1. Introduction
Gregor Mendel grew up in an area of central Europe called Moravia, where there was great interest in plant and animal breeding. As a young man, Mendel was intrigued by the observation that for some traits, when two individuals with different phenotypes are crossed, all members of the first generation express the trait characteristic of only one of the parents. The trait is faithfully expressed and is not a combination of the traits from the two parents. But when members of that first generation are crossed, the trait that had been lost suddenly reappears, perfectly expressed, in the next generation in a predictable and reproducible fashion. This “lost” trait is seen in one-quarter of the individuals produced, while the remaining three-quarters continue to express the trait seen in the previous generation. While studying in Vienna, Mendel formulated his hypothesis that heredity is determined by units that occur in pairs. He returned to his monastery at Bruno, and after spending 8 years carefully selecting seven traits in pisum that follow this pattern, proceeded to test and demonstrate the truth of his hypothesis. Note that it took Mendel many years to select a few traits that follow this inheritance pattern. That is because most traits in peas and humans do not obey these deterministic rules of expression that we now refer to as “Mendelian inheritance”.
Mendel’s great contribution was the discovery of unitary inheritance, which was in dramatic contrast to the then existing theory of inheritance by a blending of characters. Modern interpretations are usually summarized as the principles of segregation and independent assortment. The principle of segregation states that alleles are paired and each gamete normally receives only one member of each pair. Exceptions to this principle include trisomy and uniparental disomy. Independent assortment refers to the fact that pairs of genes tend to segregate independently of each other. The exception to this principle is genetic linkage.
The concepts of dominant and recessive defined by Mendel refer to the pheno-typic expression of alleles in heterozygotes, not to intrinsic characteristics of gene loci. Therefore, the older practice used by some early geneticists of referring to a locus as dominant or recessive should be avoided.
In diploid organisms, a homozygote (adj. homozygous), strictly speaking, is an individual that has two identical alleles at a gene locus, while a heterozygote (adj. heterozygous) has two different DNA sequences at a particular locus. Note that in the fields of human and medical genetics, the term “homozygote” is also frequently used to refer to an individual with a homozygous phenotype, even though the genotype may consist of two different disease-causing mutations. Such individuals are compound heterozygotes or allelic compounds.
Inheritance patterns are determined by one or more genes. From a functional standpoint, genetic factors can be thought of as monogenic or multigenic and deterministic or nondeterministic. Medical geneticists traditionally subdivide inheritance patterns into (1) chromosome abnormalities (multigenic, deterministic), (2) single-gene disorders (monogenic and usually deterministic, but sometimes nondetermin-istic), and (3) multifactorial or complex conditions (multigenic, nondeterministic). One also must consider the roles of environment, epistasis, and chance in the production of phenotypic expression.
Exceptions and variations on the simple, basic themes of monogenic inheritance commonly involve issues of penetrance and expression.
1.1. Penetrance
Penetrance (P) refers to the proportion of gene carriers that express a particular trait. P ranges from 0 to 1 (or 0 to 100%). Traits or conditions with decreased or incomplete penetrance (P < 1.0 or < 100%), therefore, fall into the category of monogenic nondeterministic inheritance.
1.2. Expression
Expression refers to the observable characteristics of the phenotypic trait in question such as quantity, quality, severity, age of onset, body part or organ system involved, and so on. Variable expression may occur within families (intrafamilial variability) or between/among different families (interfamilial variability). Intrafa-milial variability may be due to epistasis, environment, chance, or mosaicism. Interfamilial variability may be due to epistasis, environment, chance, and allelic or locus heterogeneity.
Penetrance and expression may be affected by the gender of an individual, the age of an individual (i.e., time), transmission through families, environmental factors, the effects of other genes (epistasis), stochastic factors (chance), and the presence of mosaicism.
• Examples of sex-influenced expression include male pattern baldness, breast and ovarian cancer, and anomalies of internal and external genitalia.
• For all disorders of postnatal onset, penetrance will depend on age. It should be noted that the term “variable penetrance” is commonly misused for the concept of “variable expressivity”. In fact, the term “variable penetrance” is only appropriate for conditions of postnatal onset, where there are different penetrance figures at different ages.
