1. Introduction
Newborn screening (NBS) has evolved significantly since its introduction in 1962 for one biochemical genetic disorder, phenylketonuria (PKU). It now includes endocrinopathies, hemoglobinopathies, congenital infections, cystic fibrosis, and congenital deafness as well as many biochemical genetic disorders. The wide coverage of biochemical genetic disorders has been possible because of the recent addition of tandem mass spectrometry (MS/MS) to the technology of newborn screening. This article briefly reviews newborn screening and then focuses on the advances in neonatal identification of the biochemical genetic disorders.
2. Background
Soon after Robert Guthrie developed newborn screening as a means of preventing mental retardation in PKU, he realized that several other biochemical genetic disorders fit the same mold, that is, the disorders are capable of producing mental retardation but are potentially preventable by newborn identification and dietary treatment. Among these disorders were homocystinuria, maple syrup urine disease (MSUD), and galactosemia – disorders that can also result in somatic complications. Consequently, he modified his bacterial assay for phenylalanine, the analytical marker for PKU, to render it responsive to increases in other metabolites and to allow for the detection of these disorders (Guthrie, 1968).
The keys to these additions and further developments in newborn screening are the dried blood filter paper specimen, now known as the Guthrie specimen, and the small amount of blood needed for the assays. Consequently, tests can be added to newborn screening without the requirement for another specimen, provided the test can be performed on whole blood eluted from a small disk punched from the specimen. Over the years, many investigators have become aware of the possibility that this system could be used to screen newborns for disorders in their areas of interest, and several have succeeded in modifying existing assays to meet the specifications of newborn screening. The results have been newborn screening for congenital hypothyroidism (Dussault et al., 1975), sickle cell disease (Garrick etal., 1973), and congenital adrenal hyperplasia (Pang etal., 1977). However, until very recently, the only biochemical genetic disorder among these additions has been biotinidase deficiency (Heard et al., 1984).
This omission was not by choice. Guthrie and his group tried in vain to expand biochemical genetic coverage. Several programs adopted neonatal urine screening using paper or thin-layer chromatography to expand coverage (Bamforth et al., 1999). This resulted in additional coverage but required collection of the specimen at several weeks of age rather than within the first day of life and examined only amino acids and a very limited number of organic acids. Consequently, many serious disorders were not covered, or not covered in time for effective therapy, and the disorders that were most frequently identified were benign (Wilcken etal., 1980). Thus, coverage of many important biochemical genetic disorders by screening the newborn blood specimen had to await MS/MS technology.
3. Overview of tandem mass spectrometry (MS/MS)
The mass spectrometer is an analytical instrument that detects ions by virtue of their mass and charge. It has been used for many years to identify metabolites that are separated on columns in techniques such as capillary electrophoresis, gas chromatography, and HPLC. This requirement for prior separation of metabolites, however, meant that mass spectrometry could not be used in screening since separation would be much too time consuming for the rapid throughput that routine screening requires. However, MS/MS is applicable to newborn screening because it directly analyzes the individual components of complex mixtures, eliminating the need for prior chemical separation. Developed in the late 1970s (McLafferty, 1981), MS/MS employs two mass spectrometers in tandem separated by a collision cell. It takes advantage of the principle that molecules fragment into predictable species depending on their structural properties. Millington etal. (1990) adapted this technology to identify metabolites in the Guthrie dried blood spots (DBS) of newborn infants. Naylor subsequently used this system to greatly expand the number of biochemical genetic disorders identifiable in routine newborn screening (Naylor and Chace, 1999).
