Introduction
The techniques described below are those that are most commonly encountered in forensic laboratories. For fundamental details relating to matter-radiation interactions, instrumentation, data acquisition, and data interpretation readers are directed to the reference works listed under Further Reading.
Infrared Spectroscopy
The key activity taking place during infrared spectroscopy is interaction of infrared radiation with bonds between atoms in the specimen. Absorption of radiation causes the bonds to attain a vibrational state of higher energy. As the vibrational state of a bond depends on many parameters, such as its strength, and the relative weights of the atoms at its termini, different bonds require different stimulation and therefore absorb radiation at different frequencies throughout the infrared range. Spectroscopy in the mid infrared region (4000-500 cm—1) is commonly employed in forensic science. A single infrared spectrum can, potentially, provide a wealth of information relating to a specimen.
Infrared spectroscopy is unsurpassed as a universal technique for the identification of the class of compounds present within a specimen. For example, it is very easy to identify fibers on a generic basis such as acrylics, nylons and polyesters, or paints on a generic basis such as alkyds, acrylics, urethanes, nitrocellulose, etc. With the advent of computerized spectral databases and chemometrics it is now possible to extend the technique to allow subgeneric discrimination.
However, in a few circumstances infrared spectroscopy might not allow easy discrimination within a class, particularly if mixtures of members of the class are present. Figure 1 gives the spectral data for three common phthalate ester plasticizers. Although dimethyl phthalate is easily distinguished from either diisooctyl phthalate or dihexyl phthalate, it is very difficult to distinguish the latter closely related esters from each other. Consider, then, two polymer specimens, one composed of polyvinyl chloride plasticized with diisooctyl phthalate, and another composed of polyvinyl chloride plasticized to the same extent with a mixture of diisooctyl phthalate and dihexyl phtha-late. Infrared spectroscopy would not be capable of distinguishing these two specimens. Another good example of the limitation of the technique is differentiation of paints within the alkyd class.
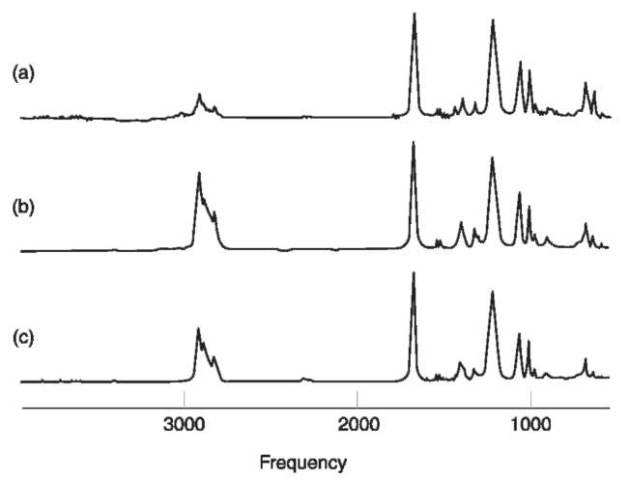
Figure 1 Infrared spectral data from three phthalate ester plasticizers. (a) Dimethyl phthalate; (b) diisooctylphthalate; (c) dihexylphthalate.
The limit of detection, using infrared spectroscopy, of a substance in the presence of another is about 5%. Infrared spectroscopy is therefore not a good technique for the detection or identification of trace copolymers, residual solvents, low-level pigments, or some additives in polymer samples (e.g. fibers or paints), nor is it of any use for the detection or identification of manufacturing impurities, or trace contaminants in illicit drugs. Above this limit of detection, however, infrared spectroscopy can be a reliable qualitative tool, and even semiquantitative analysis can be performed. For example, by using a set of calibration data comprising various known mixtures of heroin in sucrose, relatively accurate quantitative analysis of unknown mixtures of heroin in sucrose can be performed. Unlike chromatographic techniques (such as liquid chromatography or gas chromatography) that are somewhat independent of matrix identity, matrix contribution must be accounted for in quantitative infrared spectroscopy. It would not be possible, for example, to estimate the level of heroin in glucose using calibration data obtained from mixtures of heroin in sucrose. Specific calibration sets must be established for each type of analysis.
Infrared spectroscopy can be used to elicit structural information that might be difficult or impossible to arrive at using other techniques. One area of great utility is the characterization of stereoisomers. For example, the diastereoisomers ephedrine and pseu-doephedrine are not distinguishable by mass spectrometry, and depending on the stationary phase used, might exhibit identical retentions in a gas chromatograph. However, infrared spectral data for these two compounds are easily distinguishable. In a similar fashion infrared spectroscopy is very efficient as a means of discrimination between positional isomers of aromatic substances (e.g. the various isomers of dimethoxyamphetamine, or trimethyl benzene) and between other stereoisomers (e.g. the isomeric group cocaine, allococaine, pseudococaine, and pseudoallo-cocaine; or the group butyl nitrite, isobutyl nitrite, and secbutyl nitrite, or the isoheroins and heroin).
It is often of some importance to ascertain whether a drug is present as free base or a salt. The N-H stretch in an amine salt is found between 2500 and 3000 cm-1, whereas the N-H stretch in its free base is found well above 3000 cm-1. As a consequence, spectral properties for drug free bases and their salts are very different in the 2500-3500 cm-1 region.
Infrared spectroscopy is a technique that can provide information relating to the total composition of the specimen, unlike chromatographic techniques,which usually require an extraction step and have restrictions due to volatility, solubility, etc. Such techniques therefore can only provide partial information as to the specimen. In the examination of paint for example, infrared spectroscopy can provide information as to the polymeric binder as well as the inorganic extenders and pigments. Figure 2 shows the spectrum of a paint specimen, and spectra for kaolin and silica. Spectral features due to those minerals as well as the polymer are evident in the spectrum of the paint. Inorganic halides are virtually transparent to infrared radiation, therefore the presence of, for example, common salt or potassium bromide in a specimen could not be deduced. Metals and some of their oxides also do not give useful data.
The very feature that can make infrared spectroscopy useful (i.e. sensitivity to a wide range of substances) might also be a disadvantage. Figure 3 shows the spectrum obtained from a sample of colored matter (apparently electrical wire insulation) taken from a pair of wire cutters suspected of being used to disable a burglar alarm. Spectral features due to the polymer (PVC) are almost nonexistent, the majority of peaks are due to plasticizer (a phthalate ester, or a mixture of them) and calcite. As a means of comparison between the recovered chips of plastic and insulation taken from wire in the burglar alarm, infrared spectroscopy is particularly poor. Spectral features due to a very important component of the material, the polymer, are masked by peaks due to additives.