• Transmission through a family can be associated with a change in the size and, therefore, the deleterious effects of trinucleotide repeat expansion mutations, a process called anticipation. Imprinting causes expression to be determined by the gender of the transmitting parent (see Article 36, Variable expressivity and epigenetics, Volume 1).
• Environmental factors may be required for phenotypic expression. For example, the autosomal recessive inborn error of metabolism phenylketonuria is characterized by mental retardation. But a genetic deficiency in the activity of the enzyme phenylalanine hydroxylase requires dietary phenylalanine to produce this deleterious phenotype.
• Epistasis is assumed to be a ubiquitous principle, based on developing knowledge about the complexities of genetically determined biomolecular interactions in cells and tissues and on data collected from plant and animal experiments. For most or all traits, expression of an allele probably depends at some level on interactions with other gene products.
• A factor traditionally ignored in discussions about the cause of genetic disease is chance. Kurnit et al. (1987) used computer modeling to show that some of the non-Mendelian familial clustering of anomalies usually attributed to concepts such as “reduced penetrance” and “multifactorial inheritance” may be accounted for by simple, random chance. Central to this concept is the idea that biological processes, like many other phenomena that we encounter daily, are error-prone, nonlinear systems. The complex processes of embryologic development and physiologic homeostasis may be very sensitive to small perturbations, which are by themselves “within normal limits”, that subsequently, by chance, are amplified by the randomness that is inherent in all such systems.
• Mosaicism describes the situation in which an individual has cells with more than one genotype. For example, females are mosaic for X-linked gene expression, because some of their cells express the father’s X and other cells express the mother’s X. Individuals with sporadic (i.e., nonfamilial) cases of autosomal dominant disorders may have less severe manifestations than those who inherit the condition from a parent because in some sporadic cases, the mutation occurred in the postfertilization zygote and only a fraction of body cells carry the mutant allele. Postzygotic chromosome rearrangements cause a mosaic pattern to be found on karyotyping (see Article 18, Mosaicism, Volume 1). Postzygotically acquired mutations may be evenly distributed throughout the body (e.g., mild osteogenesis imperfecta caused by mosaicism for a severe lethal disease mutation), limited to specific parts or sides of the body (e.g., segmental neurofibromatosis type I), or limited to specific tissues of origin (e.g., tissue-specific mutations and chromosome rearrangements associated with malignancy).
2. Multigenic deterministic inheritance
This category includes abnormalities that involve large segments of the genome, traditionally called “chromosome abnormalities” in standard texts (see Article 11, Human cytogenetics and human chromosome abnormalities, Volume 1, Article 12, The visualization of chromosomes, Volume 1, Article 13, Meiosis and meiotic errors, Volume 1, Article 16, Nondisjunction, Volume 1, Article 17, Microdeletions, Volume 1, Article 22, FISH, Volume 1, Article 67, Approach to rare monogenic and chromosomal disorders, Volume 2, Article 71, Advances in cytogenetic diagnosis, Volume 2, and Article 87, The microdeletion syndromes, Volume 2). Examples include trisomies, partial trisomies, partial monosomies, and smaller duplications and deletions. Cytogenetically detectable abnormalities are found in a little over 0.5% of newborn babies, and in a much higher percentage of miscarriages and fetal deaths. Submicroscopic cytogenetic rearrangements include microduplications and microdeletions that cause “contiguous gene” syndromes.
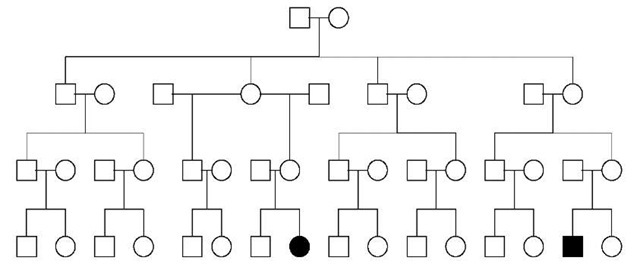
Figure 1 Pedigree with only two second cousins affected out of 30 relatives in a four-generation family. Such pedigrees are frequently assumed to be explained by complex or multifactorial inheritance. Note that this pedigree is also consistent with several other possible mechanisms of inheritance including the following: an unbalanced chromosomal rearrangement where intervening, unaffected relatives carry the balanced rearrangement; autosomal dominant with incomplete penetrance; X-linked inheritance with incomplete penetrance in heterozygous females; and mitochondrial inheritance where expression depends on an environmental exposure such as aminoglycoside-induced deafness (compare with Figure 2)
Submicroscopic microdeletions and microduplications may be vertically transmitted from generation to generation as are the autosomal dominant disorders described below. Syndromes caused by chromosome abnormalities may be inherited through families in a pattern suggesting complex or multifactorial inheritance (Figure 1). For example, cousins may have unbalanced karyotypes and be affected, while intervening, phenotypically unaffected relatives are asymptomatic carriers of a balanced chromosomal rearrangement.
