Many types of evidence (drugs, paint, explosive residue, fire debris, soil, biological fluids, etc.) encountered in the forensic science laboratory consist of complex mixtures of substances. The complexity of these materials is a double-edged sword to the forensic scientist. The more complex and variable a mixture, the greater its probative value when comparing known and questioned samples. Complex mixtures also create analytical problems, as most compounds need to be relatively pure in order to be identified by analytical techniques, such as spectroscopy. It is the ability of the forensic chemist to separate that allows him/her to identify or compare a material. Separation techniques discussed in this article can be classified into four groups: physical, chemical, chromato-graphic and electrochemical.
Physical Separations
Physical separations are commonly used in forensic chemistry. Examination of a mixture under a stereoscope may yield variation of its component particles. These particles may vary in their shape, size, color, opacity, texture or other physical properties, which can be observed microscopically. The particles may then be physically separated from the mixture.
Particle selection or ‘picking’is the major separation technique used in most areas of trace evidence. A skilled examiner can recognize and pick out critical particles in explosive residue. Paint examiners can select uncontaminated particles out of a mixed smear of paint for examination and comparison. This type of separation can also be utilized in mixtures containing cocaine hydrochloride. This salt form of cocaine has a translucent shale-like appearance, which is easily distinguished from the coarser grains of most diluents and excipients used as cutting agents in cocaine mixtures.
Forensic geologists have long used particle size to separate soil components for analysis. A novel example of this is the use of a mesh screen for separation of sand grains onto an SEM stub for automated elemental analysis and mineral identification (Fig. 1). Manual separation of particles should always be explored prior to attempting a chemical separation.
Volatile Materials
Volatile materials are often present in drug and fire debris cases submitted to forensic laboratories. These materials (primarily solvents and flammables) may be easily separated from relatively nonvolatile substances by techniques such as distillation, sublimation, headspace analysis, absorption-elution and solid-phase microextraction.
Distillation and sublimation are techniques occasionally used in the analysis of drugs. Amines such as amphetamine and methamphetamine are relatively volatile and may be flash distilled to separate the drug from a complex mixture. Dimethylsulfone is currently one of the most common cutting agents used with methamphetamine in the United States. It is far more volatile than methamphetamine, sublimating at 90-100°F (35-38°C). It can be easily removed from methamphetamine by placing the mixture on a watch glass and sublimating the dimethylsulfone over a steam bath. The purified methamphetamine can then be analyzed via infrared spectrometry.
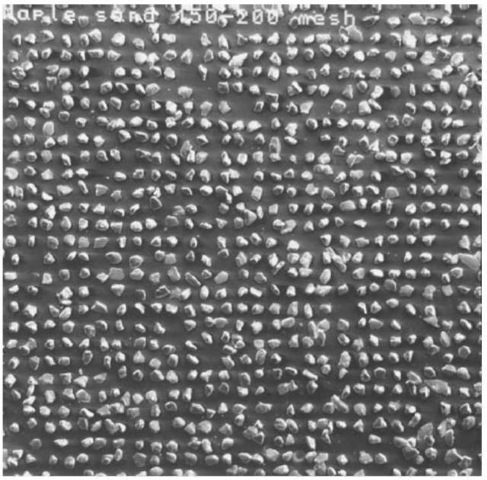
Figure 1 Photograph of sand particles separated using a mesh screen and mounted on a SEM stub.
Examination of fire debris for the presence of ignitable liquids (gasoline, fuel oil, etc.) requires that the residual amounts of these liquids be separated from the debris. Samples of debris are sealed into airtight nylon bags or paint cans and heated to volatilize the ignitable liquid into the headspace of the container. The volatile in the headspace can then be analyzed directly by removing a portion with a syringe. Often the concentration of the volatile in the headspace is insufficient for direct analysis. In these cases, a process known as absorption-elution may be used to concentrate the volatile. In this process, the volatiles are absorbed by a material (usually activated charcoal) and then extracted from the absorbent in a concentrated form by a solvent. The process of absorption can be active or passive. In active or dynamic absorption, the material containing the volatile is heated and the vapor forced through a vessel containing the absorbent. A typical setup may consist of the charcoal housed inside a disposable pipette or hypodermic needle connected to a vacuum pump. The open end of the vessel is placed in the headspace of the sample and the air containing the ignitable liquids is drawn through the vessel. The charcoal would then be removed for solvent extraction of the captured volatiles (elution). Passive absorption entails placing the absorbent material directly inside the airtight container while heating to volatilize the ignitable liquids. Vaporized molecules of the volatiles contact the absorbent in a random fashion. Because the volatiles are not forced to contact the absorbent, the time of exposure of the absorbent to the vapor must be dramatically increased in order to ensure proper absorption.