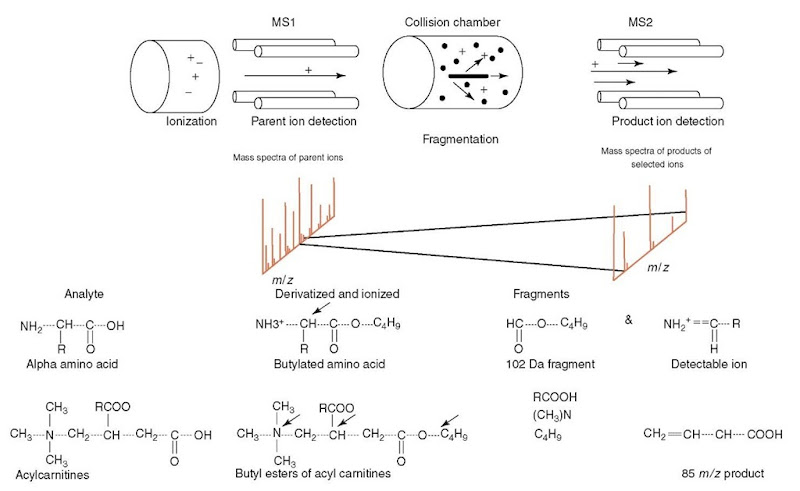
In its current application to newborn screening, MS/MS measures amino acids and acylcarnitine conjugates of organic and fatty acids in the Guthrie blood specimen. Figure 1 depicts the elements of MS/MS and the chemical characteristics of derivatization and fragmentation of the amino acids and the acylcarnitines. For a detailed description of the MS/MS methods and analysis, the reader is referred to articles by Chace et al. (1999, 2002, 2003). The process of newborn screening begins with methanol extraction of blood from the specimen, followed by butanol derivatization of amino acids and acylcarnitines. The butyl ester derivatives are ionized by electrospray. The ions enter the first MS, where the parent molecules are sorted by mass and charge and are then directed into the collision chamber. In this chamber, collision-induced disassociation (CID) produces characteristic fragments because of collision between an inert gas and the ionized particles. These fragments are then guided into the second MS where they are sorted by mass and charge (m/z) and detected as signals. Computer algorithms enable correlation between the mass spectra of the fragments in the second MS and the spectra of the intact parent molecules in the first MS. The data thus acquired can be scanned for characteristic fragments so that parent molecules corresponding with these fragments can be selectively analyzed. In general, amino acids are identified by their loss of a 102-Da fragment, while acylcarnitines are always identified by the presence of their common m/z 85 fragment ion. Different scanning modes can analyze the same data for other specific fragments, thereby resulting in identification of multiple classes of compounds in the same specimen. Quantitative analysis is performed using the ratio of the metabolite to its isotopic analog (internal standard is added at the beginning of the process). Software programs are formatted to analyze the spectra, perform the calculations to determine the concentrations, and flag the abnormal values. Though the automated system detects metabolite abnormalities, personnel familiar with metabolic disorders are essential for recognizing the metabolite profiles that indicate a disorder.
Figure 1 Schematic diagram of a tandem mass spectrometer and the path of the molecules and ions. Depicted below the diagram is the spectrum obtained from MSI and MS2. which is later correlated by the analyzer. Also shown are amino acid and acylcarnitine molecules and their fragments
MS/MS analysis requires only about 2 minutes per specimen, making it ideal for high-volume programs such as newborn screening. Nevertheless, the system has certain technical limitations: (1) The equipment needs proper maintenance and tuning, making trained technicians vital for its operation. (2) The volume of blood used in calculating the metabolite values is the volume assumed to have been extracted from the standard-sized disk of the Guthrie specimen used in the analysis, but the actual volume can vary in different specimens, depending on the extraction efficiencies. This probably accounts for most of the variation seen with MS/MS analysis. (3) The derivatization can produce hydrolysis of acylcarnitines, falsely reducing the estimate of its endogenous quantity (and falsely increasing the estimated level of endogenous carnitine).
4. Expanded newborn screening
This is the term generally applied to the increased number of biochemical genetic disorders identifiable in newborn screening that is made possible by MS/MS technology. They are broadly classified into amino acid, organic acid, and fatty acid oxidation disorders.
4.1. Prevalence
The reported frequency of identified cases in expanded newborn screening has varied from 1:2400 among 250,000 screened infants in Baden-Wuirttemberg, Germany (Schulze etal., 2003) to 1:4000 among 1.1 million infants who were screened primarily in Pennsylvania (Chace et al., 2002) and 1:5000 among 164,000 of those who were screened in Massachusetts (Zytkovicz etal., 2001). In New South Wales, Australia, a frequency of 1:6400 cases among 362 000 screened infants has been reported, but it excludes PKU (Wilcken etal., 2003), one of the most frequent of the detected disorders.
4.2. Amino acid disorders
This group of disorders is marked by the accumulation of characteristic amino acids; each disorder is the result of an inherited defect in an enzyme that is responsible for conversion of an amino acid into its product. Disorders traditionally screened, such as, PKU, MSUD, and homocystinuria, plus disorders that were not previously screened, such as, tyrosinemia, hyperglycinemia, hydroxyprolinemia, and several urea cycle defects, are identifiable by MS/MS. The incidence of the amino acid disorders detected by MS/MS screening has been reported as 1:3800 in Germany (Schulze et al., 2003), 1:7400 in Pennsylvania (Chace et al., 2003), and approximately 1:8000 in Massachusetts (Zytkovicz et al., 2001).
The clinical phenotype of the amino acid disorders is usually insidious in development. In PKU, for instance, the neonate is clinically normal and remains so until developmental delay, irritability, and autistic features appear during the latter half of the first year. Homocystinuria may present with a similar picture, subsequently producing somatic features that include ectopia lentis, skeletal changes, and thromboembolism. Tyrosinemia type I can produce liver disease in early infancy, whereas tyrosinemia type II can produce developmental delay without liver involvement. MSUD, however, can cause profound neonatal disease characterized by metabolic acidosis. This phenotype is characteristic of the organic acid disorders (in which category MSUD is often included). Other exceptions are the urea cycle disorders, in which the affected neonate may have severe hyperammonemia, producing lethargy that can rapidly progress to coma. Nevertheless, in all of the amino acid disorders, clinical signs of disease may not have appeared during the first few days of life. Expanded newborn screening, revealing a specific amino acid elevation, can identify these infants and preventive therapy can be effective.