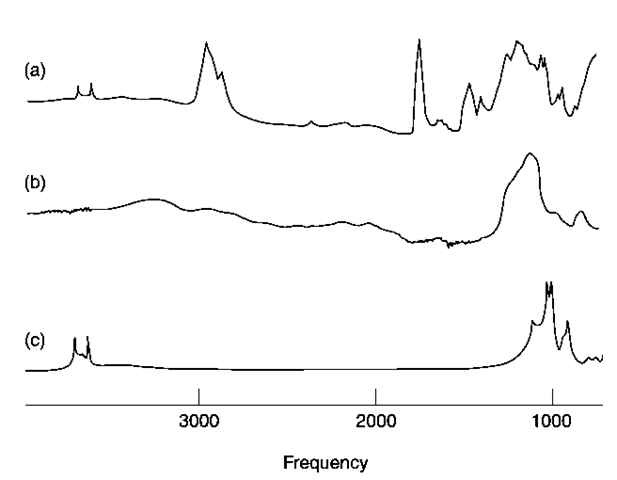
Figure 2 Infrared spectroscopy of paint films allows identification of inorganic compounds as well as the polymeric binder. In this example neither the binder nor the inorganic substances dominate the spectrum, (a) Paint film; (b) infrared spectrum for silica; (c) infrared spectrum for kaolin.
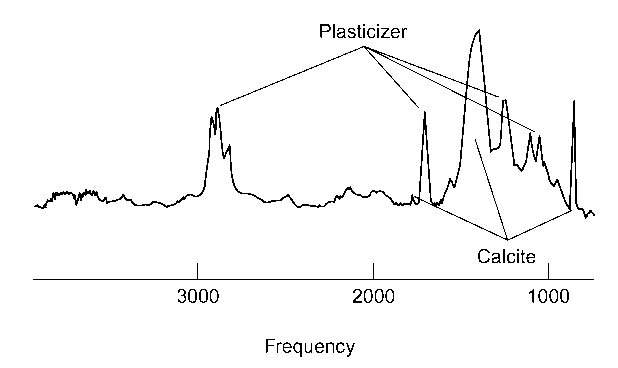
Figure 3 Infrared spectrum recorded from a small piece of plastic electrical insulation. Peaks indicated are due to plastici-zer or calcite. In this spectrum the inorganic fillers dominate, therefore the polymeric substance (polyvinylchloride) is very difficult to identify.
Undercoats and primers can be very high in inorganic solids such as calcite, talc, kaolin etc. It is often the case that the presence of these substances precludes infrared spectroscopy as a means of identifying the organic binder, or makes it very difficult. This does not mean that infrared is a poor technique for discrimination of highly filled paints, but it does mean that any discrimination is likely to be based primarily on fillers, and not on polymeric composition. Interpretation of results should, therefore, bear this in mind, or another technique to identify the polymer composition should be carried out (such as pyrolysis-gas chromatography-mass spectrometry).
Infrared spectroscopy can be carried out on microscopic specimens (down to about 10 um square) using an infrared microscope. This instrument is simply a device which condenses the infrared beam from its standard size (usually about 10 mm) to much smaller dimensions (about 0.18 mm). This task is accomplished with a microscope, which is also used to allow high magnification viewing of the specimen and accurate positioning of it in the beam. The technique is referred to as infrared microscopy or, more accurately, infrared microspectroscopy.
Infrared microspectroscopy is the usual technique employed in the analysis of small samples of paint (e.g. motor vehicle accidents, breaking and entering), single textile fibres (evidence of contact), particulate material (explosives residues such as ammonium nitrate, smokeless powder, etc.), and concentrated extracts of low dosage drugs (e.g. an extract from an LSD (lysergic acid diethylamide) dose evaporated to a microscopic spot). It is possible to record spectra from a few nanograms of material using an infrared microscope.
In practice, infrared microspectroscopy differs little from infrared spectroscopy. Sample preparation is a little more tedious, as manipulations must be carried out with the aid of a microscope. In most instances steps must be taken to reduce the thickness of the specimen to about 10-30 um, otherwise bad artifacts will be evident in the spectrum. This can be accomplished by using a sharp blade, flattening the specimen between diamond plates, rolling the specimen with a small metal roller, microtoming the specimen, etc. Even though it is possible to see very small objects (less than 10 um) with the infrared microscope, diffraction of the infrared beam inhibits the acquisition of high quality spectra. A description of diffraction and its effects on microspectroscopy is beyond the scope of this text, the reader is referred to the bibliography for useful references.
With infrared microspectroscopy, as with any other microspectroscopic technique, the homogeneity of the specimen is an important issue. For example, if single textile fibers are contaminated with blood, adhesive from tape lifts, or mountant (if the fiber has been examined previously with the aid of a compound microscope), then the contaminant is likely to give rise to unwanted peaks in the spectral data. In the case of some paint samples the granule size of some inorganic fillers, such as calcite, can be relatively quite large. Therefore, on the microscopic scale some paints are quite heterogeneous. Effects of heterogeneity can be noticed if one is forced to analyze a very small specimen of highly filled paint (e.g. architectural undercoats), or if a very small probe beam is used. Very small specimens might not exactly represent the composition of the macroscopic film from whence it came, by chance the specimen might contain more or less calcite, for example. Similarly, if a relatively large specimen is taken for analysis, but only a very small portion of it is probed by the infrared beam, the spectrum produced will depend on exactly where the microbeam strikes the specimen. In some locations the beam might encounter mostly calcite, whereas in other locations the beam might encounter mostly polymeric binder. In infrared microspectroscopy, therefore, one should take steps to insure that an accurate representation of the specimen has been achieved. If it has been possible to recover a relatively large specimen, then as much of it as possible should be analyzed. On the other hand, if only tiny specimens can be recovered it is wise to analyze a few of them (separately) in order to fully represent the film from whence they came. A single, minute paint chip presents a problem one must be aware that it might not be representative of its source.
The foregoing has described infrared spectroscopy transmission-absorption techniques, that is, infrared radiation is allowed to pass through the specimen, and absorption of radiation is measured. There are two other techniques in widespread use in forensic laboratories; diffuse reflectance spectroscopy (DRIFTS) and internal reflectance spectroscopy (IRS, sometimes referred to as attenuated total reflectance (ATR) spectroscopy).