Solid-phase microextraction (SPME) is a relatively new technique that has been used increasingly in forensic work. In this technique, a special syringe containing a spring-loaded fiber coated with a bonded phase is used. The fiber in the syringe can be contracted and retracted. The sample containing the volatiles is sealed in an airtight container. The syringe is then inserted into the headspace of the container and heated for a period of time. The volatiles are adsorbed onto the fiber, which is then retracted into the syringe. The syringe is then used for injection in a gas chromatograph. The fiber is contracted from the syringe into the injector port and the heat of the injector port elutes the volatiles from the fiber (Fig. 2). SPME may also be used to concentrate analytes from a liquid solution onto the SPME fiber. This technique is increasingly being used in the areas of solvent, drug, fire debris and high explosives analysis.
Chemical Separations Solvent extraction
Although chemical separations have increasingly become chromatographic in nature, solvent extraction is still frequently used in the forensic laboratory, especially in drug analysis, where chemical separations are generally followed by confirmatory qualitative techniques such as infrared spectrometry. Solvent extractions can be either single-phase or multiphase in nature. The theory and process of both types of solvent extractions are based on many criteria, the most important of which is solubility.
In a single-phase extraction, components of a mixture are separated based on the solubility of the components in a solvent. In this type of extraction, solvent selection is critical. The target component of the mixture must either be soluble in the solvent, while all remaining components are insoluble or vice-versa. The solvent is added to the mixture and then filtered to separate undissolved solute and solvent. If the target compound is soluble in the solvent it is then recovered by evaporation of the solvent. If the target compound is insoluble in the solvent and the other components are soluble, the filter paper is then dried and the material recovered. This process is also known as ‘solvent washing’. In complex mixtures, it is usually not possible to find a suitable single solvent to separate all components of the mixture. If this is the case, several solvents are selected and applied in a serial manner to effect the separation. The selection of solvent generally proceeds from the solvents in which few compounds are soluble (hexane, ether) to those in which a moderate number are soluble (acetone, methylene chloride, chloroform) to those in which most substances are soluble (alcohols, water). Use of acidified or basified solvents (ANOR) may increase the specificity of the extraction.

Figure 2 Photograph of a SPME fiber inserted into headspace of a sampling vial.
In multiphase or liquid-liquid extractions, components of a mixture are separated based primarily on their solubility in two immiscible solvents. This technique is also sometimes referred to as partition chromatography. The mixture to be separated is added to a solvent (generally aqueous) and a second solvent (usually organic) is then added. The components of the mixture then partition into the solvent or solvents in which they are most soluble. This type of extraction is extremely flexible, as solvent components can be selected, mixed, their pH changed, heated or cooled, used in vastly different proportions, etc. to effect the desired separation. The most commonly modified variable in multiphase extractions is the pH of the aqueous solvent phase, which is varied by addition of strong acids or bases. The solubility of components dissolved in this phase is directly affected by these pH changes. By adjusting the pH of the aqueous phase to make a particular analyte relatively insoluble in the aqueous phase, the stage is set for the analyte to be extracted into the second solvent, which is then added. Drugs in particular are amenable to separations based on changes of pH in multiphase extractions, lending to a terminology based on their behavior on extraction. ‘Acidic drugs’ are relatively insoluble in acidic solutions and may generally be extracted from acidic aqueous solutions into an organic solvent. ‘Basic drugs’ are relatively insoluble in basic solutions and may generally be extracted from basic aqueous solutions into an organic solvent. ‘Neutral drugs’ are generally unaffected by changes in pH and they will usually stay in whichever phase they are more soluble. An example of such an extraction is the separation of a mixture of codeine, butalbital and acetaminophen. Butalbital is an acidic drug; codeine and acetaminophen are basic drugs. The drug mixture is added to some acidic water, mixed and methylene chloride added. The butalbital will partition into the organic phase, which can then be drawn off and evaporated to recover the drug. The codeine and acetaminophen remain in the aqueous phase, which is then made basic with the addition of a strong base,such as NaOH. Additional methylene chloride is then added. Although acetaminophen is a basic drug, it is mostly insoluble in methylene chloride; therefore, most will remain in the aqueous phase. The organic phase containing the codeine and a small amount of acetaminophen is drawn off and additional basic water added. The remaining acetaminophen is partitioned into the aqueous phase, in which it is more soluble, and the organic phase can then be drawn off and evaporated to recover the codeine.