3. Monogenic deterministic inheritance
Frequently called “Mendelian” inheritance patterns, these are well covered in standard text topics of genetics. They include the “single-gene disorders”, autosomal and X-linked dominant and recessive inheritance, and mitochondrial patterns. The major features are summarized below.
3.1. Autosomal dominant inheritance
This occurs when one allele of a heterozygous pair causes a phenotypic trait. Many classic human genetic disorders are caused by this mechanism and follow this pattern. An organism heterozygous for the trait-causing allele has a 50% chance, or one chance out of two, of passing that allele on to each offspring. Transmission in families, therefore, is from one generation to the next in a pattern that is sometimes called “vertical inheritance”. Males and females are typically both affected and affected equally unless there is sex-limited expression. Male-to-male transmission occurs, distinguishing this from X-linked dominant inheritance.
Mendel’s definition of dominant expression was that the heterozygote appeared identical to the mutant homozygote. In human genetics, autosomal dominant diseases are relatively rare (frequencies generally less than one in several thousand). Therefore, heterozygote matings are extremely rare, and the human homozygous phenotype is unknown for most human “dominant” disorders. A few are known. Huntington disease appears to be a true Mendelian dominant because homozygote and heterozygote expressions are similar. On the other hand, the condition known as classic achondroplasia, traditionally called an autosomal dominant, is not. Homozygous offspring from two heterozygous parents are typically much more severely affected, and usually die at birth or in the first few weeks of life. Heterozygous achondroplasia is compatible with long survival and reproduction, while homozygous achondroplasia is a genetic lethal. Other terms used to describe this phenomenon include “incomplete dominant” and “semidominant” inheritance.
A paternal age effect is observed for some autosomal dominant traits. This refers to the phenomenon where new mutations are associated with advanced paternal age because mutations due to unrepaired DNA copy errors increase proportionally with increasing paternal age.
3.2. Autosomal recessive inheritance
This occurs when two phenotypically normal parents carry mutant alleles at the same locus and produce a phenotypically affected offspring that inherits a mutant allele from both parents. Penetrance in heterozygotes is zero, because they appear identical to the wild-type homozygotes. Because affected sibs may be produced by heterozygous parents, this pedigree pattern has been referred to as “horizontal inheritance”. Carrier parents have a 25% risk, or one chance out of four with each pregnancy, of having a child who is affected by virtue of being homozygous or an allelic compound. Unaffected sibs of affected patients each have a two-third chance of being a carrier.
Consanguinity is frequently a cause of both parents carrying the same rare recessive allele. Parental consanguinity is not significantly increased, however, for common recessives such as sickle-cell disease, cystic fibrosis, or hemochromatosis. Consanguinity can result in a phenomenon called “pseudodominant inheritance”. This occurs when a homozygous (or compound heterozygous) affected individual mates with an unaffected heterozygote, producing an affected offspring. A parent and child are affected but the condition is autosomal recessive because the het-erozygote is not affected. Pseudodominant inheritance of common recessive traits does not require consanguinity.
3.3. X-linked inheritance
This occurs when a phenotype is caused by an allele on the X chromosome. Because of the pedigree patterns produced, this type of inheritance is sometimes called “diagonal inheritance”. X-linked inheritance is characterized by the lack of male-to-male transmission and the fact that affected males pass their mutation-bearing X chromosomes on to all of their daughters but to none of their sons. Traditionally, human geneticists talk about X-linked recessive and dominant disorders. The former is characterized by no apparent manifestations in carrier females, but sometimes, subtle findings are apparent if a careful evaluation is performed. The latter term is used when both males and females are significantly affected, even though on close examination differences may be found.