The major confirmatory test following any newborn screening abnormality is a plasma amino acid analysis. Table 1 lists the amino acid disorders that can be identified in expanded screening according to the screening abnormalities that bring them to attention and highlights their biochemical and clinical phenotypes.
4.3. Fatty acid oxidation disorders (FAOD)
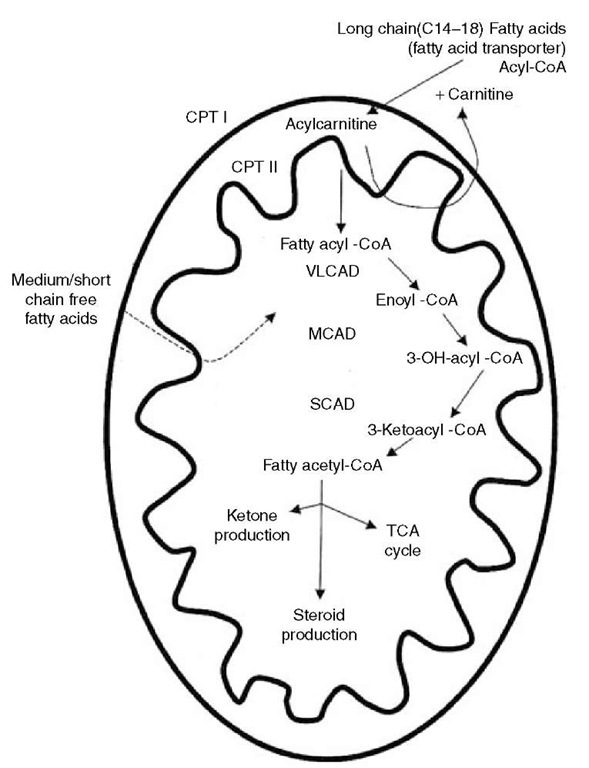
Fatty acid oxidation primarily occurs in the mitochondria and is critical for energy generation in the fasting state or during exercise. The sequence of fatty acid oxidation from transport into mitochondria through j-oxidation is depicted in Figure 2. It begins with the mobilization of very long chain and long chain fatty acids from adipose tissue. In the cytosol of cells, they link to coenzyme A (CoA) under the influence of fatty acyl-CoA synthetase to yield fatty acyl-CoA. The fatty acyl-CoA moves to the mitochondrion, as does carnitine, which has entered the cell via a carnitine transporter. On the outer mitochondrial membrane, carnitine palmitoyltransferase I (CPT I) catalyzes an exchange of carnitine with the CoA to yield fatty acylcarnitine, releasing CoA. The fatty acylcarnitine is transported to the inner mitochondrial membrane by a translocase where CPT II decouples and releases the carnitine and links the fatty acid to CoA again. The resulting fatty acyl-CoA moves into the mitochondrial matrix where it is j-oxidized sequentially by six enzymes, including very long chain acyl-CoA dehydrogenase (VLCAD), long chain hydroxyacyl-CoA dehydrogenase (LCHAD), medium chain hydroxyacyl-CoA dehydrogenase (MCHAD), medium chain acyl-CoA dehydrogenase (MCAD), short chain hydroxyacyl-CoA dehydrogenase (SCHAD), and short chain acyl-CoA dehydrogenase (SCAD). The successive removal of two carbons via the j-oxidation cycle results in acetyl-CoA that is used to generate energy through the TCA cycle and the synthesis of ketones. Acetyl-CoA is also required for the synthesis of steroids.
