1. Introduction
Normal cellular growth is a delicate balance between cellular proliferation and cell death. Cancer is a disruption in this homeostasis, resulting in an imbalance favoring cellular proliferation over cell death. In the last several years, it has become clear that this homeostasis is controlled by genes (see Article 65, Complexity of cancer as a genetic disease, Volume 2). Thus, although some cases of cancer result from genetic predisposition due to germ-line genetic alterations that increase an individual’s risk of developing cancer, cancer is a genetic disease of somatic cells (see Article 88, Cancer genetics, Volume 2). Both activation of oncogenes and loss (deletion, mutation, or inactivation) of tumor suppressor genes appear to be common in solid tumors, and altered regulation of oncogenes is prevalent in hematologic malignancies (Cavenee and White, 1995; Weinberg, 1996). This dysregulation is frequently caused by chromosomal abnormalities.
To describe the dysregulation of cancer genes, an automobile analogy serves us well. Oncogenes are dominant-acting cellular genes normally involved in the processes of cellular growth and proliferation. When one copy of an oncogene is mutated or otherwise deregulated, it functions in a manner similar to a “stuck accelerator pedal”, resulting in uncontrolled cellular proliferation. Examples of oncogenes that are dysregulated in cancer cells include CCND1 and MYC (Schwab, 1999). Tumor suppressor genes are recessive-acting “gatekeepers” of cellular proliferation; they may inhibit cell growth or promote cell death (Kinzler and Vogelstein, 1997). When both parental alleles of a tumor suppressor gene are mutated, lost, or otherwise inactivated in a somatic cell, it is as if the cell has “lost its brakes”. Examples of such tumor suppressor genes altered in cancer cells are TP53, RB1, and CDKN2A (Sidransky, 1995). An additional family of genes critical to carcinogenesis is the DNA repair genes or “caretakers” of the genome (Kinzler and Vogelstein, 1997). These genes, including ATM, MSH2, and MLH1, maintain the “integrity” of the genome, playing key roles in DNA repair processes. Their role in cancer is comparable to experiencing a car problem when your mechanic was unavailable to repair it, thus leading to additional engine (DNA) damage.
Tumors develop as a result of deregulation of not one but multiple cancer-related genes. Alteration of three to six genes is thought to be necessary for tumor development. Mutations in additional genes result in increased cellular proliferation, disorganization, tumor progression, invasion, and metastasis (Vogelstein and Kinzler, 1993). Fearon and Vogelstein (1990) first proposed a linear pathogenetic schema for colon cancer, based on the concept that tumors develop and progress as a result of a distinctly ordered accumulation of tumor suppressor gene losses and oncogene activations, which endow a clonal population of cells with a proliferative advantage. The genetic changes in tumors may be conceptualized as a series of evolutionary events, which may have neutral, deleterious, or advantageous effects on the proliferation of a clone of cells. Neutral or deleterious genetic changes may result in stagnation or cell death, whereas advantageous events may result in a proliferative advantage, an increase in recruitment of blood vessels to the developing tumor, and/or the ability to metastasize. The Darwinian concept of “survival of the fittest” (Darwin, 1860) may be modified to “survival of the baddest”, that is, those cells that can proliferate the most efficiently, vascularize, and metastasize, but they eventually defeat themselves by killing the host.