3.4. Autosomal and X-linked intermediate expression
When carrier females have a phenotype much milder than that of affected males, a term such as X-linked intermediate may be used. In fact, because of random variation in Lyonization, a variable degree of phenotypic expression can be expected in females who carry alleles for X-linked disorders and traits.
Phenotypes may be dominant or recessive depending on how one defines them. For example, the disease sickle-cell anemia is autosomal recessive but the phenomenon of in vitro sickling is a dominant trait.
Some genes may produce a wide range of mutant phenotypes. Mutations at the locus for the tissue nonspecific alkaline phosphatase gene may be recessively expressed (i.e., no phenotype in the heterozygote but severe hypophosphatasia in the homozygote or allelic compound) or dominantly expressed as childhood or adult-onset hypophosphatasia. Heterozygous carriers of alleles that cause the recessive, multisystem disorder homocystinuria may have a degree of hyperho-mocyst(e)inemia that places them at increased risk for adult vascular disease. It seems reasonable to predict that future research may find subtle phenotypic effects associated with heterozygosity for a number of other recessive disorders.
3.5. Y-linked inheritance
Holandric inheritance occurs when a phenotype is caused by an allele on the Y chromosome and is characterized by exclusive and obligatory male-to-male transmission. As of November 2004, the OMIM database (OMIM, 2000) listed 45 genes or phenotypes in the Y-linked catalog.
3.6. Mitochondrial inheritance
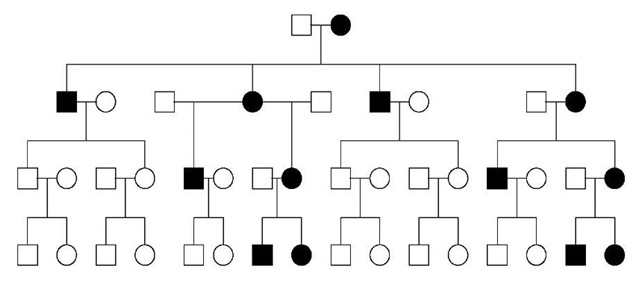
When a phenotype is caused by an allele in the mitochondrial genome, a pedigree phenomenon called “maternal inheritance” is produced, whereby all of the offspring of an affected woman are either affected or nonpenetrant carriers and none of the offspring of affected men are affected or carriers (Figure 2). Variable expression is due to the fact that within an organism’s cells there is a mixture of normal and mutant mitochondria (heteroplasmy). Lower levels of mutant mtDNA are associated with milder or minimal symptoms, while higher levels cause more severe disease expression.
Figure 2 Pedigree showing maternal inheritance of a trait caused by a mutation in mitochondrial DNA. All offspring of affected women are also affected, while none of the offspring of affected males are affected
4. Monogenic nondeterministic inheritance
This category is frequently covered in standard texts as exceptions to Mendelian inheritance. When lifetime penetrance is incomplete, some individuals who carry disease-causing genes will be unaffected. Conditions with lifetime penetrance figures that range from 99% down to 10% or so usually are recognized and classified as monogenic disorders with incomplete penetrance. That is, they are recognized as having a major single genetic factor that causes the disorder. On the other hand, it is not clear how to classify conditions or traits caused by a single major genetic factors with penetrances of 1, 5, or even 10%. These can be thought of as susceptibility genes that predispose an organism to develop a particular trait or disease. Figure 1 shows a pedigree of a family segregating such a low penetrance, disease-susceptibility gene.
The less deterministic a single major gene effect, the more likely interactions with other gene products and environmental factors will significantly affect expression. Acute intermittent porphyria is an autosomal dominant condition with incomplete penetrance that depends on environmental exposures. Similarly, hemochromatosis is an autosomal recessive susceptibility genotype with low penetrance that interacts with environmental factors to produce disease. A classic example of an autosomal dominant structural anomaly with incomplete penetrance is split-hand split-foot malformation. In this case, there is some experimental evidence that phenotypic expression is affected by other genes (i.e., the individual’s genetic background). Since the factors that influence penetrance and expressivity may be a mix of genes and environmental factors, the monogenic model, in practice, may actually be a complex “mixed” model of major single-gene effects on a polygenic background, where liability may be determined by more than one gene.