Table 1 Amino acid disorders
| Major screening analyte(s) | Disorder | Enzyme defect |
| Phenylalanine | Phenylketonuria (PKU) | Phenylalanine hydroxylase |
| Leucine/isoleucine/ hydroxyproline | Maple syrup urine disease (MSUD) | Branched-chain a-ketoacid dehydrogenase complex |
| Leucine/isoleucine/ hydroxyproline Methionine | Hydroxyprolinemia Homocystinuria | Hydroxyproline oxidase Cystathionine /J-synthase |
| Methionine | Hypermethioninemia (MAT I/III deficiency) | Methionine adenosyl transferase (MAT I/III) |
| Clinical phenotype | General treatment |
| Mental retardation | Dietary restriction of phenylalanine |
| Autism Hyperactivity Seizures Lethargy | Dietary restriction of branched-chain amino acids |
| Failure to thrive
Coma Seizures Maple syrup odor in urine and cerumen Asymptomatic |
None |
| Mental retardation Arachnodactyly | Dietary restriction of
methionine Supplemental B6 (for B6 responsive) |
| Osteoporosis Ectopia lentis Thromboembolism Asymptomatic
(Possibly rare cognitive reduction) |
None known |
| Tyrosine | Tyrosinemia type I | Fumarylacetoacetate hydrolyase |
| Tyrosine | Tyrosinemia type II | Tyrosine aminotransferase |
| Citrulline | Citrullinemia (Urea cycle defect) | Argininosuccinic acid synthetase |
| Citrulline | Argininosuccinic acidemia (Urea cycle defect) | Argininosuccinic acid lyase |
| Arginine | Arginase deficiency (Urea cycle defect) | Arginase |
| Glycine | Nonketotic hyperglycinemia (NKH) | Glycine cleavage enzyme |
| Hepatic disease
Hypoglycemia Hypophosphatemic rickets Keratoconjunctivitis |
2-(2-nitro-4-
trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC); dietary restriction of phenylalanine and tyrosine Liver transplant Dietary restriction of phenylalanine and tyrosine |
| Palmer/plantar keratosis Cognitive reduction Hyperammonemia | Dietary restriction of protein |
| Mental retardation Failure to thrive Lethargy, coma
Hyperammonemia Failure to thrive Lethargy, coma Mild-moderate hyperammonemia Mental retardation |
Supplements: L-arginine Sodium benzoate Sodium phenylacetate Dietary restriction of protein Supplements: L-arginine Sodium benzoate Sodium phenylacetate Dietary restriction of arginine and protein |
| Spastic diplegia Seizures
Hypotonia Marked lethargy |
Supportive care Sodium benzoate |
Figure 2 Metabolism of fatty acids illustrating entrance into mitochondrion and intramitochon-drial j-oxidation. Abbreviations: CPT, Carnitine palmitoyltransferase; VLCAD, very long chain acyl-CoA dehydrogenase; MCAD, medium chain acyl-CoA dehydrogenase; SCAD, short chain acyl-CoA dehydrogenase; TCA, tricarboxylic acid
Disorders have been described for each of these transporters and enzymes except fatty acyl-CoA synthetase. The reported frequency of the FAODs in expanded newborn screening is 1:10 000 in Germany (Schulze et al., 2003) and Massachusetts (Zytkovicz et al., 2001), and 1:13 000 in Australia (Wilcken et al., 2003). The most frequently detected in expanded newborn screening has been medium chain acyl-CoA dehydrogenase deficiency (MCADD), with an incidence of 1:20 000, which is similar to that for PKU.
The most striking clinical feature in the FAOD phenotype is intolerance to fasting or to vigorous exercise. Normally, reduction of glucose in the fasting state or the requirement for additional glucose during exercise triggers the compensatory production of ketones to supply energy. In the FAOD, the inability to meet this requirement for energy through the j -oxidation of fatty acids results in muscle weakness; lethargy; fatty liver; inhibition of gluconeogenesis, exacerbating the hypoglycemia; and a high risk of sudden death. Cardiomyopathy is a particular feature of carnitine transporter defect and of defects in the j -oxidation of very long chain and long chain fatty acids. A key laboratory feature is hypoketotic hypoglycemia. Other laboratory findings during acute episodes include metabolic acidosis, hyperammonemia, and elevations of the liver enzyme levels. Early detection by expanded newborn screening combined with treatment that includes avoidance of fasting, adherence to a low-fat diet, and carnitine supplementation has been effective in preventing sudden death and other complications in these disorders (Waisbren et al., 2003).
In expanded newborn screening by MS/MS, the carnitine and acylcarnitine patterns reveal the presence of an FAOD and, usually, the specific defect. Since carnitine transport defect limits both the transport of free carnitine from the intestinal lumen and its reabsorption in the kidneys, the blood carnitine concentration is very low. In CPT-I deficiency, acylcarnitines are not formed, so the concentration of free carnitine is high but that of the acylcarnitines are low. In CPT-II deficiency, the acylcarnitines are unchanged, so the long chain acylcarnitine levels are increased. For the j -oxidation defects, the levels of long chain acylcarnitines are increased in very long chain acyl-CoA dehydrogenase deficiency (VLCADD), long chain hydroxyacylcarnitines levels are increased in long chain hydroxyacyl-CoA dehydrogenase deficiency (LCHADD), medium chain acylcarnitines, particularly octanoylcarnitine (C8), are increased in MCADD, and the short chain acylcar-nitines, primarily butyrylcarnitine (C4), are increased in short chain acyl-CoA dehydrogenase deficiency (SCADD). After an acylcarnitine abnormality has been ascertained, usually by repeat screening, confirmatory testing is performed by analyses of plasma acylcarnitines and urine acylglycines. The individual fatty acid oxidation disorders are summarized in Table 2.
In contrast to amino acid concentrations, which increase in a time-dependent manner, the acylcarnitines decrease significantly with passage of time and with initiation of feeding. This occurs within the first week of life. The rate of decrease of the long chain acylcarnitines is more rapid than that of the short chain acylcarnitines (Chace et al., 2003). Effective screening, therefore, requires collection of the Guthrie specimen within 24-48 h of life and lowering of the cutoff value for repeat specimens. The concentrations of multiple acylcarnitine markers and relative molar ratios are also important in the interpretation of a positive result.