Cancer is a disease in which genetic alterations, most frequently chromosomal rearrangements, are acquired in somatic cells, resulting in loss of critical cellular checks and balances, resulting in dysregulation, proliferation, and ultimately, malignant transformation. According to Hanahan and Weinberg (2000), the “hallmarks of cancer” are defects in cell growth and proliferation, evasion of apoptosis, genome instability, sustained angiogenesis, and invasion and metastasis. Human cancer cells are characterized by cytogenetic alterations: numerical chromosomal alterations (aneuploidy), structural chromosomal alterations, and more frequently in carcinomas than hematologic malignancies or sarcomas, chromosomal instability. Aneuploidy results from the numerical chromosomal alterations. Further segmental chromosomal gains and losses come from structural chromosomal alterations, including reciprocal and nonreciprocal translocations, homogeneously staining regions, amplifications, and deletions. The cytogenetic aberrations in hematologic malignancies and sarcomas are frequently clonal, that is, the same abnormalities are present in multiple cells, all the progeny of one cell with a primary genetic alteration. In carcinomas, it is not unusual to find that each cell within a tumor has a different karyotype owing to chromosomal instability, which can be defined simply as numerical and structural chromosomal alterations that vary from cell to cell. Chromosomal instability is thought to be the means by which cells develop the features that enable them to become cancer cells (Hanahan and Weinberg, 2000). In spite of chromosomal instability, cancer cell karyotypes remain stable over time, probably because their genotypes have been optimized over their evolution for growth, making it less likely that additional genetic alterations will confer an additional growth advantage (Albertson et al., 2003).
2. Historical perspective and technical background
Chromosomal alterations and karyotypic instability in human tumor cells have been investigated for more than a century. In 1890, David Hansemann reported that tumor cells with an abnormal chromosome constitution (aneuploidy) had abnormal mitotic spindles with extra poles and other chromosome segregational abnormalities (Hansemann, 1890). This aneuploidy theory was formalized in the early 1900s when Theodor Boveri, while studying chromosomal segregation in Ascaris worms and Paracentrotus sea urchins, suggested that malignant tumors arise from a single cell that has acquired an abnormal chromosome constitution (Boveri, 1929). Chromosomal cytology was advanced in the 1920s and 1930s by two technical improvements by plant cytologists, the squash technique that eliminated the difficulty in reconstructing and analyzing chromosomes from serially sectioned preparations and the application of the mitotic inhibitor, colchicine, derived from the Mediterranean Colchicum plant, to disrupt the mitotic spindle and increase the yield of mitotic cells available for chromosome analysis. In spite of these improvements, mammalian cytogenetics did not blossom until the 1950s, when hypotonic treatment was found to facilitate chromosome spreading, enabling visualization, counting, and analysis of individual chromosomes. In 1955, Tjio and Levan applied these new advances in cytogenetics to their cultures of human fetal lung tissues and discovered that the human chromosome number is 46 (23 pairs of autosomes plus the two sex chromosomes, XX in females or XY in males), rather than the previously reported 48. During this time period, Theodore Hauschka, Sajiro Makino, and Albert Levan each observed that tumor cell lines expressed both numerical and structural chromosomal aberrations. Soon afterward, Jerome Lejeune founded the field of clinical cytogenetics, by reporting that children with Down syndrome (then called mongolism) had 47 chromosomes due to trisomy 21. Cancer cytogenetics advanced in 1960, when Nowell and Hungerford reported the first chromosome abnormality in malignant human cells, the Philadelphia (Ph) chromosome, in chronic myelogenous leukemia (CML). Their finding supported Boveri’s theory that cancer cells express chromosomal alterations. Major breakthroughs in cytogenetics occurred in the 1970s as a result of the development of chromosomal banding methods that enabled the identification of individual chromosomes as unique entities, based on their staining patterns (see Article 11, Human cytogenetics and human chromosome abnormalities, Volume 1 and Article 71, Advances in cytogenetic diagnosis, Volume 2). Classical karyotyping was formalized at the Paris conference in 1971 (and a subsequent meeting in Edinburgh early in 1972), when a uniform system of human chromosome identification based on the new chromosome banding methods was established. Current karyotyping practice involves analysis of 20 trypsin-Giemsa banded metaphase cells and karyotyping of at least two per clone. Cytogenetic nomenclature divides chromosomes into arms (p for petit and q, the next letter in the alphabet after p), and arms into regions, and regions into bands and subbands (ISCN, 1995). Thus, chromosomal locations are pronounced by chromosome number, arm, region, and band. For example, chromosomal band 11q13 is pronounced, “eleven q one three” rather than “eleven q thirteen”, since 13 refers to region one band three and there are not 13 bands on the long arm of chromosome 11. Numerous additional classical cytogenetic banding methods have been developed to define chromosome structure and specific chromosome abnormalities.