In IRS the infrared beam is caused to reflect inside a special crystal. At the point of reflection the beam breaks through the crystal surface slightly (the order of about 1 um) to form what is referred to as an evanescent wave. The specimen is placed on the crystal at the point of the evanescent wave and any absorption of the wave by the specimen is recorded as the IRS spectrum (Fig. 4). There are some important considerations with IRS. First, the magnitude of the evanescent wave is dependent on the wavelength of the radiation; long wavelength infrared generates a bigger evanescent wave, short wavelength infrared a smaller wave. The outcome is that IRS spectra, compared to standard transmission spectra, show increased absorbances of peaks at long wavelength. Second, the penetration of the evanescent wave into the specimen is small because the wave itself is small, therefore IRS is a surface analytical technique. IRS will overrepresent surface contamination or surface modification, if the specimen is laminated or graded in some way then IRS will provide data relating to the surface only. For comparative work therefore, IRS data should not be compared with transmission data. Furthermore, the properties of IRS should be borne in mind when examining sheath-and-core bicomponent fibers, and identifying polymers with surface films, surface contamination or surface oxidation.
Diffuse reflectance is a special type of reflection from the specimen; the origin of the phenomenon is not of great relevance here. The important issue is that during the process of diffuse reflection the infrared beam interacts with the specimen and some radiation is absorbed. It is this absorbed radiation that is measured and plotted, versus frequency, as the DRIFTS spectrum.
The main virtue of DRIFTS is the ease with which samples can be prepared. All that is required for powdered specimens is to insure that the particle size is reasonably small, the spectrum can be acquired directly from the specimen raked flat in a small cup. Solutions of drugs, for example extracts in dichlor-omethane, can be applied to ground potassium bromide in the sample cup and their spectral data collected after the solvent has evaporated. Large specimens of polymer can be abraded using silicon carbide paper, and the spectrum of the dust thus produced can be acquired directly from the surface of the paper. DRIFTS is representative of the surface of a specimen, therefore, depending on the exact method of specimen preparation, the precautions listed above for IRS might need to be borne in mind.
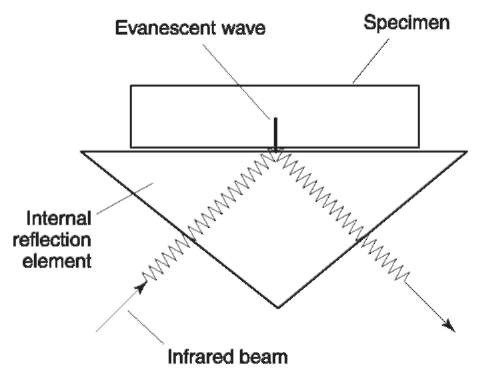
Figure 4 Principle of internal reflection spectroscopy. The infrared beam enters a special crystal and is internally reflected offone of its surfaces. At the point of internal reflection an evanescent wave penetrates the crystal surface into the specimen, which is in very close contact with the surface. The beam, which has suffered some absorption via the evanescent wave then travels on to the detector.
Visible and Ultraviolet Spectroscopy
Visible (Vis) spectroscopy and ultraviolet (UV) spectroscopy are usually referred to as a single technique (UV-Vis). This is because the two types of radiation are adjacent in the electromagnetic spectrum, the molecular transitions that give rise to the spectroscopic phenomena are of the same nature, and usually a single instrument is capable of acquiring both types of spectral data. The key activity taking place during this technique is interaction of visible light or ultraviolet radiation with electrons within molecules in the specimen. Absorption of radiation causes molecules to attain an excited state of higher potential energy. The energy of the excited state depends on the types of bonds and atoms present in the specimen, therefore different electrons in different substances require different stimulation, and therefore absorb different frequencies of radiation. This probe, however, is not as sensitive to subtle changes in molecular structure as say, infrared spectroscopy or nuclear magnetic resonance (NMR), therefore UV-Vis spectroscopy is a technique with relatively limited discriminating power. As a consequence, UV-Vis spectroscopy does not feature prominently in forensic laboratories as an identification technique.
A major use is screening powders for the presence of illicit drugs. In this application UV-Vis is quite successful because the technique has a very low limit of detection, powders are usually simple mixtures, and illicit drugs give good signals.
UV-Vis is a very useful, if somewhat limited, quantification technique, again particularly for illicit drugs. Of all the spectroscopic techniques it is easiest with UV-Vis to make use of Beer’s law, which states that the absorbance of radiation at a given frequency is proportional to both the distance the radiation travels through a specimen and the concentration of the species that absorbs the radiation.
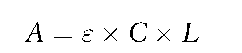
where A = absorbance; s = extinction coefficient; C = concentration of the absorbing species and L = path length of radiation through the specimen.
The constant of proportionality, s, in Beer’s law is called the extinction coefficient. It is a measure of how well a compound absorbs radiation at a given wavelength; it is as characteristic of that compound as other physical constants such as melting point, boiling point or molecular weight. The chemical literature abounds with extinction coefficients for many substances, including pure drugs and their salts in a variety of solvents at a variety of wavelengths. Many useful solvents such as water, methanol, ethanol, and acetonitrile are virtually transparent throughout the visible and ultraviolet spectrum. It is, therefore, very easy to prepare a solution of the illicit preparation to an accurate concentration in a solvent that does not interfere with the absorption spectrum. Using Beer’s Law, the absorbance of the specimen, the optical path length (usually 1 cm in standard sample cells), and the extinction coefficient of the drug, the concentration of drug present in the solution of the illicit preparation can be calculated. The attraction of such an analytical strategy is that it is very inexpensive, rapid, and simple. Furthermore, if the UV-Vis spectrometer has been properly calibrated using absor-bance and wavelength references, a pure reference drug standard is not absolutely necessary in order to quantify the drug because extinction coefficients are universal constants.
This facility for accurate, standardless quantification is unique to UV-Vis spectroscopy; chromato-graphic techniques (e.g. GLC, HPLC, CZE) require a reference substance in order to calibrate the response with respect to amount, and for other spectroscopic techniques (e.g. infrared spectroscopy) extinction coefficients are not available. UV-Vis spectroscopy is not generally applied to the quantitative assay of illicit drug preparations, however, because the technique in general has severe limitations for the analysis of uncharacterized mixtures. However, when a mixture is well characterized and there is some degree of separation between the spectral peaks arising from the compounds in the mixture, UV-Vis spectroscopy can be useful.
Another example is the estimation of carboxyhaemoglobin in human blood. The spectral characteristics (i.e. absorption maxima and extinction coefficients) for carboxyhaemoglobin and interfering blood pigments (e.g. oxyhaemoglobin, methaemoglobin) are well known. For a blood specimen of unknown car-boxyhaemoglobin saturation it is a simple matter to deconvolute mathematically the spectral contributions due to interfering blood pigments from the bands due to carboxyhaemoglobin.