Table 2 Fatty acid oxidation disorders
| Major screening analyte(s) C4 (butyrylcarnitine) | Disorder
Short chain acyl-CoA dehydrogenase deficiency (SCADD) |
Enzyme defect
Short chain acyl-CoA dehydrogenase (SCAD) |
| C8 (octanoylcarnitine) | Medium chain acyl-CoA dehydrogenase deficiency (MCADD) | Medium chain acyl-CoA dehydrogenase (MCAD) |
| C8 (octanoylcarnitine) | Multiple acyl-CoA
dehydrogenase deficiency (MADD) |
Multiple acyl-CoA
dehydrogenation defects due to decreased electron transfer flavoprotein (ETF), or decreased electron transfer flavoprotein ubiquinone oxidoreductase (ETF-QO) |
| C8:l (octenoylcarnitine) | [Also known as glutaric acidemia II (GA II)] | |
| C14
(tetradecanoylcarnitine) |
Very long chain acyl-CoA dehydrogenase deficiency (VLCADD) | Very long chain acyl-CoA dehydrogenase (VLCAD) |
| Clinical phenotype
Variable presentation: may be asymptomatic Asymptomatic when well With fasting and/or illness: vomiting, lethargy, seizures, coma, hypoketotic hypoglycemia, metabolic acidosis, hepatomegaly Can cause sudden death Hypoketotic hypoglycemia Metabolic acidosis “Sweaty feet” odor Muscle weakness Neonatal form has occasional dysmorphic features and hypotonia Variable presentation: Cardiomyopathy (hypertrophic) |
General treatment
Avoid fasting Diet: low fat, high carbohydrate (CHO) Avoid fasting Frequent CHO feedings (q4 hours until 4 months; q6 hours until 8 months; q8 hours thereafter) Possible supplementation with carnitine Avoid fasting Dietary restriction of fat Frequent CHO feedings Supplement with riboflavin and carnitine Avoid fasting Low fat, high CHO diet |
| C14:1
(tetradecenoylcarnitine) |
|||
| C160H (hydroxyde-canoylcarnitine) | Long chain hydroxyacyl-CoA dehydrogenase deficiency (LCHADD) | Long chain hydroxyacyl-CoA dehydrogenase (LCHAD) | |
| C18:10H (hydroxyoc-tadecenoylcarnitine)
C180H (hydroxyloctade-canoylcarnitine) |
|||
| CN (carnitine) | Carnitine palmitoyltransferase I (CPT I) deficiency | Carnitine palmitoyltransferase I (CPT I) | |
| C16 (palmitoylcarnitine) | Carnitine palmitoyltransferase II (CPT II) deficiency
or |
Carnitine palmitoyl-transferase II (CPT II)
or |
|
| C18:l
(octadecenoylcarnitine) |
Carnitine/acylcarnitine translocase deficiency | Carnitine/acylcarnitine translocase | |
| Sudden death | Frequent CHO feedings, medium chain triglycerides (MCT) |
| With illness: vomiting, lethargy,
seizures, coma Hypoketotic hypoglycemia Hypoketotic hypoglycemia |
Carnitine Avoid fasting |
| Mild hyperammonemia Cardiomyopathy | Low fat, high CHO diet Supplement with carnitine |
| Sudden death
Associated maternal syndrome |
Early severe hypotonia |
| Renal tubular acidosis | Hemolysis, Elevated liver enzymes and Low Platlets (HELLP) |
| Infantile form: | Dietary restriction of high fat foods |
| Fasting hypoketotic hypoglycemia | Supplement: Frequent CHO feedings, MCT supplements, carnitine |
| Lethargy
Hepatomegaly Seizures |
|
| Infantile presentation: | Dietary restriction of high fat foods |
| Hypoketotic hypoglycemia | Supplement: Frequent CHO feedings, carnitine |
| Lethargy | |
| Seizures
Cardiomyopathy |
|
Table 3 Organic acid disorders
| Major screening analyte C3 (propionylcarnitine) | Disorder
Propionic acidemia |
Enzyme defect Propionyl-CoA carboxylase |
| C3 (propionylcarnitine) | Methylmalonic acidemia | Methylmalonyl-CoA mutase |
| C5 (isovalerylcarnitine) | Isovaleric acidemia | Isovaleryl-CoA dehydrogenase |
| C5
(2-methylbutyrylcarnitine) |
2-methylbutyryl-CoA
dehydrogenase deficiency |
2-methylbutyryl-CoA
dehydrogenase (short/branched-chain acyl-CoA dehydrogenase) |
| Clinical phenotype Metabolic acidosis | General treatment
Dietary restriction of threonine, isoleucine, valine, methionine |
| Hyperammonemia Vomiting Failure to thrive Metabolic acidosis | Dietary restriction of isoleucine, valine, methionine (supplemental B12 for Bi2-responsive form) |
| Hyperammonemia Vomiting Failure to thrive Metabolic acidosis “Sweaty feet” odor | Dietary restriction of leucine Supplemental glycine and L-carnitine |
| Vomiting Lethargy Failure to thrive Possibly Asymptomatic | Dietary restriction of protein |
| Possibly Failure to thrive, hypoglycemia, metabolic acidosis | Supplementation with L-carnitine |
| C5DC (glutarylcarnitine) | Glutaric Acidemia I | Glutaryl-CoA dehydrogenase | |
| C50H (3-
hydroxyisovalerylcarnitine) |
3-Hydroxy-3-methylglutaryl (HMG)-CoA lyase deficiency | 3-Hydroxy-3-methylglutaryl-CoA lyase | |
| C50H (3-
hydroxyisovalerylcarnitine) |
3-Methylcrotonyl-CoA carboxylase (3-MCC) deficiency | 3-Methylcrotonyl-CoA carboxylase | |
| Dystonia
Acidosis and vomiting |
Dietary restriction of lysine