Cytogenetic techniques critical to our understanding of genetic alterations in cancer cells are listed in Table 1. Molecular cytogenetic analysis, which blossomed in the late 1980s and continues to advance, has revolutionized the field of cytogenetics, particularly, cancer cytogenetics. The primary technique used for molecular cytogenetic characterization of chromosomes is fluorescence in situ hybridization (FISH) (Landegent et al., 1987; Cremer et al., 1988; Trask, 1991; Lichter and Ried, 1994; see also Article 22, FISH, Volume 1). This method involves hybridization of one or more fluorescently labeled DNA probes to metaphase chromosomes and/or nuclei. The most common form of FISH involves hybridizing a fluorescently labeled DNA probe (or a hapten-labeled DNA probe that can later be detected by a fluorescent tag) to a gene or DNA segment of interest and a different colored fluorochrome-labeled control probe (that maps to the same or a different chromosome) to metaphase spreads from a tissue or cells of interest. Cancer-related applications include mapping tumor-related genes or enumeration of gene or chromosome copy number in the context of deletions, amplifications, or ploidy changes. FISH hybridization to interphase nuclei (in paraffin or frozen sections, touch preparations, cytology brush preparations, or from harvested, dissociated tissue) is quite useful in cancer cytogenetics. Optimally, 200 cells are examined for this type of analysis. The results might also provide an indication of ploidy.
Table 1 Techniques useful in cancer cytogenetics
| Cytogenetic technique | Application | Tissue preparation |
| Trypsin-Giemsa | Classical karyotyping | Metaphase spreads from |
| banding (G-banding) Fluorescence in situ hybridization (FISH) | Molecular cytogenetic analysis of targets defined by the DNA probe utilized, e.g., centromeres by a-satellite DNA, cosmid or bacterial artificial chromosome (BAC) clones of genes, etc. for detection of chromosome or gene copy number or gene mapping | cultured cancer cells Metaphase spreads or interphase nuclei from cultured cells, paraffin or frozen sections, touch preparations, cytology brush preparations, or dissociated tissue biopsies |
| Chromosome painting | Molecular cytogenetic analysis of specific chromosomes or chromosome segments | Metaphase spreads from cultured cancer cells |
| Spectral karyotyping or multiplex FISH | Molecular karyotyping using a set of chromosome-specific DNA probes, each labeled with a different dye or dye combination, to distinguish each of the human (or mouse) chromosomes as unique | Metaphase spreads from cultured cancer cells |
| Comparative genomic hybridization (CGH) | Comprehensive screen of the genome for segmental gains and losses using DNA isolated from test tissue (e.g., tumor cells; labeled in green) and control DNA (labeled in red) | Normal control male
metaphase chromosomes; high molecular weight DNA isolated from cancer cells |
Interphase FISH eliminates the necessity and time of cell culture and enables enumeration of gene copy number compared to a control probe on the same chromosome in large populations of cells by molecular karyotyping of interphase nuclei even in the absence of metaphase chromosomes. However, FISH analyses only identify alterations of the targeted DNA probe(s), and do not detect other alterations, such as chromosomal abnormalities that may be important for a particular diagnosis or prognosis in a cancer patient. Therefore, at the present time, it is critically important that FISH be used as an adjunct to classical cytogenetic analysis and not as a “stand-alone” test. Another FISH method, chromosome painting, utilizes a cocktail of fluorochrome-labeled DNA probes from a chromosome or selected chromosomal region to localize those sequences in metaphase spreads from a tissue of interest (Pinkel et al., 1988; Ried et al., 1998). Chromosome painting enables identification of the origin of chromosomal segments involved in translocations and characterization of marker chromosomes, the origin of which is refractory to definition by classical cytogenetic methods. When examined using standard fluorescence microscopy, painting of two or more differentially labeled chromosomes in interphase nuclei results in beautiful multicolored nuclei, but provides little specific structural information owing to overlap of the chromosomal domains, which can only be resolved by computerized optical sectioning using confocal or other sophisticated computer-controlled microscopy.