Purification via chemical reaction
Another chemical technique used in forensic work is purification by salt formation or derivatization. Both techniques involve the addition of a chemical to react with the target component to form a complex, which has properties allowing it to be separated from the rest of the mixture. An example of this is the separation of inorganic acids from a solution. These types of liquids are often encountered in poisoning cases (e.g. a suspect put an acidic solution in a victim’s drink) or in clandestine laboratories where acids are utilized in the production of illicit drugs. Knowing that an acid plus a base yields a salt plus water, concentrated ammonium hydroxide is added to the acidic solution, which forms an ammonium salt with the acid. As these salts are insoluble in acetone the addition of this solvent precipitates the ammonium salt from solution. Infrared analysis of the ammonium salt allows the analyst to determine the composition of the acid originally in solution. The production of ammonium chloride indicates that hydrochloric acid was present, ammonium sulfate indicates that sulfuric acid was present, etc.
There are hundreds of chemicals used to derivatize compounds, with the added benefit that the complex formed by derivatization is generally more stable than the underivatized compound. An example of this additional stability is the silylation of psilocybin with BSTFA (N, 0-bis-(trimethylcilyl)-trifluoroaceta-mide). Psilocybin (the active drug present in psilo-cybin mushrooms) undergoes thermal decomposition on heating due to the loss of the dihydrogen phosphate ester group; therefore, due to the heat of the injector, gas chromatography-mass spectrometric (GC-MS) analysis produces chromatograms and spectra that are identical with psilocin. Silylation of the psilocybin prior to GC-MS analysis ‘locks in’ the phosphate group, eliminating its thermal instability on injection.
Separation of enantiomers
Enantiomers (isomers that are mirror images of each other) are traditionally difficult to separate. Enantiomers have identical melting points, boiling points,density, dissociation strengths, reaction rates, solubilities, etc. The only variable in which they can be distinguished is the direction of their refraction of plane polarized light (optical activity). Products formed by the reaction of enantiomers with optically inactive reagents are identical, as are the rates of reaction. The reaction rate of enantiomers with reagents that are themselves optically active are not identical and may be so different that one isomer does not react at all. Derivatization of enantiomers with optically active reagents may lead to their separation and their isomeric determination. This is usually accomplished by a chromatographic method following derivatiza-tion. Reaction with an optically inactive derivatizing reagent may also yield information about the optical activity of a compound, albeit indirectly. An example of this is in the determination of the optical isomer of methamphetamine. Under US Federal sentencing guidelines, D-methamphetamine (+ rotation) and D,L-methamphetamine (racemic) carry tenfold the penalty as L-methamphetamine (— rotation), necessitating the determination of the optical isomer. The methamphe-tamine can be derivatized with phenylisothiocyanate (PIT). The resultant derivative precipitates with the racemic mixture but not with the single enantiomers; therefore, if the reaction results in precipitation of the derivative, a racemic derivative of D,L-methampheta-mine-PIT forms and its chemical structure is confirmed via infrared spectrometry. If no precipitation occurs, standard L-methamphetamine can be added to the PIT-sample complex and if precipitation occurs after the addition, it can be concluded that D-metham-phetamine was initially present. If no precipitation occurs after addition of D-methamphetamine, the entire process must be repeated with the addition of standard D-methamphetamine instead of L-metham-phetamine. If precipitation occurs upon the addition of D-methamphetamine to the PIT-sample complex, it can be concluded that L-methamphetamine was initially present.
Microchemical tests
Although superseded in many disciplines by instrumental methods, microchemical tests are an excellent example of the use of chemical reactions for the separation and identification of some compounds. Typically microchemical tests are performed by mixing a small drop containing the substance to be tested with a small drop of reagent. After a few seconds to minutes, characteristic crystals of the resulting compound can be observed and identified via polarized light microscopy. An excellent reference for micro-chemical tests is Chamot and Mason’s Handbook of Chemical Microscopy, Vol. II, where scores of microcrystal tests are described and can be used to separate and identify components of mixtures. The quickness, specificity and sensitivity of microchemical tests make them ideal for forensic scientists testing low explosive residue. Using a scheme of microchemical tests to determine the anions and cations present enables the analyst to identify the inorganic oxidizers present in the residue. Some of the more useful tests are summarized in Table 1.