and tryptophan Supplemental L-carnitine, riboflavin |
| Macrocephaly Vomiting | Intravenous (IV) glucose |
| Lethargy Hypotonia Seizures Hypoglycemia Metabolic acidosis Asymptomatic, or: | Restrict protein, fat IV glucose |
| Lethargy Hypotonia Hypoglycemia Metabolic acidosis | Restrict protein |
4.4. Organic acid disorders (OAD)
The defect in an OAD is usually in an enzyme within an amino acid degradative pathway that is responsible for conversion of an organic acid derivative of the amino acid to another organic acid. The result of the defect is an increased concentration of the unconverted organic acid, some of which can conjugate with free carnitine to produce an increased acylcarnitine level detectable by MS/MS. The reported frequency of OADs is 1:15 000 in Germany (Schulze et al., 2003), 1:30 000 in Australia (Wilcken et al., 2003), and 1:55 000 in Massachusetts (Zytkovicz et al., 2001). The unconjugated organic acid (and secondary organic acid metabolites) accumulate to very high levels and produce metabolic acidosis. This can present in the neonate as poor feeding, lethargy, vomiting, tachypnea, and seizures. During such acute episodes, substantial hyperammonemia is also often present. Later complications include developmental delay, mental retardation, and other neurologic abnormalities. A notable exception to this dramatic presentation is glutaric acidemia type I (GA I), in which the neonate appears to be normal (except for macrocephaly) but suddenly becomes dystonic later in the first or second year of life, often during an acute febrile illness or after an immunization. Expanded newborn screening by MS/MS has identified these neonates presymptomatically by an acylcarnitine abnormality and has improved their outcome through appropriate dietary therapy (Waisbren et al., 2003). Confirmation of an acylcarnitine screening abnormality that suggests an OAD includes urine organic acid analysis. The individual organic acid disorders are summarized in Table 3.
5. Considerations in MS/MS screening
5.1. Amino acid disorders
MSUD: An elevation of levels of leucine and variable increases in levels of valine, isoleucine, and alloisoleucine are the characteristics of MSUD. Leucine, isoleucine, and alloisoleucine have the same molecular weight and, therefore, give the same signal in the MS/MS analysis. Nevertheless, since all these metabolites are elevated in MSUD, an increase in the signal intensity of “leucine”, especially when accompanied by increased valine levels, is suggestive of this disorder. The false-positive rate for MSUD can be reduced by considering the ratios of “leucine” to phenylalanine and “leucine” to alanine. Increased levels of hydroxyproline also increases the signal intensity of “leucine” since its molecular weight is the same as leucine and the isoleucines. Consequently, hydroxyprolinemia, which is a benign disorder (Kim et al., 1997), can suggest MSUD in MS/MS screening. An important difference, however, is the likely presence of increased valine levels in MSUD and normal valine levels in hydroxyprolinemia. Homocystinuria: Homocystinuria results in a primary elevation of homocysteine levels, with a secondary increase in methionine. Homocysteine cannot be detected by MS/MS, so methionine is the marker metabolite for this disorder.
As a secondary metabolite, however, the accumulation of methionine lags behind that of homocysteine. Consequently, during the first days of life, the methionine level can remain normal or below the cutoff level for identifying it as being abnormal even if the infant has homocystinuria (Peterschmitt et al., 1999). This is especially likely when the Guthrie screening sample is collected at less than 24 hours after birth.
Tyrosinemias: Tyrosinemia I results in only a moderate elevation of tyrosine. Thus, in order to increase the sensitivity of the screening, a low threshold for identifying increased tyrosine must be set. Nevertheless, since many or most affected infants have normal blood tyrosine levels during the first days of life, they are still missed. This was recognized several years ago in Quebec and the newborn screening program was stimulated to apply an enzyme assay screening method for succinylacetone, the specific marker metabolite for tyrosinemia I, to the Guthrie specimen (Grenier etal., 1982), resulting in almost complete ascertainment. Unfortunately, succinylacetone is currently not identifiable by MS/MS. Tyrosinemia II may more likely be identified on the basis of increased tyrosine. When increased tyrosine is flagged in newborn screening, the vast majority of infants have transient neonatal tyrosinemia, a benign finding (Wilcken et al., 2003).