Two new variations of chromosome painting that are extremely useful in cancer cytogenetics are spectral karyotyping (SKY) and multiplex FISH (M-FISH) (LeBeau, 1996; Schrock et al., 1996; Speicher and Ward, 1996; Speicher et al., 1996). These methods distinguish the 24 different human chromosomes (22 auto-somes and two sex chromosomes) using a set of chromosome-specific DNA probes, each labeled with a different array of one or more fluorescent dyes and FISH hybridization to metaphase chromosomes. The detection and classification of the rainbow of probes utilizes different strategies, both involving CCD cameras and computer algorithms, with SKY taking advantage of Fourier spectroscopy to resolve the multicolored probes and M-FISH using multiple optical filters and image super-imposition. Combinations of these classical and cytogenetic methods are essential to fully characterize the karyotypic alterations in cancer cells.
A common problem in cancer cytogenetics is a low mitotic index and the consequent lack of metaphase chromosomes to analyze and karyotype. Comparative genomic hybridization (CGH) is a form of FISH that comprehensively screens the genome for gains and losses of DNA segments (Kallioniemi et al., 1992; Du Manoir et al., 1993). The advantages of CGH are that it provides an overall picture of genomic imbalances in a tumor, eliminates the need for cell culture, and thus overcomes the primary difficulty of karyotyping tumors. The disadvantages of CGH are that it cannot detect balanced chromosomal rearrangements, such as translocations or inversions, and it cannot suggest the manner of loss in terms of chromosome rearrangements (i.e., whether loss of a segment is due to a deletion or an unbalanced translocation). Further, CGH can only detect relatively large genetic aberrations (such as gains of at least 2mB and losses on the order of 10 mB). Moreover, the smallest abnormalities are only detectable by CGH when present in the vast majority of the cells (~85%) in the population(s) from which the DNA was isolated, and larger abnormalities must be present in the majority of cells (~70%). Like other molecular methods that average the results, CGH analysis of tumor specimens is hampered significantly by the genetic heterogeneity seen within tumors and the frequent admixture with normal cells. This procedure is facilitated by commercially available imaging instrument and computer systems combined with sophisticated proprietary software. Newer advances include array CGH, a method involving the use of DNA microarrays containing specifically spaced, mapped markers distributed throughout the genome that are then hybridized and the ratios are linked to segmental gains and losses (Pinkel et al., 1998). The advantage of array CGH over cytogenetic CGH is higher resolution. The significant disadvantage at present is that high-resolution arrays are not readily available to the scientific community.
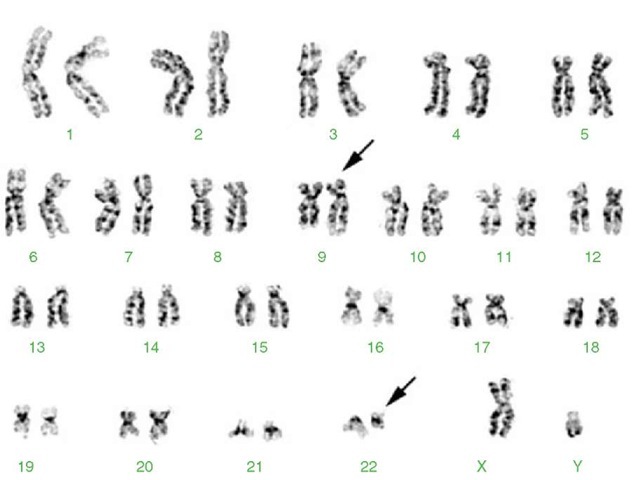
Advances in cancer cytogenetics have led from the discovery of the Ph chromosome to the characterization by Janet Rowley of the Ph chromosome as a reciprocal translocation between chromosomes 9 and 22, t(9;22)(q34;q11.2) (Figure 2). Identification of the Ph translocation, whether by classical or molecular cytoge-netic methods or molecular genetic techniques, is now essential for diagnosis of patients with CML, although the Ph chromosome may also be observed in some patients with acute lymphocytic leukemia (ALL) and less frequently, acute myeloid leukemia (AML). Characterization of the Ph chromosome in CML led to cloning of the Philadelphia translocation breakpoint and the discovery that the translocation creates a hybrid gene consisting of 5′ regulatory and coding sequences of the BCR