1. Introduction
The programmed exclusion of chromosomes from the genome is a remarkable developmental phenomenon that in insects is very diversified between different families and between individual species. The best-known examples are found in Diptera such as Sciara (Sciaridae), Heteropeza, Miastor, Mayetiola, Wachtiella (Cecidomyiidae), Acritocopus (Chironomidae), and also in Homoptera coccids (reviewed in White, 1973). A common feature is that specific chromosomes are eliminated from presomatic cells at early cleavage divisions by the time of somatic/germ-line separation. In some species, there is an additional chromosome loss from germ cells. In all cases, this process leads to a reduction in the number of chromosomes in the somatic tissues compared to the germline.
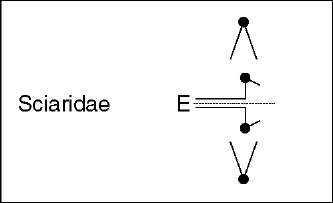
Somatic embryonic elimination involves chromosomes of the regular complement (mostly sex chromosomes) and/or chromosomes that are restricted to germline. In both cases, the timing of chromosome elimination in early embryos is species specific. A classic example of sex chromosomes elimination is that of Sciara where the loss of one or two X chromosomes at early cleavages determines the sex of the embryo (Metz and Moses, 1926). That this type of elimination is preceded by imprinting was discovered in Sciara when it was found that the discarded X chromosomes are invariably of paternal origin (Crouse, 1960; reviewed in Gerbi, 1986). Despite its importance, the parent-of-origin-specific mark(s) involved in this elimination system is still unknown. A substantial number of examples, including cecidomyiids species, show elimination of sex chromosomes linked to sex determination (Nicklas, 1959; reviewed in White, 1973). An extreme case is found in coccids diaspididae where the somatic elimination of the entire paternal chromosome set produces solely male embryos (reviewed in White, 1973). On the other hand, germ-line-limited chromosomes, present in certain sciarids and most cecidomyiids and chironomids (“L”, “E”, or “K” chromosomes, respectively), are all discarded from the presumptive somatic nuclei at early cleavages in both sexes. This is independent of their number, which can be extremely high and varies greatly between species. In this respect, the elimination of germ-line-limited chromosomes
in early cleavages reduces the chromosome number in the future somatic nuclei to a level characteristic of the Diptera in general. This may be particularly important, since the developmental pattern in Diptera has been adjusted to a low number of chromosomes in relatively small nuclei (Nicklas, 1959). Most of the germ-line-limited chromosomes are of heterochromatic nature and contain repetitive DNA sequences. The functional role of these chromosomes in the germline remains obscure. However, experimental elimination of the E chromosomes in pole cells of cecidomyiids species revealed that their presence is necessary for the female gonad development (Geyer-Duszynska, 1959).
In germ cells, chromosome elimination commonly involves the loss of the whole paternal regular set of chromosomes during male meiosis. In Sciara species, an exceptional and unique type of paternal X-chromosome nuclear exclusion also takes place in embryonic germ cells of both sexes (reviewed in Goday and Esteban, 2001).
Despite many years of study, chromosome loss in insects is far from being exhaustively analyzed. An intriguing question is whether the cellular and molecular mechanisms underlying these processes share common traits between different insects. With this in mind, we discuss here relevant data coming from examples in Diptera, Sciaridae, Cecidomyiidae, and Chironomidae.
2. Mechanisms of chromosome elimination in the soma
In all species, chromosome loss at early embryonic mitotic divisions is produced by an abnormal segregation of the chromosomes. A regular cytological feature of this event is the occurrence of “lagging chromosomes” at anaphase, so that these chromosomes fail to enter the daughter nuclei.