Urea cycle defects: Three of the five urea cycle disorders (i.e., citrulline-mia, argininosuccinic acidemia, and arginase deficiency) can be identified by expanded newborn screening. Citrullinemia and argininosuccinic acidemia are identified on the basis of increased citrulline levels, more pronounced in cit-rullinemia than in argininosuccinic acidemia. Arginase deficiency is identified by increased arginine levels. The other two urea cycle disorders, carbamyl phosphate synthetase deficiency (CPSD) and ornithine transcarbamylase deficiency (OTCD), could be determined by decreased citrulline levels, but, at present, this identification is limited by an excessively high false-positive rate.
5.2. Fatty acid oxidation disorders
MCADD: The levels of the primary analyte of MCADD, octanoylcarnitine (C8), can also be elevated because of valproate therapy or medium chain triglycerides (MCT) oil supplementation and is also an indicator of the fatty acid disorder multiple acyl-CoA dehydrogenase deficiency (MADD). The C8/C10 ratio, which is usually elevated in MCADD, is useful in distinguishing MCADD from these other causes of elevated C8. MCADD is also usually accompanied by elevations in C6, C10, and C10:1.
SCADD: Elevations in butyrylcarnitine (C4) levels, characteristic of this disorder, are also seen in the fatty acid disorder, MADD.
VLCADD: Elevations of C14 (tetradecanoylcarnitine) and C14:1 (tetradecenoyl-carnitine) are the primary abnormalities of this disorder, though they can also be present in MADD, CPT-II, and carnitine-acylcarnitine translocase deficiency. VLCADD may also be accompanied by elevations of C16, C18, and C18:1.
LCHADD: Elevations of the primary analytes of LCHADD, C16OH (hydoxy-hexadecanoylcarnitine), C18OH (hydroxyoctanoylcarnitine), and C18:1OH (hydroxyoctadecenoylcarnitine), can be minimal, making LCHADD difficult to diagnose. Elevations of C16, C14, C14:1, and C14OH may also be present.
CPT-I deficiency: This is the only FAOD with elevated levels of free carnitine. Also present in CPT-I deficiency are decreased levels of C16, C18, and C18:1.
CPT-II deficiency and carnitine-acylcarnitine translocase deficiency: The levels of the primary analytes for these disorders, C16 (hexadecanoylcarnitine), C18 (octadecanoylcarnitine), and C18:1(octadecenoylcarnitine), can also be elevated in VLCADD and LCHADD but are more prominent here. Additionally, free carnitine is decreased, and the ratio of C16 to carnitine is a useful diagnostic marker.
Carnitine transporter defect: In this disorder, free carnitine level is decreased to less than 5% of normal levels.
MADD (Glutaric Acidemia II): The acylcarnitines levels are only mildly increased in MADD, making the detection of this disorder difficult. MADD is characterized by increased C8 and C8:1, but variable elevations of C6, C5, C5DC, C4, C14, and C14:1 are also seen.
5.3. Organic acid disorders
Propionic acidemia (PA) and methylmalonic acidemia (MMA): C3 (propionyl-carnitine) level is elevated in both these conditions, but the level is typically higher in PA. In fact, in MMA the C3 elevation may be just at or even below the screening cutoff, rendering MMA very difficult to detect. Since cobalamin (B12) is a cofactor for methylmalonyl-CoA mutase, vitamin B12 deficiency can also result in increased methylmalonic acid and C3. The ratio of C3 to C2 increases the sensitivity and specificity for detection.
Isovaleric acidemia (IVA): The indicating metabolite of isovaleric acidemia in newborn screening is C5 (isovalerylcarnitine). 2-methylbutyryl-CoA dehydrogenase deficiency (2-MBDD) also produces an elevation of C5 but is not as striking an elevation as does IVA. However, pivalic acid, a constituent of certain antibiotics, produces a metabolite that is also detected at the m/z of C5. Neonates of mothers who ingested pivalic acid can thus mistakenly be thought to have IVA or 2-MBDD on newborn screening (Abdenur et al., 1998).
Glutaric acidemia I (GA I): The primary analyte in this disorder, C5DC (glutarylcarnitine), a dicarboxylic acid, has two negative charges that need to be neutralized by derivatization before analysis in MS/MS. Elimination of the derivatization step can thus decrease the sensitivity for C5DC.
Hydroxymethylglutaryl (HMG) CoA lyase and 3-methylcrotonyl carboxylase (3-MCC) deficiencies:The level of the marker metabolite for these two disorders, C5OH (3-hydroxyisovalerylcarnitine), can also be elevated in 3-methylglutaconic aciduria I. Furthermore, a peak at m/z 318 indicating C5OH can also be produced by methylbutyrylhydroxy acylcarnitine, which is the marker metabolite for methyl acetoacetyl-CoA thiolase (j-ketothiolase) deficiency.