gene on chromosome 22 joined with 3′ coding, polyadenylation, and termination sequences from the ABL proto-oncogene on chromosome 9. This BCR/ABL rearrangement may be identified by FISH, a molecular cytogenetic method described earlier (Figure 5). The new chimeric gene formed by the Philadelphia transloca-tion codes for a protein with enhanced tyrosine kinase activity. Identification of this protein led to the development of an effective new drug, Gleevec®, which inhibits this tyrosine kinase, blocks cellular proliferation, and induces apoptosis in cells expressing the Ph chromosome. Thus, clarification of the cytogenetic aberrations in a cancer enables not only diagnosis, but molecular characterization of the breakpoints, identification of the genes involved, description of the molecular pathology of the malignancy, and development of targeted therapies. As noted recently by Rowley (2001), cytogenetic identification and subsequent molecular cloning of translocation breakpoints has become one of the most efficient tools for identifying genes involved in malignant transformation.
3. Clinical applications of cancer cytogenetics
Over the past four decades, it has become clear that acquired, nonrandom chromosome abnormalities are associated with specific malignancies, particularly hema-tologic malignancies and sarcomas, although other alterations are characteristic of carcinomas, either individually or as a group (Table 2; Figures 1-5). The diagnosis of hematopoietic and lymphoid malignancies has been codified recently by the World Health Organization (WHO) (Jaffe et al., 2001). Cytogenetic studies are valuable for determining the diagnosis and prognosis of specific cancers and serve as independent etiologic or prognostic risk factors even when age, white blood cell count, classification, and cell surface markers are evaluated. The new WHO classification of hematopoietic malignancies is the first to formally recognize the importance of genetic alterations in the diagnosis of leukemias, especially with regard to AMLs. Several types of AML are classified specifically on the basis of the cytogenetic aberrations present in the cells (Table 2; Figure 1). A cytogenetic alteration may be used to diagnose a particular malignancy on the basis of the specific association of the aberration with the disorder even when other diagnostic criteria are equivocal. Moreover, specific chromosomal abnormalities in malignancies may be associated with specific etiologies, such as loss of one chromosome 5, one chromosome 7, or deletion of the long arm of one chromosome 5 or one chromosome 7 in patients with AML after exposure to organic chemicals (such as dry cleaning or furniture refinishing solvents) or mutagenic therapy. Specific chromosomal alterations are often associated with a good prognosis. In AML, the t(8;21), t(15;17), and inv(16) rearrangements (Table 2) are usually associated with a favorable response to therapy and long-term survival (Grimwade et al., 1998). A complex karyotype, loss of one chromosome 5 or one chromosome 7, deletion of the long arm of one chromosome 5 or one chromosome 7, and/or abnormalities involving the long arm of chromosome 3 are associated with a relatively poor prognosis in AML. In addition to the diagnostic and prognostic usefulness of cyto-genetics, a change in the karyotype of the abnormal cells from a patient often precedes progression of their malignancy. Disease relapse in a patient following bone marrow transplantation may also be heralded by the reappearance of host cells with acquired chromosomal abnormalities that may have been observed prior to transplantation. Thus, chromosomal abnormalities are common in human cancer cells and may provide useful diagnostic and prognostic information.
