Introduction
Spectroscopy is an analytical technique that uses electromagnetic radiation to probe the atomic or molecular composition of substances. This article outlines the basic principles of spectroscopy for non-specialists.
Light and Electromagnetic Radiation
Light is a phenomenon that is a special case of a much wider phenomenon called electromagnetic radiation. As the name indicates, electromagnetic radiation is a travelling disturbance in space that comprises electric and magnetic components. Unlike the field associated with a common magnet or the earth, the magnetic field associated with electromagnetic radiation is constantly changing its direction and strength. The electric field component undergoes exactly the same behavior. Figure 1 illustrates what would be observed if it was possible to see the electric and magnetic components of a ray of radiation as it passes. It is the interaction of the electric and magnetic components of radiation with matter that are responsible for all the phenomena we associate with radiation, such as human vision, sunburn, microwave cookery, radio and television, and of course spectroscopy.
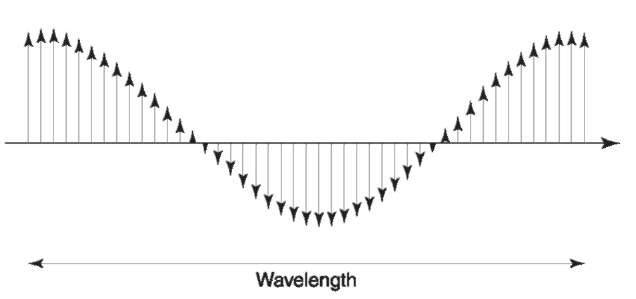
Figure 1 Representation of a ray of electromagnetic radiation traveling through space, left to right. The small vertical arrows represent the strength and direction of the electric field component of the radiation at discrete locations (the magnetic field behaves in an analogous manner). The disturbance is periodic, with the distance between field components of identical strength and direction being the wavelength of the radiation.
There are some important fundamental features of electromagnetic radiation. The periodicity of the disturbance, or the distance between adjacent troughs or adjacent peaks, is referred to as the wavelength of the radiation. The number of times the electromagnetic field undergoes a complete cycle per unit time is called the frequency. The speed with which radiation can travel is limited, and moreover, radiation of all wavelengths travels at a single speed. This is referred to as the speed of light, universally denoted by the symbol C. The speed of light (and every other radiation) is dependent on the substance through which it travels, and reaches a maximum in a vacuum. The speed of light is a constant of proportionality between frequency and wavelength as described by equation 1.
C = v x A
where v is radiation frequency, X is radiation wavelength and C is speed of light.
The interpretation of this equation is that wavelength and frequency are related. Electromagnetic radiation of long wavelength must have a low frequency, whereas radiation of short wavelength must have a high frequency. At the long wavelength (low frequency) end of the electromagnetic radiation spectrum are radio waves, at the other is high frequency (short wavelength) radiation such as gamma rays. Between these extremes lie other forms of radiation, including microwaves, infrared, ultraviolet, and that narrow band of radiation that can be directly observed by humans, visible light.
Radiation carries energy with it, a phenomenon which is made use of in the domestic microwave oven, for example. The amount of energy carried is proportional to the frequency of the radiation. As frequency and wavelength have an inversely proportional relationship, energy carried is inversely proportional to wavelength. Radiation of high frequency and short wavelength (e.g. x-rays, gamma rays) therefore is of high energy, whereas low frequency radiation which must have long wavelength (e.g. radio waves) carries little energy.
Matter
Given that radiation is an electromagnetic phenomenon, the main feature of matter as far as its interaction with radiation is concerned is the distribution of its electric and magnetic components.
From the subatomic scale to the molecular, matter exhibits a variety of electric and magnetic features with which radiation can interact. Figure 2 depicts some of these features for a molecule of ammonia. The forces that hold the molecule together arise from attraction between negatively charged electrons shared between the positively charged nuclei of the nitrogen and hydrogen atoms. The nuclei of each of the hydrogen atoms contain a single charged particle, a proton. The share of electrons between the hydrogen nuclei and the nitrogen nucleus is not equal; nitrogen commands a larger share. Each nitrogen-hydrogen bond therefore has a heterogeneous distribution of charges resulting overall in a positively charged end (towards the hydrogen atom) and a negatively charged end. Such a bond is described as having a dipole moment or being polarized. As each nitrogen-hydrogen bond has a similar polarization there is an additive effect with the result that the entire molecule is polarized or has a dipole moment.
Each nucleus of the hydrogen atoms is comprised of a single positively charged proton, each acts like a small bar magnet, or is said to possess a magnetic moment. Potentially the entire ammonia molecule, its bonds, its protons, and its electrons each have the necessary prerequisites to interact with radiation.
Interaction Between Radiation and Matter
In the previous section it was shown that matter can be thought of as possessing many minute electric and magnetic moments on the microscopic sale. Radiation, which is also electromagnetic in nature, therefore potentially can interact with matter on the microscopic scale. If interaction is to be significant, however, there has to be a match between the frequency of the electromagnetic oscillations executed by the radiation and oscillations of the electromagnetic moments within the matter. The situation is analogous to that of pushing a person on a swing. Say the person on the swing completes a cycle once every second. Efficient transfer of energy takes place when pushes are in unison with the motion of the swing (i.e. with a frequency of one per second). If the frequency of pushing is three per second, then two of the three will accomplish nothing, energy transferral is not efficient.