A major application of visible spectroscopy is the objective color comparison of evidential material such as fibers or paint. The human eye and brain interpret the visible spectrum of light reflected from an object as its color. However, the human eye is a low-resolution transducer, it can be fooled into interpreting two closely related colors as identical. Figure 5 shows visible spectra acquired from two different turquoise wool fibers. Even though the spectra from the two fibers are noticeably different, the eye perceives the two fibers to be identical in color. In the modern forensic laboratory, therefore, visible spectroscopy is invaluable as a means of confirming whether two objects are of the same color, or refuting the observation.
Conventional UV-Vis spectrometers cannot acquire spectral data from microscopic samples of paint, nor from short pieces of single textile fibers. The instrument used is referred to as a visible micro-spectrometer, it functions in the same way as the infrared microscope described above. Unlike the infrared microscope, visible microspectrometers allow analysis and visualization of objects less that 10 um in size. This is possible because visible light suffers less diffraction than infrared radiation. Visible spectroscopy should be considered a color analysis technique, not a pigment analysis technique. This is because visible spectroscopy is not always capable of resolving different compounds (or different blends of compounds) that have the same color. Reliable pigment analysis is achieved only when visible spectroscopy is used in conjunction with other techniques. For example, thin layer chromatography (TLC) could be attempted on pigments extracted from fibers, or scanning electron microscopy-energy dispersive x-ray microanaly-sis could be used to characterize pigments in paint.
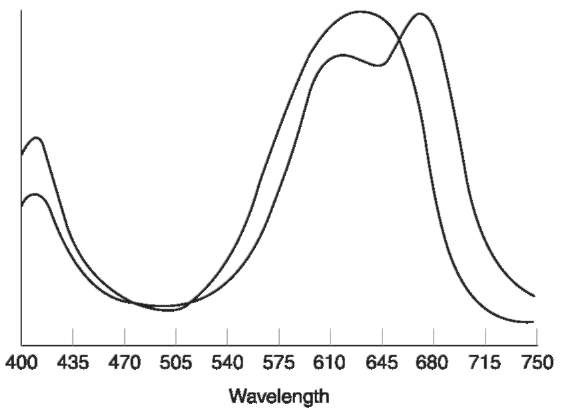
Figure 5 Visible microspectra of two different, metameric turquoise wool fibers. Although these fibers are of an identical color to the human eye, they are easily distinguished using visible microspectroscopy.
Pigments absorb radiation in the UV region as well as in the visible region, and paints designed for outdoor application can contain compounds that are active in the UV. Therefore, compared to visible spectroscopy, UV-Vis spectroscopy can be expected to offer enhanced discrimination. However, the standard optics present in visible microspectrometers are usually made of glass, and these strongly attenuate UV radiation. It is possible to purchase microspect-rometers equipped with quartz optics, which allow the spectrometer to cover the UV and visible range. When faced with the high cost of quartz optics however, forensic laboratories tend to rely on visible microspectroscopy and enhance pigment discrimination with a simple, inexpensive technique such as TLC.
Nuclear Magnetic Resonance Spectroscopy
In NMR spectroscopy the absorption of radiation (radio waves) by the specimen causes the nucleii of atoms in it to attain excited states of spin. The exact energy of the excited spin states depends very markedly on the molecular environment the atoms are in. Nuclei in different environments therefore absorb different frequencies within the radio wave region. There is such a sensitive correlation between environment and the signal associated with the nuclei that NMR is capable of yielding an enormous amount of information regarding molecular structure, and therefore unsurpassed specimen discrimination.
In a forensic context NMR finds its greatest application in the analysis of organic compounds. It can be described as a wide range technique, applicable to volatile, low molecular weight compounds as well as involatile, relatively high-molecular-weight substances. Substances of high polarity or low polarity can be analyzed, and it is usual to conduct the analysis at near to room temperature, therefore thermally labile substances can be analyzed. A few factors have, however, conspired to minimize the impact of NMR spectroscopy on forensic science. First, instrumentation is expensive to purchase and, in the case of instruments equipped with superconducting magnets, expensive to maintain. Second, analytical mixtures must be relatively simple as commercial instruments with interfaces to chromatographic techniques are not available. Finally, the technique has a relatively high limit of detection, and unfortunately that limit is inversely proportional to the cost of the instrument.
Analysis is usually conducted in one of two modes, proton mode which detects signals from the nuclei of hydrogen atoms, and carbon-13 mode which detects signals from natural abundance 13C nuclei. In carbon-13 mode it is usual to see a signal for every none-quivalent carbon atom within the specimen. Therefore, if the specimen is a pure sample heroin, for example, 21 distinct signals at characteristic frequencies are observed. This is an extremely high level of discrimination. In proton mode, the situation is analogous. Let us now consider the case where the specimen is an equimolar mixture of acetyl codeine, O6-acetylmorphine, and heroin. In this case the carbon-13 spectrum would contain 60 signals, some resolved, some not. If the task was a target analysis of the specimen for the presence of heroin, then it would be difficult but successful. If the task was identification of an unknown specimen, it would be very difficult indeed. Therefore one of the strengths of NMR, the provision of enormous structural detail, becomes a limitation in the case of complex mixtures. NMR is therefore not applicable to the identification of hydrocarbon fuels, for example. In polymers and oligomers many atoms have very similar environments, therefore many signals overlap. NMR therefore is not valuable for the forensic discrimination of paints or fibers.
NMR is not a technique that can be applied to trace analysis, for example detection of explosives in bombing debris, or drugs in body fluids.
NMR can be of great value for target analysis of illicit drugs because the analytes are usually present in high concentration in a simple mixture. Specimen preparation is very simple; the substance can be dissolved in a suitable solvent (heavy water, deutero-chloroform, deuterated methanol) or basic drugs can be extracted from alkaline aqueous solution using a suitable solvent (deuterochloroform, carbon tetrachloride). If the instrument is calibrated and an internal standard is included in the protocol, then the test can be quantitative as well as qualitative. The extremely high level of discrimination gives the test a very high probative value. Due to the nature of material encountered, NMR is also very useful in the identification of precursors and intermediates found in clandestine laboratories.