Table 2 Most common clonal chromosomal abnormalities in malignancies0’6
| Malignancy | Consistent chromosomal abnormalities | Specific genes involved |
| AML | t(8;21)(q22;q22) | AML1/ETO |
| Acute promyelocytic | t(15;17)(q22;q12) | PML/RARa |
| leukemia | ||
| AML with abnormal bone | inv(16)(p13q22)c or t(16;16)(p13;q11) | CBFB/MYH11X |
| marrow eosinophils | ||
| AML | 11q23 rearrangements | MLL |
| Chronic myelogenous | t(9;22)(q34;q11.2)d | BCR/ABLg |
| leukemia (CML) | ||
| Precursor B-cell acute | t(9;22)(q34;q11.2) | BCR/ABL |
| lymphoblastic leukemia | ||
| t(4;11)(q21;q23)<l | MLL2(AF4)/MLL | |
| t(1;19)(q23;p13) | PBX/E2A | |
| t(12;21)(p12;q22) | ETV/CBFA | |
| Burkitt lymphoma | t(8;14)(q24;q32) | MYC/IGH@ |
| Follicular lymphoma | t(14;18)(q32;q21) | IGH@/BCL2 |
| Mantle cell lymphoma | t(11;14)(q13;q32) | CCND1/IGH@ |
| Anaplastic large cell | t(2;5)(p23;q35)f | NPM1/ALK |
| lymphoma | ||
| Alveolar | t(2;13)(q35;q14) | PAX3/FOXO1A |
| rhabdomyosarcoma | ||
| Ewing sarcoma | t(11;22)(q24;q12) | EWSR1/FLI1 |
| Synovial sarcoma | t(X;18)(p11;q11) | SS18/SSX1, SS18/SSX2, SS18/SSX4 |
a AML: acute myeloid leukemia; CLL: chronic lymphocytic leukemia. 6Based on Mitelman Database of Chromosome Aberrations in Cancer (2003). c Figure 1. d Figure 2. e Figure 3. f Figure 4. g Figure 5.
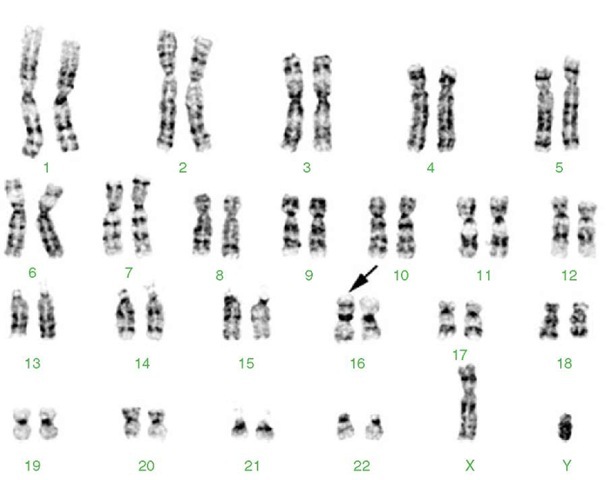
Figure 1 Karyotype from a 10-year-old female with acute myeloid leukemia (AML) with abnormal bone marrow eosinophils and the characteristic inverted chromosome 16 (arrow). This inversion results in a fusion between CBFB on the long arm that codes for a subunit of a DNA binding protein complex and the MYH11 gene on the short arm that codes for the smooth muscle form of myosin heavy chain protein. The fusion protein is thought to interact with the AML1 transcription factor and increase its ability to bind to DNA and regulate transcription. 46,XX,inv(16)(p13q22)
Cytogenetic alterations often point to the genes involved in the development and/or progression of malignancies. Numerous chromosomal translocation breakpoints have been cloned, and the genes, regulation of which has been altered by those translocations identified. These genes belong to specific functional families that are involved in the regulation of cellular growth (see Rowley, 2001, Table 1). Genes altered in cancer cells are important targets for the development of designer drugs to limit aberrant cellular proliferation. The example of the Ph chromosome was discussed earlier. Identification of the altered gene product in CML, a novel tyrosine kinase resulting from this translocation led to Gleevec®, a targeted (designer) therapy, with significant efficacy in CML and several solid tumors. Targeting of additional genes or gene families may lead to effective therapies for other malignancies.