Chromatographic techniques
Chromatography involves the separation of compounds based on their distribution between a stationary phase and a mobile phase. There are hundreds of chromatographic techniques utilizing various mobile phases, stationary phases and processes of interaction between the two. In all chromatographic techniques, substances are separated based on their affinity for the stationary phase during the interaction with the mobile phase. Adsorption to the stationary phase is usually caused by the attraction between a polar group on the stationary phase and a group of opposite polarity on the sample component. The separated compounds are then processed to visualize the separation, either chemically or more commonly via the use of a detector, which gives an electrical signal that can be graphically displayed (a ‘chromatogram’). Often chromatographic methods are used in combination with spectrometric detection to allow the identification of the compounds as they are separated.
Table 1 Common microchemical tests for inorganic ions
| Ion | Reagents |
| Aluminum | Ammonium fluoride, ammonium |
| molybdate, cesium sulfate | |
| Ammonium | Chloroplatinic acid, iodic acid, uranyl |
| acetate | |
| Arsenic | Ammonium molybdate, cesium chloride, |
| magnesium acetate | |
| Barium | Ammonium bichromate, potassium |
| ferrocyanide, squaric acid | |
| Beryllium | Chloroplatinic acid, potassium oxalate |
| Calcium | Ammonium carbonate, potassium |
| ferrocyanide, sulfuric acid | |
| Carbonate | Silver nitrate, calcium acetate |
| Chloride | Silver nitrate, thallous nitrate |
| Chromium | Lead acetate, silver nitrate, cesium |
| sulphate | |
| Copper | Potassium iodide, potassium mercuric |
| thiocyanate, uranyl acetate | |
| Cyanide | Silver nitrate, ferrous chloride |
| Fluoride | Sodium fluosilicate |
| Iron | Potassium ferrocyanide, potassium |
| thiocyanate | |
| Lead | Ammonium bichromate, potassium iodide |
| Magnesium | Uranyl acetate |
| Mercury | Potassium bichromate, potassium iodide |
| Nitrate | Silver nitrate, nitron |
| Potassium | Chloroplatinic acid, uranyl acetate, |
| perchloric acid | |
| Sodium | Uranyl acetate, zinc uranyl acetate |
| Strontium | Ammonium carbonate, squaric acid |
| Tin | Cesium chloride, oxalic acid |
| Uranium | Thallous sulfate |
| Zinc | Oxalic acid, potassium mercuric |
| thiocyanate, sodium nitroprusside |
Column or liquid-solid (LSC) chromatography LSC utilizes common laboratory materials to effect a separation. The LSC column is typically composed of a laboratory pipette filled with the stationary phase; usually pH adjusted celite or alumina. The mixture to be separated is dissolved in a solvent and the solvent added to the column. Additional solvent (mobile phase) is added until the separated compounds are eluted. The rate at which a compound travels through an LSC column is determined by the amount of time that it spends adsorbed on the surface of the stationary phase. The amount of time a compound takes to elute from the column is known as the retention time. Eluted compounds in column chro-matography may be recovered and qualitatively examined. Lysergic acid diethylamide (LSD) may be easily separated by this method using an alumina column. LSD blotter paper is first base extracted into methylene chloride. This crude extract generally contains LSD and dyes and other materials present in the blotter paper. The extract is then added to an alumina column. The LSD at this point does not flow down the column but is retained at the top of the alumina. A few drops of methanol are added which neutralizes some of the active sites in the alumina column, allowing the LSD to flow down the column. Additional mobile phase is added to maintain the flow down the column and the column is observed under UV light to visualize the characteristic fluorescent LSD band as it travels down the column. When the fluorescent band reaches the point of elution, the solvent is collected in a mortar. Potassium bromide is then added and the mixture dried and pressed into a pellet for analysis by infrared spectrometry. Because column chromatography depends on relative adsorption to the stationary phase, this method is also referred to as adsorption chromatography.
