Introduction
Alcohol (ethanol, CH3CH2OH) tops the list of toxic substances encountered in forensic toxicology for the simple reason that heavy drinking and drunkenness are incriminated in many fatal accidents, trauma deaths, suicides, crimes of violence and antisocial behavior in general. Reports from accident and emergency service departments worldwide provide ample evidence to support the negative impact of alcohol abuse and alcoholism in society. The impairment caused by overconsumption of alcoholic beverages explains many accidents in the home, in the workplace and on the roads. Accordingly, measuring and interpreting the concentrations of alcohol in blood and other biological specimens are routine procedures in forensic medicine and toxicology. The existence of threshold limits of blood-alcohol concentration (BAC) for driving a motor vehicle, such as 80 mg 100ml-1 (0.80gl-1) in UK and 50mg 100ml-1 (0.50 gl- 1) in most of the other European nations, means that the results of forensic alcohol analysis have important social and medicolegal ramifications.
Qualitative and quantitative analysis of ethanol in postmortem specimens is a relatively simple analytical procedure and with gas chromatographic methods, accurate, precise, and specific results are possible. However, difficulties arise when the concentration of alcohol in a postmortem blood specimen is interpreted and conclusions are drawn about a person’s state of inebriation at the time of death. Translating a BAC determined at autopsy (called necropsy in UK) into the amount of alcohol in the body at the time of death is subject to considerable uncertainty.
A major problem associated with postmortem alcohol analysis is the risk that the alcohol, at least in part, was generated or destroyed between the time of death and the time of the autopsy or after taking the specimens and performing the toxicological analysis. This becomes a major dilemma when decomposed bodies are examined and requests are made for alcohol analysis. The blood-glucose concentration increases after death owing to mobilization and hydrolysis of glycogen stores in the liver and muscle tissue thus providing abundant substrate for microbial synthesis of alcohol. This occurs by various processes when the conditions of time, temperature and number and nature of available microorganisms are optimal.
After death the process of autolysis begins and body compartments are progressively destroyed. Within a few hours of death, bacteria and microorganisms begin to spread from the gastrointestinal canal through the portal system, and eventually reach deep into the vascular system. If alcohol was present in the stomach at the time of death, e.g. if the deceased had consumed alcohol before a fatal accident, this might diffuse into surrounding tissues such as the liver, heart, lungs and major blood vessels. For this reason the analysis of alcohol in stomach contents is a common practice in forensic toxicology to compare with blood-alcohol concentration thus providing a clue as to whether the person had died shortly after drinking alcohol.
Analysis of Alcohol in Postmortem Specimens
The methods suitable for analyzing alcohol in postmortem specimens are essentially the same as those used when specimens are taken from living subjects, e.g. drinking drivers or emergency service patients. The units used to report the concentration of alcohol determined in blood and other body fluids differ from country to country, e.g. mglOO ml-1 (UK), g 100ml”1 (USA), gkg-1 or mgg (Scandinavia and Germany) and gl-1 or mgml-1 (mid- and southern Europe). Examples of the kind of units used to report blood-alcohol concentrations for clinical and forensic purposes are given in Table 1.
Quantitative methods for the determination of alcohol in blood and urine have been available for more than 1OO years. Although by modern standards the first efforts were rather primitive, the results at least took the guesswork out of deciding whether gross intoxication might have contributed as a cause of death. Ethanol had to be separated from the biological matrix by distillation or diffusion followed by oxidation with excess potassium dichromate in strong sulfuric acid and back titration of the amount of oxidizing agent remaining with sodium thiosulfate and iodometric titration. The major uncertainty with wet-chemical oxidation methods stemmed from the fact that other organic volatiles that might have been present in postmortem blood were oxidized along with ethanol leading to falsely high blood-alcohol concentrations being reported. The wet-chemistry oxidation procedures were replaced by milder and more selective enzymatic oxidation in the 1950s and under these conditions only a few other alcohols (propan-1-ol, isopropanol, butan-1-ol) represented any real interference problem.
In the 1960s gas chromatographic (GC) methods were developed for the analysis of alcohol in blood and urine and these have dominated ever since. GC methods had the distinct advantage of providing a qualitative screening analysis based on the retention time of the substance together with a quantitative analysis based on the detector response as reflected in peak area or peak height on the resulting chromato-gram. The response of the flame ionization detector (FID) is remarkably linear over the range of concentrations (0-800 mg 100 ml-1) encountered in postmortem specimens. The limit of quantitation of alcohol in postmortem blood specimens under routine conditions is about 10mg100ml-1 (0.1 gl-1) and analytical results below this threshold are generally reported as negative in postmortem work.