Figure 2 Karyotype from a 33-year-old male presenting with chronic myelogenous leukemia (CML). 46,XY,t(9;22)(q34;q11.2). See text for details about this translocation. The derivative chromosomes 9 and 22 are marked by arrows
4. On the origin of chromosomal abnormalities in human cancers
Although specific numerical and/or structural cytogenetic alterations have been associated with particular malignancies, the mechanisms by which those abnormalities occur have yet to be delineated.
Numerical chromosomal alterations can arise by random loss or gain of chromosomes, but also as a result of defects in the process by which equal numbers of chromosomes are distributed to daughter cells at mitosis, that is, chromosome segregation. Recent studies have shown that several factors can result in segregation defects, including abnormal kinetochore-spindle interactions, premature chromatid separation, centrosome amplification, multipolar spindles, and abnormal cytokinesis. Research by a number of investigators has recently advanced our understanding of the relationship between centrosomal defects, multipolar spindles, unequal chromosome distribution, and chromosomal instability. Investigations of several centrosomal proteins have been important in our understanding of segrega-tional defects in human cells. Examination of Aurora A kinase and other proteins (pericentrin, NuMA, y-tubulin, and polo kinase) involved in centrosome function and the spindle checkpoint are in progress (reviewed in Gollin, 2004). Their role in chromosomal instability and implications in the diagnosis, prognosis, and therapy of human tumors will be revealed in the coming years. Further, these proteins are candidate targets for drug development.
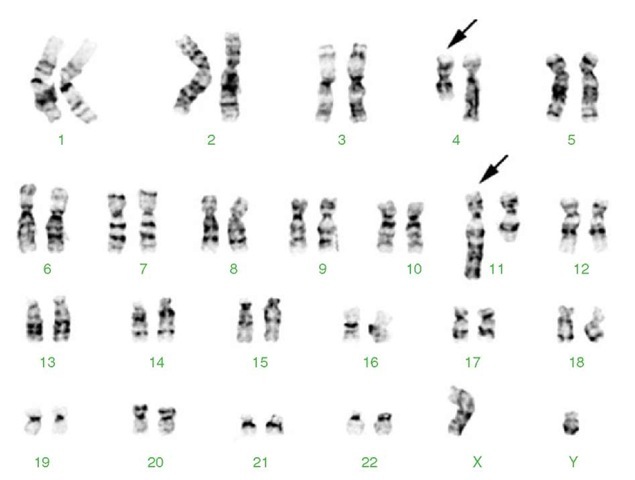
Figure 3 Karyotype from a young boy with acute lymphoblastic leukemia. This translocation results in fusion of the MLL gene at 11q23 and the MLL2/AF4 gene at 4q21, resulting in an abnormal chimeric transcript. The leukemia in these patients usually has aggressive clinical features, hyperleukocytosis, a B-precursor immunophenotype with coexpression of myeloid markers, and a poor response to conventional chemotherapy. 46,XY,t(4;11)(q21;q23). The derivative chromosomes 4 and 11 are marked by arrows
Structural aberrations in cancer cells include structural chromosomal alterations, including reciprocal and nonreciprocal translocations, homogeneously staining regions, amplifications, and deletions. The etiology of structural chromosomal abnormalities is not clear. However, we do know that (1) environmental agents, such as cigarette smoking, benzene, and other chemicals, cause DNA double-strand breaks; (2) repeated sequences, including duplicons and other genomic repeats may play a role in chromosomal rearrangements in cancer (Saglio et al., 2002; Lupski, 2003; and discussed in Rowley, 2001); and (3) patients with DNA repair defects are prone to develop cancer (Shiloh, 2003; Gollin, 2004). Thus, structural abnormalities in cancer may be related to one or a combination of factors, including the formation of DNA double strand breaks, and then either recombination between homologous genomic motifs and/or defects in the DNA damage response. Structural chromosomal abnormalities in cancer cells often result in further structural alterations and instability due to chromosomal breakage. Factors involved in structural and numerical chromosomal instability in cancer cells are reviewed in more detail in Gollin (2004). Further studies on the mechanisms behind chromosomal alterations in human tumors are underway in many labs around the world.