Planar chromatography This technique is a form of liquid-solid chromatography in which the stationary phase is held on a plane rather than in a column. The plane may consist of a glass plate coated with the stationary phase (thin-layer chromatography) or a piece of filter paper or cellulose (paper chromatogra-phy). Both techniques are commonly used in the forensic laboratory as a screening method and in the analysis of drugs and inks. Thin-layer plates are commercially available with a wide variety of stationary phase coatings to suit most applications. As with other chromatographic techniques, the compounds are separated based on their affinity to the stationary phase. The more tightly bound a substance is to the stationary phase, the less distance it travels up the plane. This rate of travel of the compound in relation to the rate of travel of the mobile phase (Rp) may be used to aid in the identification of the compound. When compared to the travel of a standard under the same conditions, the distance traveled can be measured and if equivalent it is indicative the sample and the standard could be structurally similar. In planar chromatography, the mixture of components to be separated is first dissolved in a small amount of suitable solvent. It is best to select the most volatile solvent in which the mixture is soluble in order to reduce spot size. A portion of the dissolved material is spotted onto the stationary phase with a micropipette. The plate or paper is then placed into a jar containing the mobile phase, which is at a level slightly below that of the spotted samples. The mobile phase travels up the planar surface via capillary action and carries the spotted compounds with it. The spots can be visualized by spraying chemical reagents onto the plane. In planar chromatography, the analyst is limited to the following variables in order to effect a separation: composition of stationary phase and mobile phase, the length of the plane and development time. Thin-layer chromatography is a nondestructive technique; the stationary phase spot containing the compound of interest can be scraped off and extracted to recover the isolated compound.
High performance liquid chromatography (HPLC)
HPLC is a subdivision of LSC that uses a stationary phase that has a very small particle size in a commercially produced column that is tightly packed. The contact of the mobile phase to the stationary phase is greatly increased, resulting in superior resolution. The flow of mobile phase through such a tightly packed column is highly restricted, necessitating a quality pump capable of sustaining a high inlet pressure. HPLC can be run in either normal phase (stationary phase more polar than mobile phase) or reverse phase (stationary phase less polar than mobile phase). Since no heat is used, HPLC is ideal for the separation of volatile compounds such as high explosives and some drug samples. Advances in the last decade have allowed the outlet of an HPLC to be mated to the inlet of spectroscopic instruments such as ultraviolet, mass or infrared spectrometers, allowing automated separation and identification of complex mixtures. In addition to the variables listed under planar chromatography, column flow rates and temperature can be varied in order to effect a separation. The ability to alter the mobile phase composition during a run is also useful in effecting separations. HPLC is a nondestructive technique and after elution the sample can be recovered by collection of the mobile phase.
Gas chromatography (GC) Probably the most widely used separation technique, gas chromatography is the subdivision of chromatography where the mobile phase is a gas (known as the ‘carrier gas’). The stationary phase is either a solid adsorbent (GSC) or, more commonly, a liquid coated on the walls of the column (GLC). The separation of compounds on a GLC column is based on the relative solubility of the sample components in the stationary phase. Samples run on a GC must be first converted into a gas at the temperature of the column. This may be accomplished by dissolving the sample in a solvent and injecting the sample with a syringe into a heated (~300°C) injector port at the inlet of the column. Samples which cannot be dissolved (paint, fibers, plastics) can be converted into the vapor phase with the use of a pyrolysis unit (~800°C) at the inlet of the column. Once introduced to the column, the sample is carried through the column by the carrier gas. The column is heated in an oven to prevent precipitation of the sample and to finely control the elution of compounds from the column. Heating the column increases the solubility of the mobile phase in the stationary phase, which as a result decreases the retention time of compounds on the column. The temperature of the column can be programmed from low to high temperatures making separations highly efficient. Exiting the column, the separated substances are detected instrumentally, most commonly by a flame-ionization detector (GC-FID) or a mass spectrometer (GC-MS). The use of a mass spectrometer is most advantageous since it may allow the analyst to identify compounds as they are eluted. GC is routinely used in the forensic laboratory for the analysis of flammable compounds, toxicological samples, drugs, paint and other organic compounds (Fig. 3). Mobile phase flow and column temperature, which can be programmed to change during a run, are common variables used to effect a GC separation. GC is considered a destructive technique as there is no practical way to recover samples once in the vapor phase.