One of the greatest benefits of NMR spectroscopy is the predictive structural information that can, with skill, be elucidated from the spectrum of an unknown substance. From the carbon-13 spectrum the number of distinct carbon atoms within the unknown molecule can be deduced. By sophisticated pulse techniques these can be identified as methyl, methylene, methine, or quaternary residues, and by careful examination of the position of each peak, the presence of heteroatoms and carbon-carbon multiple bonds can be inferred. This information allows a good picture of the component bits and pieces of the unknown molecule. Proton NMR allows the connectivity of the moieties to be deduced, and a complete picture of the molecular structure built up. Using this technique it is possible to identify new designer drugs and unusual precursors or intermediates in clandestine laboratories.
X-ray Fluorescence
In this technique the specimen is irradiated with X-rays. This causes electrons associated with atoms in the specimen to attain an excited state of potential energy. From this excited state the electrons can relax, or release that potential energy, a process which results in the specimen emitting energy (fluorescence) in the form of new X-rays. The X-rays are emitted at energies that are characteristic of the atoms present in the specimen. Therefore the X-ray fluorescence (XRF) spectrum carries information as to the chemical elements present in the specimen.
XRF spectrometers can be manufactured for the analysis of microscopic specimens. Given that it is necessary in forensic science to have the capability for microanalysis this section will deal only with micro-XRF.
In this technique X-rays are delivered to the specimen via a light guide. It is possible to bring the beam into ‘focus’ in a spot about 10 um in diameter. The specimen is viewed under low magnification with the aid of a video camera, which allows control over the area illuminated by the X-ray beam and therefore the region in the specimen that is analyzed.
The X-ray beam even in a micro-XRF spectrometer penetrates a long way into the specimen and interacts with a relatively large volume of the specimen. Therefore, with thin, microscopic specimens, such as small flakes of glass or thin pieces of paint, most of the X-ray beam will pass right through and fluorescence will be minimal. If the specimen is constructed of thin laminates then special sample preparation, such as the preparation of cross-sections or subsamples dissected from the entire specimen, must be undertaken unless a composite spectrum of all the laminations is required.
Given the sampling and imaging idiosyncrasies of XRF, it is useful for the elemental analysis of relatively large, easy-to-find specimens such as thickly layered paints, big chips of glass, fibers, and pieces of automotive accessories. Compared to scanning electron microscopy (SEM)-X-ray microanalysis, XRF has slightly lower limits of detection for elements in a homogeneous specimen, therefore there is the potential for a slightly higher level of discrimination in the analysis of metal alloys and glass for example. Compared to techniques such as atomic absorption spectroscopy, however, detection limits are relatively high.
Many specimens in forensic science are quite heterogeneous on the microscopic scale and useful information might arise from probing the microstructure. This facility is not especially well catered for by XRF due to the low contrast images produced by the video system, the limited range of specimen magnification achievable, and the large volume of specimen that is excited by the probe beam. Illustrations of the limitations of the technique are provided by paint comparison and firearm primer analysis.
In paint comparison, analysis of a relatively large volume of specimen using XRF indicates the elements present down to the limit of detection for the technique. Let us assume that XRF indicates the presence of Ca, S and Ba. The technique does not allow us to discriminate between paints that contain calcium sulphate and barium sulfate, or barium sulfate and calcium carbonate, or a mixture of calcium sulfate, calcium carbonate and barium carbonate. In order to resolve this dilemma what is needed is the ability to distinguish individual particles within the paint film and analyze them. However, with the video imaging system associated with XRF it is difficult to discern individual filler particles unless they are very coarse grained, and the relatively large volume excited by the probe beam means it can be difficult to restrict excitation to individual particles.
With firearm primer particles on a tape-lift the analyte is composed of minute particles (usually less than 10 um) scattered thinly over a relatively large area. It is not possible with XRF to enhance specimen image contrast (i.e. by techniques such as backscat-tered electron imaging as used in SEM-X-ray micro-analysis) or achieve high magnification, therefore it is a very difficult task to locate analyte particles. Furthermore, the particles are likely to be much smaller than the probe beam, and in any event the volume of specimen excited by the beam is relatively large, therefore useful fluorescent yield will be low, and contribution from the mounting medium could be large.
Compared to micro-XRF, analysis of heterogeneous specimens is likely to be more efficient using SEM-X-ray microanalysis (see below).
The application of XRF to the analysis of explosives residues further illustrates the versatility of the technique, but also a shortcoming that is common to any X-ray microanalytical technique. For example, XRF can be used to identify particles containing chlorine arising from chlorate, perchlorate or hypochlorite-containing improvised explosive devices. Unfortunately it is not possible to use XRF to distinguish these oxyanions from each other, nor from chloride, which is not characteristic of explosives residues. Similarly, in cases of black powder explosions, XRF can be used to identify the presence of potassium and sulfur, but it is not possible to prove whether the sulfur is present as one of its oxyanions, or as elemental sulfur, or sulfide.
Molecular Fluorescence
As described above, when molecules absorb visible or ultraviolet radiation they are elevated to an excited state of high potential energy. From that state the molecule can undergo a variety of actions in order to relax. One activity is to emit radiation of lower energy than that which caused the excitation in the first place. The emitted radiation (fluorescence) consists of many frequencies of characteristic intensity. Like the UV-Vis spectrum, the emission spectrum (i.e. the plot of emitted radiation intensity versus wavelength) of a substance can be a means of char-aterizing that substance.
Fluorescence has three important applications in forensic science. Perhaps the most widespread use is not in relation to spectroscopy at all, but image or specimen enhancement in the fields of ink comparison, latent fingerprint enhancement, and classification of textile fibers. Another use is in relation to chromatographic detection associated with sepa-Dration techniques, such as high performance liquid chromatography and capillary electrophoresis, where extremely low limits of detection can be realized. Finally, molecular fluorescence can be used as a spectroscopic technique (spectrofluorimetry).
Although spectrofluorimetry is related to UV-VIS spectroscopy, there are some significant differences. One distinguishing property of fluorescence, a feature that is both its biggest asset and its biggest drawback, is that not all molecules exhibit this behavior. In general terms, only aromatic compounds or highly conjugated systems are fluorescent, particularly if oxygen or nitrogen atoms are attached. The presence of electron-withdrawing groups (e.g. halogen atoms, nitro groups) diminishes or even completely quenches fluorescence in these substances. On the positive side therefore, techniques based on fluorescence can have very low limits of detection because spurious background signals are not likely to arise. As the excitation radiation is of a shorter wavelength that the fluorescence, it is possible to enhance detection further by filtering out interference from the exciting radiation. Spectrofluorimetry has a potential for high discrimination due to the low number of fluorescent compounds. Furthermore, the exact nature of the emission spectrum is linked to the nature of the excitation, and this has a positive effect on discrimination. For example, if two compounds have similar emission spectra, there is the potential for further discrimination if different excitation is required to produce that emission. By means of sophisticated instrumentation (a description of which is beyond the scope of this article) it is possible to scan the excitation radiation in a stepwise or continuous fashion and record emission data.