In sciarids, as classically described for L- and X-chromosome elimination processes in S. coprophila (Dubois, 1933; reviewed in Gerbi, 1986), the chromosomes begin their movement toward the poles at anaphase but are incapable of complete chromatid separation and remain at the equatorial plate (see Figures 1 and 2). These early observations led to the proposal that alterations in centromeric activity cause chromosome loss in sciarids (reviewed in Gerbi, 1986). In S. coprophila, moreover, it was found that a cis-acting locus or controlling element (CE) located in a heterochromatic block near the centromere of the X chromosome regulates the elimination process (Crouse, 1960). The molecular nature of the CE is still undetermined, but when the CE is translocated to an autosome it is able to direct its elimination in paternally inherited translocations (Crouse, 1979). Recent analysis of L- and X-chromosome elimination kinetics in S. coprophila through confocal microscopy showed that the centromeres remain attached to the spindle and stretched toward the poles at anaphase, while the chromatids remain joined at a region on the long arm of the X chromosome (de Saint-Phalle and Sullivan, 1996). It was proposed that anaphase lag (of X and L chromosomes) is caused primarily by a CE-controlled failure of chromatid separation rather than by a CE-controlled centromere dysfunction (de Saint-Phalle and Sullivan, 1996). To find the specific biochemical alterations in the chromatid separation processes at the anaphase transition would be extremely interesting to further develop this conclusion.
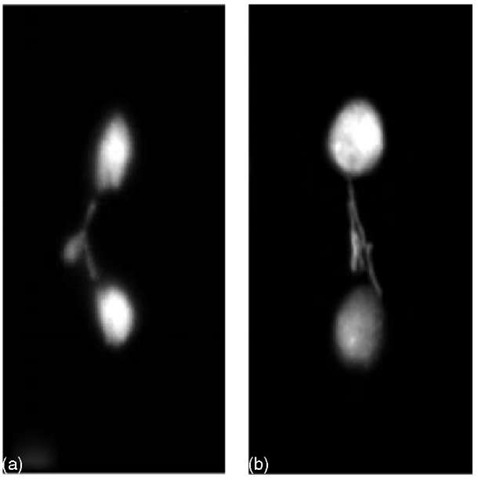
Figure 1 X-chromosome elimination from the soma in S. ocellaris embryos. DAPI-stained early syncitial somatic divisions. Lagging X chromosomes in somatic division undergoing elimination. Only one X chromosome is discarded from cells of female embryos (a), while two X chromosomes are discarded from cells of male embryos (b)
Another yet undiscovered issue is the nature of the cytoplasmic factor(s), produced by the mother and distributed within the egg, involved in regulating the number of X chromosomes eliminated in sciarids. As demonstrated in sciarid species, this factor(s) is produced in the oocyte during oogenesis (reviewed in Gerbi, 1986 and Goday and Esteban, 2001). Two main models have been put forward to explain the number of eliminated X chromosomes (de Saint-Phalle and Sullivan, 1996; Sanchez and Perondini, 1999). The one-factor model (de Saint-Phalle and Sullivan, 1996) is based on a maternal factor that regulates the differential X-chromosome elimination pattern through the quantity of this factor present in the egg. In the alternative, two-factor model (Sanchez and Perondini, 1999), a hypothetical chromosomal factor interacts with the X chromosome(s) causing its (their) elimination. In this model, the number of X chromosomes to be eliminated is controlled by a maternal factor that regulates the amount of free chromosomal factor interacting with the X chromosomes (reviewed in Goday and Esteban, 2001).
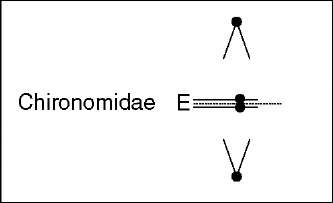
In contrast to Sciara, in the chironomid Acritopus during Ks-chromosomes elimination, the Ks sister chromatids are not stretched in the direction of the spindle poles, and their centromeres appear not to separate while somatic chromosomes move to the poles (Staiber, 2000). Since Ks chromosomes stay in the equatorial plate, it was concluded that their sister chromatids remain joined at their centromeric regions rather than at the chromosomes arms or the telomeres (see Figure 2). Moreover, it was proposed that proteins responsible for centromeric cohesion might be involved in this type of chromosome behavior (Staiber, 2000).
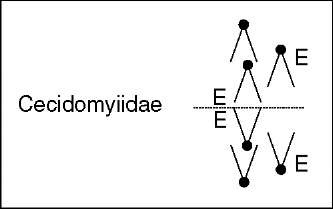
Figure 2 Chromosome elimination in embryonic somatic cells. Diagram summarizing the”lagging chromosomes” phenotypes, relevant cytological events, and proposed elimination mechanisms. E denotes the chromosomes undergoing elimination
Interestingly, a specific highly repetitive DNA family located in the paracentromeric heterochromatin of the Ks chromosomes was identified in A. lucidus (Staiber et al., 1977). Whether these repetitive DNA sequences are involved in identifying the eliminating chromosomes as proposed (Staiber et al., 1977) remains unknown.