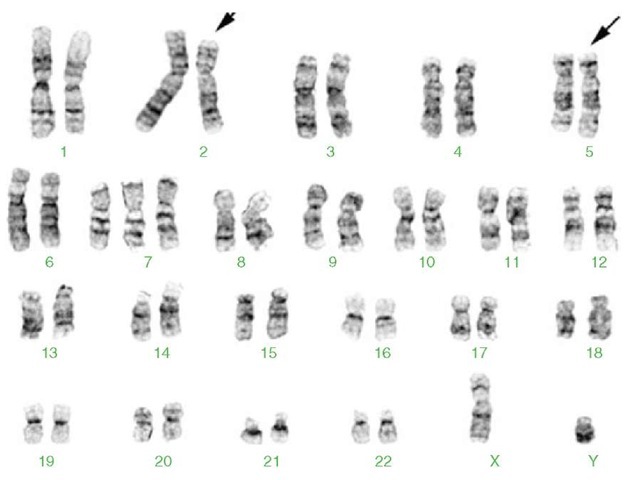
Figure 4 Karyotype from a male with anaplastic large cell lymphoma with the t(2;5)(p23;q35) and trisomy 7. This translocation results in fusion between the anaplastic lymphoma kinase (ALK) gene on 2p23 and the nucleophosmin (NPM) gene on 5q35. 47,XY,t(2;5)(p23;q35),+7. The derivative chromosomes 2 and 5 are marked by arrows
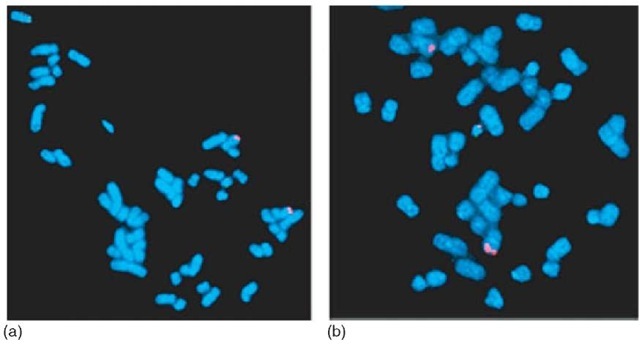
Figure 5 FISH for the BCR/ABL rearrangement, usually seen in chronic myelogenous leukemia. The cells are hybridized with the LSI BCR/ABL ES (extra signal) probe (Vysis, Inc., Downers Grove, IL). (a) Negative control (no rearrangement). Two red signals for the ABL oncogene (9q34) on distal 9q. Two green signals for the BCR gene at 22q11.2. (b) Metaphase from a CML patient with the t(9;22)(q34;q11.2). BCR/ABL rearrangement (adjacent red and green signals on the small Philadelphia chromosome in the center of the metaphase spread), one red signal on the normal chromosome 9, a residual red signal on the rearranged chromosome 9, and one green signal on the normal chromosome 22
5. Conclusion
Cancer cytogenetics has become a very important area of clinical cytogenetics, critical to the diagnosis of hematologic malignancies and helpful in the differential diagnosis of solid tumors. Research in cancer cytogenetics will continue to play an important role in the identification of oncogenes and tumor suppressor genes and examination of their function(s) in the cellular regulatory and signaling pathways. With the results of the Human Genome Project and the advances in molecular biology laboratory methodology and drug development, our prospects for significant advances in cancer diagnostic and prognostic testing and therapy based on the results of cancer cytogenetic studies are great. Yet, it is likely that classical as well as molecular cytogenetic studies will continue to be carried out on malignancies, as they provide a relatively rapid, inexpensive overall view of genomic alterations in a malignancy, useful for diagnosis, prognosis, and choice of therapeutic regimen.