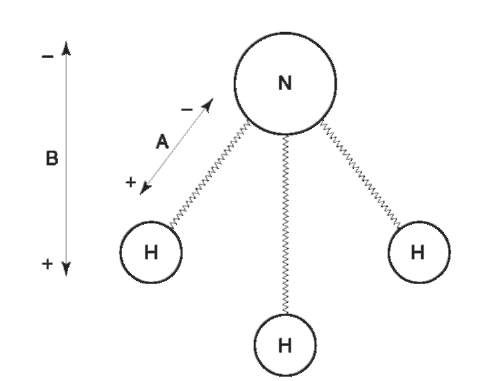
Figure 2 Electrical moments in the ammonia molecule. ‘A’ represents the electrical field resulting from unequal sharing of electrons between nitrogen and hydrogen nuclei. ‘B’ represents the overall field resulting from combination of three ‘A’ moments.
Spectroscopy is a tool that makes use of interactions between matter and radiation. The outcome of the interaction allows scientists to infer analytical information regarding the atomic or molecular composition of the matter.
Spectrometers
The interaction between radiation and matter can lead to the occurrence of a variety of phenomena; two important ones as far as spectroscopy is concerned are absorption and emission.
An appreciation of absorption can be gained by considering the properties of the lens in a pair of sunglasses. Through the lens the image of the outside world is duller, some of the radiation (light) encountering the lens does not pass through – the lens absorbs it. The basis of absorption spectroscopy is the measurement of the manner in which matter absorbs radiation.
If a substance absorbs radiation it can re-emit it in a modified form; this is referred to as emission. The basis of emission spectroscopy is the measurement of the manner in which matter emits radiation. The output from measurement of either emission or absorption is called the spectrum of the substance.
Figure 3 depicts the important features of simple instrumentation that can be used for absorption spec-troscopy, and a typical spectrum. Although all absorption spectrometers might not be exactly described by Fig. 3, as far as this article is concerned the important issue is the process leading to the spectroscopic output, and the features of the output itself, rather than the exact mechanism by which it was generated.
Three basic elements are present: a radiation source; a variable ‘filter’; and a detector. It is usual for the source to emit radiation of many frequencies within a region of the entire electromagnetic spectrum, for example the infrared region, the visible light region, x-rays, etc. The region over which the source operates in a given spectroscopic technique is often used as a means to name that technique. For example, if an infrared source is used, then the technique is referred to as infrared spectroscopy.
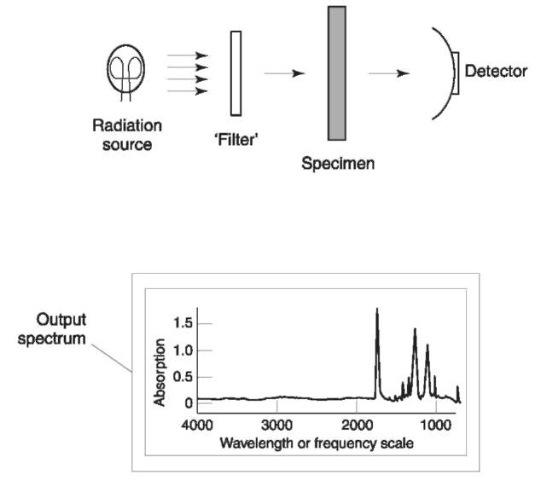
Figure 3 Schematic diagram of a spectrometer. Radiation from the source is passed through a ‘filter’, then the specimen, and then on to the detector. The spectral output is a plot showing the range of frequencies (or wavelength) that strike the specimen, versus the extent of absorption of each frequency.
The filter selectively modifies radiation from the source, ideally it should allow radiation of only one frequency at a time to pass through. The radiation from the filter is then allowed to pass through the specimen to be analyzed. The detector then collects the radiation that is transmitted by the specimen, where its intensity is measured. Spectroscopic measurement of the specimen is performed by ‘sweeping’ the filter through its entire frequency range allowing (ideally) the detector to measure the radiation transmitted by the specimen one frequency at a time. A graphical representation of measurements made by the detector is the spectrum of the specimen; typical output is shown in Fig. 3.
The horizontal axis in the spectrum defines the range of frequencies of the radiation that strikes the specimen. The vertical axis indicates how much of each frequency is absorbed by the specimen (i.e. a peak at a certain frequency indicates that the specimen absorbs at that frequency).
The ideal of spectroscopy is that because different substances undergo different motions on the microscopic scale, then the spectrum of each must have a unique pattern of peaks. The reality is that substances with very similar structures could behave in a very similar manner, with the result that instrumentation is not capable of discriminating them.
Throughout this article the term spectrometer has been used to describe the device that acquires a spectrum, whereas spectroscopy has been used to describe the technique. This usage is common in modern publications, but it is by no means the only terminology that might be encountered. The words spectrograph, spectrophotometer, and spectroscope are used to describe apparatus, whereas spectrometry and spectrophotometry are used interchangeably with spectroscopy.