The elimination of E chromosomes in cecidomyiids displays varied chromosome-lagging patterns (see Figure 2). In examples of Miastor, Micophila, and Heteropeza, E chromosomes remain at the equator as a result of the apparent absence or diminution of the usual mid-anaphase tension (Nicklas, 1959; Nicklas, 1960; White, 1973). In several of the observations, it is clear that among the chromosomes to be lost there are chromatid pairs that remain totally separate and yet fail to exhibit normal mid-anaphase movement. In Mayetiola and Wachtliella examples, all E chromatids separate completely but fail to continue anaphase along with S chromosomes (Geyer-Duszynska, 1959; reviewed in White, 1973). A remarkable case is that of Heteropeza pygmaea where until mid-anaphase both the E and S chromosomes segregate to the poles as in a normal cleavage division. Time-lapse cinemicrography revealed that the velocity of the E chromosomes, however, is less than that of the S chromosomes. After variable amounts of anaphase movements, the E chromosomes return toward the equator with their kinetochores being still oriented toward the poles (Camezind, 1974). From these, and other cytological observations, it was generally accepted that functional defects in the centromeres of E chromatids were responsible for causing elimination in cecidomyiids. So far, nothing is known about the biochemical and molecular organization of the E-chromosomes’ centromere with respect to those of S chromosomes; neither about specific DNA sequences candidates to constitute molecular chromosome landmarks for determining elimination in cecidomyiids. Both kinds of studies would no doubt shed light on the mechanisms and control of elimination.
Figure 2 shows a schematic diagram summarizing the main “lagging chromosomes” phenotypes and the emphasized elimination mechanisms.
3. Mechanisms of chromosome elimination in the male germline
In Sciaridae and Cecidomyiidae, there is an additional elimination of chromosomes in male germ cells during spermatogenesis. In this elimination event, the paternal chromosome set is discarded in Sciaridae. In Cecidomyiidae, elimination includes a haploid set of somatic chromosomes (presumably paternally derived) plus all, or nearly all, E chromosomes (reviewed in White, 1973 and Gerbi, 1986). In male meiosis I, the absence of homolog pairing, synapsis, and metaphase alignment is a common characteristic for both groups.
In sciarids, in anaphase I, only maternal (and L chromosomes when present) move toward the single pole of a monocentric first meiotic spindle, and become included into the daughter nucleus. The paternal set, in contrast, segregates away from the maternal set into a cytoplasmic bud that is later cast off from the spermatocyte (see Figure 3). Several observations support the conclusion that differential kinetic behavior of Sciara maternal and paternal chromosomes is accomplished by a monopolar spindle and by non-spindle cytoplasmic bud micro-tubules (Kubai, 1982; Fuge, 1994; Esteban et al., 1997). Furthermore, unorthodox microtubule-organizing centers (MTOCs) have been found to be responsible for the assembly and polarity of microtubules in the bud regions in Sciara spermatocytes (reviewed in Esteban et al., 1997). Such microtubules class are specifically involved in capturing and retaining paternal chromosomes in the spermatocytes bud. Most interesting, the presence of organized kinetochores in paternal chromosomes appears not to be necessary for their regular elimination among sciarids. Hence, an essential role of the centromeres in this kind of elimination seems to be discardable (reviewed in Goday and Esteban, 2001).
As in Sciara, in the cecidomyiids Miastor, Heteropez, and presumably in Mayetiola, a monopolar spindle directs anaphase I (Nicklas, 1959, 1960; White, 1973; Stuart and Hatchett, 1988). A haploid set of S chromosomes orientate themselves with their centromeres directed toward the single pole while the remainder (one haploid set of S chromosomes and the E chromosomes) remain, generally less condensed, in an unorientated group on the opposite nuclear side. Two kinds of secondary spermatocytes are formed that are of very different sizes, the larger being a residual cell that contains the discarded chromosomes. As in Sciara, the chromosomes that interact with, and migrate to, the single spindle pole are the ones that will be maintained and included in the sperm nucleus. So far, it is not known if in cecidomyiids unorthodox MTOCs generating nonspindle microtubules are also involved, together with the spindle microtubules, in the elimination of chromosomes. If this is so, they may, as in Sciara, be part of the established cellular mechanism that assures the regular elimination of specific chromosomes.