On the negative side, techniques based on fluorescence will have a very limited applicability because not all compounds fluoresce. As petroleum-based greases, oils, and concentrated fuel residues contain aromatic compounds (in particular polynuclear aromatic compounds) spectrofluorimetry can be used as a means to characterize them. Some drugs (e.g. LSD, phenothiazine, quinine and quinidine) are fluorescent, and therefore amenable to qualitative and quantitative analysis using spectrofluorimetry. Some metal atoms can also fluoresce, this finds some application in glass analysis.
Raman Spectroscopy
In terms of analytical results, but not mechanism, Raman spectroscopy very closely resembles infrared spectroscopy. The Raman signal arises through scattering of a probe beam of (usually) visible light. Scattering is a phenomenon whereby incident radiation interacts with molecules in the specimen, thereby raising them to an excited state. From this excited state molecules relax and emit radiation. During this process some molecules might retain some energy, therefore the scattered radiation will be of lower energy than the probe beam, or some molecules might release more energy than they received, in which case the scattered radiation is of higher energy than the probe beam. The energy kept by the specimen results in excitation of its vibrational states, whereas energy released from the specimen comes from relaxation of its vibrational states. The scattered radiation therefore contains information relating to the vibrational states of the molecules in the specimen. The spectrometer usually detects only the scattered radiation of lower energy and generates a plot of frequency of scattered radiation versus intensity. This plot is the Raman spectrum, and the peaks present are due to vibrational excitation; it is for this reason that Raman spectroscopy is referred to as the complement of infrared spectroscopy.
As the probe beam comes from a laser operating close to the visible region it is not absorbed strongly by water or glass, as is the case with infrared radiation. Therefore, one advantage of Raman over infrared spectroscopy is that specimens can easily be analyzed as solutions in water or as films on a glass microscope slide. In infrared spectroscopy specimens must be supported on halide crystals, which are soluble in water and easily ruined, or on insoluble but expensive crystals such as diamond, zinc selenide etc.
As an analytical technique Raman spectroscopy has a lot to offer the forensic scientist, but it is not widely used. Although the discrimination of the technique is as good as infrared spectroscopy, it does have some drawbacks compared to infrared. First, infrared spec-troscopy is a well-established, mainstream technique requiring relatively inexpensive instrumentation. Second, arising from the foregoing, there is a wealth of infrared spectroscopic data compiled for many substances; e.g. fibers, drugs, minerals, paint etc. Third, the incident radiation can induce fluorescence, which can swamp the weak Raman signal. For these reasons Raman spectroscopy might best be described as a technique of emerging importance for forensic science. One area in which Raman spectroscopy promises strong potential, compared to infrared spectro-scopy, is microspectroscopy. As visible light is used as the probe beam in Raman microspectroscopy, there is less beam diffraction. Therefore much smaller specimens can be analyzed using Raman microspectroscopy (~1 um as opposed to -10 um) and spatial resolution is much better. This means that if two different substances are present side by side, such as adjacent layers of paint or the two components of a bi-component fiber, it is much easier to achieve a spectrum of one of the substances free of spectral interference from the other. As Raman spectroscopy is not a transmission technique, there is no requirement to make the specimen transmit radiation, as is the case in infrared microspectroscopy, therefore specimen preparation is very simple. Currently Raman microspectrometers operate with the probe beam impinging on the surface of the specimen, therefore the technique can overrepresent the surface chemistry of the specimen. Future developments in confocal Raman microspectroscopy could allow accurate control over the probe beam so it can be brought into focus in a very small volume within the specimen, not just on its surface. This will allow evaluation of the entire specimen, and might be a useful way of avoiding excess fluorescence arising from surface contamination, textile brighteners, etc.
Atomic Spectroscopy: Atomic Emission Spectroscopy, Atomic Absorption Spectroscopy and Related Techniques
In atomic spectroscopy atoms within the specimen are elevated to an excited state by extreme heat as provided by a flame or a plasma torch. From this excited state atoms can emit radiation (atomic emission spectroscopy), or absorb radiation from a probe beam (atomic absorption spectroscopy (AAS)). The spectrometer detects absorbed or emitted radiation that is characteristic of the elements present in the specimen. In a related technique, excited atoms produced by a plasma torch are passed on to a mass spectrometer which differentiates the elements present on the basis of their atomic weight. This technique is called inductively coupled plasma-mass spectrometry (ICP-MS), and strictly speaking it is not a spectroscopic technique. As it is closely related to the other techniques described in this subsection it will be discussed along with them.
Atomic spectroscopy finds some application in forensic science. In classical experiments, the analyte is presented to the instrument as a solution in a relatively large volume of solvent. As a means of solution analysis atomic spectroscopy offers the forensic scientist unsurpassed limits of detection, accuracy and precision for elemental analysis. This does not imply, however, that it provides the most effective means by which a forensic scientist can conduct elemental analysis. First, classical atomic spectroscopy is destructive, the sample presented for analysis is usually treated with a very strong acid to form a solution, and then irreversibly aspirated into the instrument. Second, because the sample is homogenized by dissolution, atomic spectroscopy cannot yield any information as to spatial distribution, or compounds present in the specimen. For example, a sample of paint might be found to contain Ca and S. Although this strongly suggests that the paint contains calcium sulfate, one cannot rule out the possibility that calcium carbonate and a sulfur-containing compound are present, or that the paint might contain granules of calcium carbonate and calcium sulfate. Third, any contaminant associated with the specimen will be digested along with it, and will contribute to the results. Fourth, although atomic spectroscopic techniques do have very low limits of detection, they are often not low enough to detect trace elements in trace evidence. This is because the specimen must be made into a solution of relatively large volume (usually 0.55 ml). As a consequence trace elements in, for example, small chips of glass or paint yield very dilute solutions. Finally, some techniques, such as flame atomic absorption spectroscopy, only allow sequential analysis of target elements; one analytical test provides data with respect to only one element. As it is not possible to screen a specimen for many elements in the one test the analysis is not particularly efficient, especially with regard to specimen consumption.
Paradoxically, given the very low limits of detection for these techniques, they are of greatest use in the analysis of a relatively large specimens, and given that the technique is destructive, specimens must be big enough to allow subsampling. Such specimens could be human tissue for toxicological analysis, and milligram-size pieces of glass, paint and metals.
