1999; Rakyan etal., 2003). A yellow-coated Avy female produces more yellow offspring than a pseudoagouti female, despite being genetically identical (Morgan et al., 1999; see Figure 2). That is, there is some memory of the phenotype, and, therefore, the epigenotype of the mother. The mechanism of epigenetic inheritance is not known, but it seems likely that there is incomplete clearing of the epigenetic marks between generations. Again, it is unclear how often epigenetic inheritance may be occurring, but it certainly has profound implications on the inheritance of phenotype in general. Interestingly, although epigenetic inheritance can occur through the male germline (Rakyan et al., 2003), it is more common following female transmission. It is interesting to consider whether this mode of inheritance has some adaptive value. In higher organisms, the mother will often stay with the young after birth. It may be advantageous for the phenotype of the offspring to be more closely related to that of the mother, who is presumably well adapted to the surrounding physical environment.
Figure 2 Schematic pedigrees of coat color inheritance at the Avy allele. Mice heterozygous for the Avy allele mated with congenic animals, carrying a null agouti allele. Only offspring carrying the allele are displayed. Comparing (a) and (b), there are more yellow mice observed following female transmission of the allele from a yellow parent. This is a subtle parent-of-origin effect, which shifts the proportion of each phenotype of offspring. Comparing (b) and (d), the yellow female produces more yellow offspring than the pseudoagouti female, so the range of phenotypes of the offspring is influenced by the phenotype of the dam. This is transgenerational epigenetic inheritance
A recent finding in Drosophila suggests that epigenetic inheritance of chromatin state could provide a mechanism for rapid evolution of morphological traits (Sollars etal., 2003). Sollars and coworkers found that epigenetic variants produced de novo, can cause phenotypic variations. The variants were produced by genetically altering levels of chromatin proteins. Unexpectedly, the phenotypic variation could be inherited through the female germline for successive generations, in the absence of the original genetic mutation. The inheritance resulted after only one meiotic generation, several generations faster than is required for genetic variation to drive to fixation (Rutherford and Henikoff, 2003).
The existence of variable expressivity and epigenetic inheritance, raises the possibility that phenotypic variation in humans may not be all QTL-based. Moreover, some of the evolution of quantitative traits may have been due to epigenetic, rather than genetic changes. Consider a situation in which an allele displays variable expressivity, due to differences in epigenetic state of the allele. One of the pheno-types may be selected owing to environmental change, and inherited through the germline owing to transgenerational epigenetic inheritance. However, since epige-netic inheritance is never complete, variation is not lost, which may allow for a more plastic adaptation than the selection of genetic variation affords.