Many instances of mitochondrial inheritance fit in this category also. Variable expression and even incomplete penetrance may be due to heteroplasmy. Figure 1 illustrates how mitochondrial inheritance, using the same pedigree transmission as in Figure 2, can suggest complex or multifactorial inheritance. In this case, the mitochondrial mutation required an environmental exposure for phenotypic expression. An example of this would be aminoglycoside-induced deafness.
5. Multigenic nondeterministic inheritance
The terms “complex inheritance” or “complex disease” imply that a single, causative, completely penetrant gene does not always produce the phenotype in question (see Article 57, Genetics of complex diseases: lessons from type 2 diabetes, Volume 2, Article 58, Concept of complex trait genetics, Volume 2, and Article 59, The common disease common variant concept, Volume 2). Instead, a combination of effects from more than one gene, or one or more genes with environmental (nongenetic) factors may produce the phenotype. “Complex” phe-notypes are causally heterogeneous. This usually means that over an extended population, the causes of a particular phenotype, trait, or disease may include both low frequency, high-penetrance causative (i.e., deterministic) alleles and more common, low-penetrance susceptibility alleles interacting with environmental factors. Most of the common disorders of children and adults are complex phenotypes. The common disorders of childhood include birth defects, mental retardation, and short stature. Common disorders in adults include cancer, diabetes, cardiovascular disease, hypertension, stroke, the psychoses, and so on. These conditions are frequently familial, but pedigrees usually do not suggest a straightforward pattern of Mendelian inheritance (Figure 1)
To explain these “non-Mendelian” patterns of familial recurrence, mathematical models were developed that assumed a continuous distribution of genetic “liability” to malformation or disease in the general population. “Polygenic” determination initially referred to a mathematical model in which a number of genes with small, additive effects provide an underlying genetic predisposition to malformation or disease. Some quantitative traits and clinical disorders in humans have been studied and found to be compatible with this mechanism of determination. “Multifactorial” describes models in which environmental factors interact with genetic predisposition, which is frequently thought of as polygenic. In the case of quantitative traits such as blood pressure, weight, and height, a normal curve would represent the distribution of measurements in a population. This model was adapted to account for discontinuous traits by the addition of a threshold, the point within that liability distribution beyond which individuals are affected.
5.1. Digenic causation
This designates a subset in which diseases or traits result from mutations in two genes at different loci. For example, if two unlinked genes encode polypeptide subunits of a functional protein complex, then mutations in either or both genes cause dysfunction and clinical phenotypes. While this may seem like a simple variation on the theme of monogenic inheritance, it is included under “multigenic nondeterministic inheritance” because its existence confounds standard linkage analysis. Examples include some forms of retinitis pigmentosa, nonsyndromic hearing loss, junctional epidermolysis bullosa, and autosomal dominant polycystic kidney disease.
5.2. Oligogenic causation
“Oligogenic” designates a subset of “polygenic” causation in which diseases or traits result from the effects of relatively few genes, some of which may have rather large effects. Many current research efforts are directed toward identifying a small number of interacting genes that produce clinical phenotypes and diseases, without the necessity of identifying a quantitative, polygenic background of susceptibility. An example is Hirschsprung disease (HSCR), a disorder characterized by congenital absence of ganglion cells in the wall of the colon. Eight incompletely penetrant genes from three different but interacting signaling pathways are implicated in causing HSCR.
5.3. Environmental factors
These influence both embryonic development and adult disease. For example, the risk of developing emphysema is greater in individuals with both an environmental exposure such as smoking and a genetic predisposition such as alpha-1-antitrypsin deficiency than it is for individuals with only one of these risk factors or none at all. Severe respiratory distress in asthmatics is triggered by exposure to environmental triggers. Those with a familial susceptibility to skin cancer can decrease their risk of developing cancer by modifying their sun exposure. Obesity strongly affects morbidity in individuals with inherited susceptibilities to type II diabetes.
The relative merits of hypothetical models such as polygenic, multifactorial threshold, and major single gene with incomplete penetrance have been debated vigorously over the years. It seems reasonable to assume that complex phenotypes result from many different stochastic combinations of genetic and environmental factors, where the genetic contribution may be from one or more genes, and expression is merely a possibility, pending environmental exposure(s) and/or random fluctuations of normal processes in the embryo, fetus, or adult organism.