Another strong application of atomic spectroscopy is the analysis of illicit drug powder samples. The low limits of detection that can be achieved allow many trace elements to be detected in heroin, for example. It is possible to identify the source country of the drug on the basis of the suite of elements it contains.
Some of the major shortcomings of ICP analysis can be rectified by the use of a laser ablation source. In this technique a laser beam is use to vaporize very small quantities of the specimen which are then swept into the instrument. It is possible to allow the laser beam to dwell on the specimen for some time before analysis, thereby effectively removing any surface contamination. As the laser beam can be focused to a small spot size, it is possible to sample and analyze discrete regions within the specimen. This allows some identification of the spatial distribution of compounds within the specimen. Finally, the laser ablates only a tiny amount of material, therefore the specimen is left intact for further analysis.
Spectroscopy-related Techniques
Scanning electron microscopy-X-ray microanalysis (SEM-EDX)
Scanning electron microscopy-X-ray microanalysis strictly speaking is not a spectroscopic technique, however, with respect to operation and application it is very similar to a spectroscopic technique.
The basis of the instrument is an electron microscope, which in order to generate an image of the specimen, bombards it with a beam of electrons. Many phenomena originate from the collision between electrons and the atoms in the specimen, but the most important as far as this article is concerned are scattering of the electron beam, and emission of X-rays. X-rays are emitted when the atoms within the specimen capture energy from the electron beam and attain an excited state. From this state the atoms can relax and emit X-rays. The key feature of the technique is that X-ray emission is not random; different atomic elements within the specimen emit rays with characteristic energy. Therefore the X-ray emission contains information as to the atoms present within the specimen, in this regard the technique resembles XRF.
In order to produce a spectrum from the emitted X-rays they are processed in one of two ways. One way is to pass the X-rays through a special crystal that acts like a prism and disperses the radiation in space. This technique is referred to as wavelength dispersive spectroscopy (WDS). Another technique involves collecting the X-ray emissions from the specimen and sorting them on the basis of their energy. The intensity of X-ray emission is then plotted as a function of energy; this approach is called energy dispersive X-ray microanalysis (EDX).
Although wavelength dispersive X-ray spectrometers offer superior performance in terms of resolution and limit of detection, EDX spectrometers are more common in forensic laboratories due to their relatively low cost. Here, only EDX is referred to, although the comments made are equally applicable to WDS.
Combination of EDX with scanning electron microscopy makes a versatile and powerful technique for forensic science. The electron microscope can achieve a wide range of magnifications simply by causing the electron beam to impinge on either a very small area (high magnification) or relatively large area (low magnification) of the specimen. Therefore X-rays can be generated from either a very small area (< 1 um diameter) or a relatively large area, the choice being controlled readily by the analyst depending on the analytical task at hand. In analytical range SEM-EDX is superior to XRF. The electron beam does not penetrate very far into the specimen, it is therefore possible to examine thin objects without receiving a signal from the mounting substrate, or examine individual layers of laminated materials such as paints without receiving a spectral contribution from lower layers. Therefore, with respect to spatial resolution of the specimen, SEM-EDX is superior to XRF.
A criticism often unfairly leveled at SEM-EDX is that the technique has a high limit of detection. If the specimen is a homogeneous mixture, then the limit of detection for any one particular element is of the order of a few tens of parts per million. Many atomic spectroscopic techniques, such as those based on ICP or AAS, or even XRF, have much better limits of detection than this. However, SEM-EDX still finds widespread application in forensic science simply because specimens are usually not homogeneous. Consider a tape lift a few square centimeters in size containing ten firearm cartridge primer particles each about 10 um in size. Each would weigh approximately a few nanograms, so the specimen would contain about 50 ng of analyte, say lead, barium and antimony, in total. If SEM-EDX was used at low magnification to analyze the entire tape lift it is very unlikely that any of the analyte would be detected, the particles are just too widely distributed over the tape. However, the actual particles contain very high percentage concentrations of the heavy metals, each element well above the detection limit for SEM-EDX. If the particles can be found using the microscope, and the electron beam made to dwell only on the individual particles by adjustment of the magnification, then each element present will be readily detected. Fortunately a phenomenon associated with electron microscopy makes it easy under certain circumstances to locate particles such as those from cartridge primers. Not only does electron impact cause the specimen to emit X-rays, it also results in an output of electrons with various characteristics. Some of these secondary electrons are a result of inelastic collisions between the electron beam and atomic electrons in the specimen; the electron microscope captures secondary electrons from the specimen in order to produce the standard magnified image that we associate with SEM. In addition, some electrons from the beam collide with nuclei of atoms in the specimen and are bounced straight back out of the specimen with little loss of energy. These backscattered electrons can also be detected and used to create a magnified image of the specimen. Heavy nuclei within the specimen are more likely to produce backscattered electrons, therefore bright areas in the backscattered image correspond to areas rich in heavy elements. With appropriate signal processing the backscattered electron image can be tuned to show only elements of high atomic weight. In the case of the tape lift described above, the back-scattered image can be tuned to reveal heavy elements such as lead, while light elements remain invisible.
Under these circumstances searching for primer particles is simple, especially if automation is used. Once particles are found by their bright backscatter signal, they can be analyzed readily. In principle, SEM-EDX could be used to analyze a minute particle in an enormous matrix so long as there is enough contrast between the particle and the matrix to enable it to be found. Under these circumstances SEM-EDX is capable of extremely low limits of detection.
In order to analyze the tape-lift using atomic spectroscopy, it must be digested in solvent, say 1 ml of acid. Using this approach the technique must be able to detect 50ngml_1 (50 parts per trillion) for it to be successful. It is unlikely that such limits of detection could be practically achieved for routine work. Furthermore, if results could be obtained using atomic spectroscopy, then interpretation would be difficult. Let us assume that the results indicate low levels of lead, barium and antimony. Although this could mean that the specimen contains mixed lead, barium and antimony particles characteristic of firearms primer, it could also indicate the presence of a mixture of pure lead particles, pure antimony particles, and pure barium particles, a situation which does not point to the presence of gunshot residue particles.
It is possible to analyze the tape lift for primer particles using XRF, but it is tedious and not efficient.
First the technique does not have an equivalent to a backscatter image, therefore tiny primer particles are very hard to see, and the specimen must be searched by eye manually. Second, if a particle is found it will likely occupy only a small percentage of the volume of interaction of the X-ray beam, therefore analytical results will reflect the composition of the surroundings more than the particle itself.