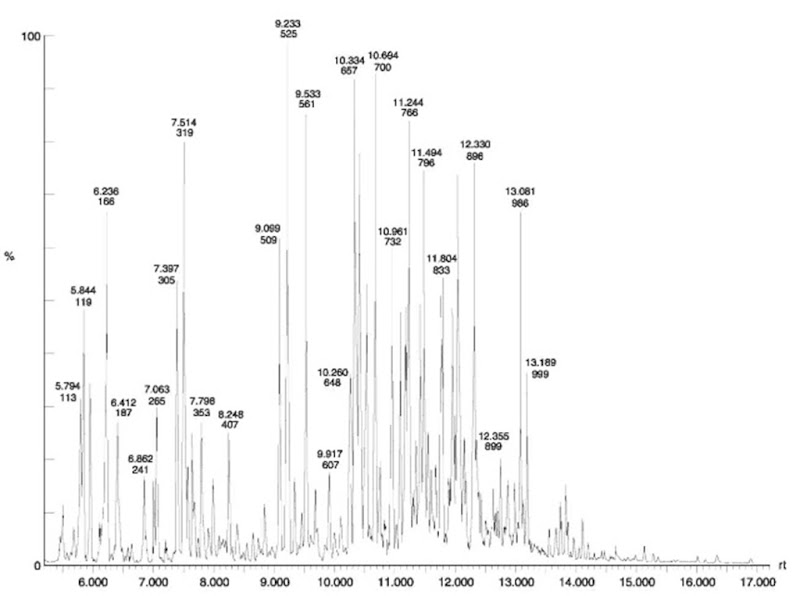
Figure 3 Gas chromatogram of unleaded gasoline.
Electrochemical Techniques
Although these techniques bear a resemblance in theory and function to chromatographic techniques, they cannot be classified as such, as the sample compounds are not partitioned between a stationary phase and a mobile phase.
Electrophoresis
This technique is a planar technique, which uses an electric potential to separate large ions according to their mass charge ratios. The sample is spotted on a gel plate (support) which is permeated with an electrolyte solution. An electric potential is applied across the support for a fixed period of time, causing positive ions to be attracted toward the cathode and negative ions to be drawn toward the anode. The rate of migration of the ions across the support increases as the charge on the ion increases, but decreases with increasing size or mass of the ion. The separation is based on the ratio of charge to mass of the components. Changing the voltage potential across the support controls the speed of migration. The separated components can be visualized by the use of visualizing reagents. Planar electrophoresis is primarily used in forensic work in the separation of proteins present in biological fluids, including DNA.
Isoelectric focusing
The addition of a series of ampholytes to the gel results in enhanced separation of closely related ions. Ampholytes are usually polyaminopolysulfonic acids, which serve as pH buffers. When a voltage is applied, each ampholyte migrates into the gel a different distance creating a continuous pH gradient across the gel. The pH of the gel increases from the anode to the cathode. Substances migrate through the gel until they reach the pH region at which the protein exists as a neutral species. At this pH (the isoelectric point or pI), the migration ceases, since the neutral species has no attraction to the charged electrode.
Capillary electrophoresis
In the past decade, there has been a tremendous advance in the application and use of capillary electrophoresis (CE), which involves an electrophoretic separation in a narrow-bore fused silica capillary tube. The capillaries used in CE generally are about 30-50 cm in length and have an internal bore of 0.010.8 mm. The capillary is filled with buffer and each end is also immersed in buffer. The sample is injected at one end and an electric potential applied across the capillary, driving the components into discrete bands. At the output end of the capillary, analytes are detected and quantified. The resolution of this technique is significantly greater than planar electrophor-esis and also has the added advantage of reduced run times. Capillary electrophoresis has gained a foothold in DNA laboratories and is capturing a large share of the HPLC market in forensic drug laboratories.
Ion mobility spectrometry (IMS)
Instruments, such as the Barringer Ionscan, are in their relative infancy but have become increasingly popular due to their portability, sensitivity and speed of analysis. IMS has been used as a forensic screening technique in the areas of drug and explosive residue. In this technique, samples are swabbed with a filter paper and inserted into a heated sampling device, which serves to ionize the sample. The ions are released into a separation region that is under the influence of an electric field. The ions move through this region at a rate proportional to their mass and against the flow of a gas. Negatively charged ions move quickly through the field toward the cathode and their time of flight recorded. This time of flight can be compared to the time of flight of a variety of substances across the field allowing tentative identification of the substance. These instruments are commonly used in airports to rapidly screen luggage for explosives and drugs. In the last few years they have been increasingly used at the scenes of clandestine laboratory and bombing investigations and prison inspections.