6. Missed cases
Most infants with biochemical genetic disorders are not identified by newborn screening. These infants usually have disorders that are currently not detectable by screening, including diseases in categories such as the lysosomal disorders (e.g., Gaucher disease and Pompe disease), the glycogen storage diseases (e.g., von Gierke disease), the mitochondrial disorders (e.g., Mitochondrial Encephalomyopa-thy, Lactic Acidosis and Stroke like episodes (MELAS), Myoclonic Epilepsy with Ragged Red Fibers (MERRF)), and others. Attempts are being made to include these categories in newborn screening, the most notable of which is the development of feasible screening methods to detect lysosomal disorders in the newborn (Chamoles et al., 2001), but, so far, methodologies suitable for routine screening have not emerged.
Much less frequent are infants with metabolic disorders that are identifiable by newborn screening but are undetected. These “missed” infants can be placed into two categories, true negatives and false negatives. In the former classification are infants such as those with tyrosinemia I who did not have increased tyrosine when the Guthrie specimen was collected, those with homocystinuria and a normal methionine level during screening (Wagstaff et al., 1991), and those with carnitine transporter defect but a carnitine level in the Guthrie specimen that was above the cutoff level for identification (Wilcken et al., 2003). In the false-negative category is the rare infant who had an abnormal level of the metabolite marker for a disorder but was missed because of program or laboratory error (Smith et al., 2001).
The important message is that any patient in whom there is clinical suspicion of a biochemical genetic disorder could have a disorder, either because it was not included in the newborn screening schedule or because it was missed in the screening process. Thus, all such patients should have laboratory testing for the disorders. These tests may include plasma amino acid analysis, urine organic acid testing, plasma acylcarnitine profiling, urine acylglycine analysis, tests for lysosomal disorders, and/or additional tests, depending on the clinical and general laboratory findings.
7. Molecular genetic techniques
The application of molecular genetic techniques to screening for a wide spectrum of disorders has been made possible by the stability of DNA in the Guthrie card (McCabe etal., 1987). Segments of genes can be amplified directly from the filter paper matrix using the polymerase chain reaction (PCR) without prior extraction. These amplified segments can be examined for mutations relevant to the disorder(s) screened using identification techniques such as restriction analysis or single-nucleotide polymorphism (SNP) methodology. Molecular testing is not currently used in primary newborn screening for multiple reasons, including the many mutations responsible for each of the biochemical genetic disorders, the greatly increased “noise” that would be generated by heterozygote detection, and the prohibitive expense. The plethora of mutations could be met by multiplex PCR coupled with microarrays using chip technology (Caggana etal., 1998), but heterozygote detection and perhaps the expense would continue to be major issues. Nevertheless, candidates for primary molecular screening include those with treatable disorders with few molecular etiologies such as factor V Leiden, sickle cell disease, and the mitochondrial MERRF and MELAS syndromes. Primary screening for severe combined immunodeficiency (SCID), fragile X syndrome, type I diabetes, acute lymphoblastic anemia, and hereditary hemochromatosis might also be considered.
Currently, molecular testing in newborn screening is used as a second tier, through examination of Guthrie cards from infants in whom the primary screening result was abnormal. The purpose of this is to sort the affected infants from those whose primary screening abnormality was due to some reason other than the targeted disorder. The most notable use of molecular testing is in screening for cystic fibrosis, wherein the primary screen, immunoreactive trypsinogen (IRT), can be increased for many reasons other than cystic fibrosis. Second tier testing of the abnormal specimens sorts out those infants who harbor one or more CFTR mutations known to be associated with cystic fibrosis. In expanded newborn screening, second tier molecular testing is also used to test the Guthrie specimen for the A985G mutation in MCAD, the major mutation associated with MCADD, when the primary screen reveals an increased level of C8. In both these examples, second tier molecular testing substantially reduces the number of false-positive results.
8. Postmortem specimens
Inherited disorders of fatty acid oxidation account for an estimated 3-6 % of sudden unexpected deaths in infancy (Boles etal., 1998). Among this group, MCADD is the most frequent, and death during the initial presentation has been noted in 25-33% of the cases (Iafolla etal., 1994). Sudden death as a result of an inherited metabolic disorder may remain unexplained even after an autopsy and, hence, may get classified as sudden infant death syndrome (SIDS). To examine the frequency of biochemical genetic disorders in such infants and children, MS/MS analysis was performed on postmortem Guthrie specimens obtained from over 7000 cases of sudden unexplained death. In 66 specimens, diagnosis of a biochemical genetic disorder was considered likely (Chace etal., 2001). The disorders included MCADD, VLCADD, glutaric acidemia I and II, CPT-II/translocase deficiency, carnitine deficiency, isovaleric acidemia/2-methylbutyryl-CoA dehydrogenase deficiency, and LCHADD. This suggests that protocols should include metabolic screening by MS/MS as part of the postmortem investigation of all cases of sudden death.
Postmortem blood specimens from all infants, even those without a metabolic disorder, are characterized by increased carnitine, acylcarnitines, and amino acids. Consequently, identification of biochemical genetic disorders from postmortem specimens must utilize criteria that are different from those used for the interpretation of metabolic profiles in newborn screening.