The tape lift described above is an example of a specimen exhibiting extreme heterogeneity. Other important evidentiary materials are likely to be more homogeneous, but nevertheless SEM-EDX can still be valuable. A good example is paint comparison. Under low magnification a sample of paint appears to be quite homogeneous. SEM-EDX at this magnification gives results analogous to those obtained by techniques such as atomic spectroscopy or XRF, that is, a breakdown of all the elements contained within the specimen down to the limit of detection. SEM-EDX can yield further information that cannot be obtained using the other techniques, however. Consider the sample of paint discussed above in relation to XRF; the presence of Ca, S and Ba are detected. Analysis on the gross scale does not allow differentiation between paints filled with calcium sulfate and barium sulfate, or barium sulfate and calcium carbonate, or a mixture of those three compounds. However, with SEM all that is required is to zoom the magnification until individual particles are visible, and then analyze those particles individually. If particle analysis indicates Ca and S to be present in the same particle, then it can be safely concluded that calcium sulfate is present, particles containing only Ca indicate calcium carbonate, particles containing Ba and S indicate barium sulfate. In order to facilitate this process further the backscatter signal can be tuned to highlight particles containing Ba.
Similar to the case described for XRF, SEM-EDX suffers from a lack of specificity with respect to oxidation state of complex anions. For example the technique cannot distinguish between chloride, chlorate, perchlorate or hypochlorite, nor between sulfur, sulfide, sulfate, or thiosulfate.
Although SEM-EDX has a few shortcomings, it is a very versatile and efficient technique for elemental analysis. Although it suffers from an apparently high limit of detection, the ability to analyze submicro-metre particles or specimen areas compensates to a great extent. If a particle or unusual region in the specimen can be found using the imaging options, then it can be analyzed, giving the technique an extremely low working limit of detection. Furthermore, the instrument allows imaging of specimens at high or low magnification with very high depth of focus. This facility can provide useful morphological information relating to trace evidence, such as fiber cross-section and surface morphology. Finally, an important consideration is that often the specimen needs little or no treatment before analysis, and the technique is not destructive.
Mass spectrometry
A characteristic of substances is the atomic weight or molecular weight of its constituent atoms or molecules. Atoms or molecules are too small to be weighed directly by conventional equipment such as a micro-balance, but their weight can be measured indirectly using a device called a mass spectrometer. In forensic science, mass spectrometry is mainly used for molecular analysis, therefore this section will deal only with this topic.
Before mass measurement, molecules within the specimen are raised to a charged state of high potential energy. The classical approach to this is to bombard the specimen with a beam of electrons; this technique is referred to as electron impact ionization. The excitation might produce a state of such high potential energy that molecules within the specimen break up into fragments. This outcome sounds counterproductive, but in reality it can yield useful information. Fortunately, fragmentation is not a random process, it occurs at the weakest bonds within compounds, or at locations where stable fragments are produced.
Figure 6 shows the mass spectrum produced by a sample of cocaine.
The peaks in the spectrum indicate fragments detected; the horizontal axis indicates the weight of the fragment detected, and the vertical axis indicates the abundance of those fragments detected. Weight displayed is a relative measure. Mass spectrometers are calibrated using the assumption that carbon atoms have a weight of 12. On this scale hydrogen atoms have a weight of 1, oxygen 16, nitrogen 14, sulfur 32 etc. In reference to Fig. 6, the peak at 303 is due to intact cocaine molecules that have not fragmented and therefore contain 17carbon atoms, 21 hydrogen atoms, four oxygen atoms and one nitrogen atom (303 = 12×17+1×21+16×4+14); this peak is referred to as the molecular ion. Other peaks relate to fragments derived from the molecule, for example the peak at 182 is due to a fragment containing 10 carbon atoms, 16 hydrogen atoms, two oxygen atoms and one nitrogen atom (182 = 12 x 10+ 1 x 16 + 16 x 2 + 14).
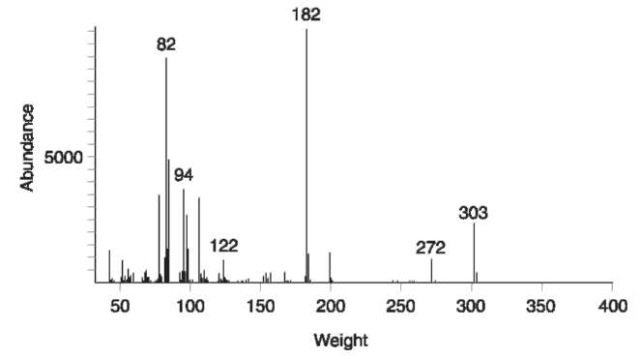
Figure 6 Mass spectrum for cocaine.
The fragmentation pattern produced by a given molecule is distinctive, and in some instances unique, it is therefore a good means of identifying compounds present in a substance. However, some collections of atoms very strongly direct fragmentation, with the result that various molecules that contain those groups produce fragmentation patterns that resemble each other. For example, the —CH2—NH2 group in primary amines very strongly directs fragmentation, with the result that, as a group, the amphetamines all produce mass spectra with strong peaks at 44, and few other fragments. With respect to compounds of forensic interest, mass spectrometry can fail to produce good discrimination, within a class, for substances such as aliphatic or aromatic hydrocarbons (as found in accelerants), nitrate esters (explosives), as well as amphetamine analogues and methyl-amphetamine analogues.
The cause of this lack of specificity is that electron impact ionization raises molecules to a state of very high potential energy. It is possible to use special excitation techniques that do not encourage fragmentation, such as chemical ionization, fast atom bombardment, laser desorption or electrospray ionization. Instead of peaks arising from fragments of molecules, mass spectra acquired using these techniques give a peak corresponding to the molecular weight of the compound. Using the example of amphetamine, the chemical ionization spectrum of this substance shows a strong peak at 135 instead of a strong peak at 44 as obtained using electron impact ionization. Therefore, although the chemical ionization spectrum is still very simple, in this example it is more discriminating than the electron impact spectrum.
Mass spectrometry is, therefore, a very versatile technique. The fragmentation pattern generated by electron impact in most instances allows good discrimination between compounds. In those instances when fragmentation occurs too readily in one direction, other ionization techniques can be brought into action.
The main limitation of mass spectrometry is that if the specimen is a mixture of compounds, then the mass spectrum acquired will be also be a mixture. Unless the task is target analysis, this can make interpretation of data and detection of trace level components very difficult. This shortcoming can be overcome by using a technique known as mass spectrometry-mass spectrometry (a discussion of which is beyond the scope of this chapter), or by combining mass spectrometry with a chromatographic technique such as gas chromatography, liquid chromatography or capillary electrophoresis.