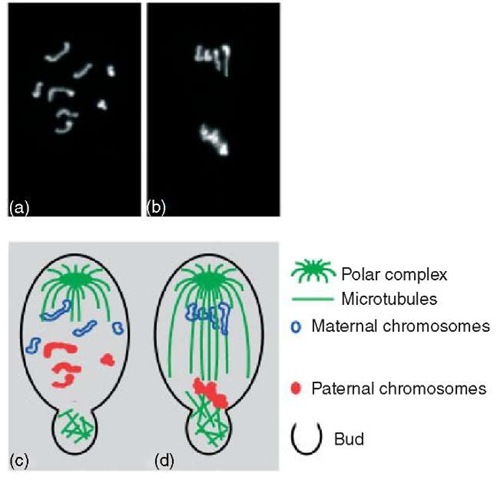
Figure 3 Chromosome elimination during first male meiosis in S. ocellaris. Upper row: DAPI-stained spermatocyte chromosomes. (a) Prophase I; (b) Anaphase I. Lower row: a diagrammatic representation of the same pictures illustrating the chromosome interactions with the microtubules of the first meiotic spindle and bud microtubules in the spermatocyte. (c) Prophasic chromosomes do not pair. Maternal and paternal chromosomes display a separate arrangement within the nucleus. A monopolar spindle is formed and nonspindle microtubules are generated in the cytoplasmic bud regions. (d) Maternal chromosomes move toward the single pole while paternal chromosomes segregate into the bud
A highly relevant feature, common to cecidomyiids and sciarids, is that there is a spatial compartmentalization of chromosomes within the meiotic prophase nuclei. Ultrastructural studies in the cecidomyiid Monarthropalpus buxi demonstrated that the non- and eliminated chromosomes are separated in the spermatogonium nucleus by a complex system of intranuclear lammellae (reviewed in Jazdowska-Zagrodzinska and Matuszewski, 1978). Similarly, in S. coprophila male germ cells, paternal and maternal chromosomal sets occupy distinct compartments in the meiotic prophase nuclei, and this accounts for their nonrandom chromosome segregation during anaphase I (Kubai, 1982). The territorial separation of the two chromosome sets most probably facilitates the proper interactions of each parental chromosome group with the microtubular system that further separates them at anaphase I (Kubai, 1982; Goday and Esteban, 2001). The existence of separate chromosomal territories is further supported by recent data indicating that histone acetylation between chromosomes of different parental origin highly differ, both in early germ nuclei as well as during first male meiotic division in Sciara (Goday and Ruiz, 2002).
| Cytological events (meiosis I) | Proposed elimination mechanisms | |
| Sciaridae Cecydomyiidae | No pairing of homolog chromosomes No metaphase alignment
Intranuclear chromosomes compartmentalization Monopolar spindle formation |
Differential kinetic behavior of chromosomes at anaphase I
Active role of monopolar spindle structure in capturing noneliminating chromosomes |
| Sciaridae | Generation of non-spindle citoplasmic microtubules | Active role of non-spindle microtubules in capturing eliminating chromosomes |
Figure 4 Chromosome elimination at male first meiotic division. Summary of relevant cytological events and proposed elimination mechanisms
The most relevant cytological events of chromosome elimination during first male meiosis and proposed mechanisms are summarized in Figure 4.
In conclusion, a common feature underlying chromosome elimination in insects is the occurrence of changes in the chromosome segregation modalities. Different tissue-specific mechanisms have evolved to achieve chromosome elimination in somatic cells and in germ cells. Modifications in centromeric functional activities and failure of sister chromatid separation are thought to account for somatic elimination events. On the other hand, chromosome elimination during gametogenesis can be conceived as the result of the combined role of a specific spindle structure (monopolar spindle) and cytoplasmic microtubules (generated by unorthodox MTOCs). The success of such a microtubule-based differential meiotic segregation seems to require a previous intranuclear specification of the chromosomal set domains.