Gas chromatography coupled with the headspace sampling technique (HS-GC) still remains the method of choice for forensic analysis of alcohol in specimens from living and dead persons. Figure 1 gives a schematic representation of the headspace analysis procedure showing two chromatographic traces with propan-1-ol and t-butanol as internal standards. In postmortem toxicology two stationary phases (SP) are necessary to enhance specificity for ethanol. These are denoted as SP-1 and SP-2 in Fig. 1. When only a single stationary phase is used for GC-analysis, there is always a risk that ethanol and some other volatile component in the blood, such as a product of putrefaction or fermentation, might have the same retention time. Making a duplicate determination of the blood-alcohol concentration with two different column packing materials for the chromatography gives two different retention times for ethanol which minimizes or eliminates the risk of obtaining coincident retention times.
Table 1 Concentration units used to report blood-alcohol concentration in clinical and forensic science.
| mg ml 1 | mg 100 ml-1 | g 100ml-1 | gkg 1 | mU |
| orgl-1 | or mg dl-1 | or g%, w/v | or mg g-1a | or mmol l-1b |
| 0.50 | 50 | 0.05 | 0.47 | 10.8 |
| 0.80 | 80 | 0.08 | 0.76 | 19.1 |
| 1.00 | 100 | 0.10 | 0.95 | 21.7 |
| 2.00 | 200 | 0.20 | 1.89 | 43.4 |
| 5.00 | 500 | 0.50 | 4.74 | 108.6 |
” The unit used in Scandinavia and Germany assuming a specific weight of 1.055 for whole blood, that is, 1 ml = 1.055g. b The SI unit used in clinical chemistry (mg ml -1 x 1000J/46.05 where 46.05 is the molecular weight of ethanol.

Figure 1 Schematic diagram showing analysis of alcohol in biological samples by headspace gas chromatography with two different stationary phases (SP1 and SP2) for packing the chromatographic columns and two different internal standards (pro-pan-1-ol and t-butanol) for diluting the specimens prior to analysis.
Examples of the retention time of ethanol compared with other low-molecular-weight volatiles with two widely used stationary phases for GC analysis are given in Table 2. If needed, the chromatographic operating conditions (e.g. oven temperature, carrier gas flow) can be optimized to give better separation of peaks for the various substances. The use of two chromatographic systems is a mandatory requirement in postmortem toxicology when ethanol is the substance analyzed. Packed columns made from glass or stainless-steel tubes which are 2 m long and 3 mm internal diameter are still widely used for forensic alcohol analysis. However, major developments have occurred in separation science for gas chromatogra-phy and today wide-bore capillary columns dedicated for blood-alcohol analysis are available such as Rtx-BAC1 and Rtx-BAC2. Whatever the chromato-graphic method used, a minimum requirement must be to determine methanol, ethanol, acetone and propan-2-ol at the same time in a single run. These are the most frequently encountered low-molecular-weight volatile substances in postmortem blood specimens.
The precision of HS-GC methods of blood-alcohol analysis expressed as coefficient of variation (CV) is often less than 1% within a single laboratory. The variation between laboratories, reflecting the repro-ducibility of the method, when aliquots of the same blood specimen are sent to specialist forensic laboratories, is about 3% CV, and in hospital clinical laboratories CVs of 6-8% are reported. In postmortem toxicology, the sampling variation and magnitude of site-to-site differences in blood alcohol concentration often exceeds these pure analytical variations.
Another way of enhancing selectivity for identification of ethanol is to make use of an independent analytical principle such as enzymatic or chemical oxidation together with the usual gas chromato-graphic analysis. However, the current trend is towards the use of mass selective detectors for verification of ethanol in blood specimens. The electron-impact mass spectrum of ethanol shows prominent ion-fragments with a base peak at mlz 31 (common for primary alcohols), mlz 46 (molecular ion) and also at mlz 45 (molecular ion -1). Compact bench-top GC-MS instruments are now affordable and these are widely available in forensic toxicology laboratories. These instruments offer excellent opportunities for unequivocal identification of alcohols and other volatiles in blood and tissue specimens obtained at postmortem as well as in the poisoned patient.
Table 2 Retention times (RT) of ethanol and other low molecular volatile substances analyzed by headspace gas chromatography on two different stationary phases commonly used in forensic toxicology laboratories. RTs relative to propan-1-ol as internal standard are shown in brackets
| Substance | Retention time (min) Carbopak C as column packing material | Retention time (min) Carbopak B as column packing material |
| Acetaldehyde | 0.56 (0.38) | 0.53 (0.29) |
| Acetone | 1.00(0.68) | 0.86 (0.46) |
| Butan-1-ol | 4.68(3.16) | 4.11 (2.22) |
| Butan-2-ol | 2.99 (2.02) | 2.53 (1.36) |
| Ethanol | 0.72 (0.49) | 0.98 (0.53) |
| Methanol | 0.49 (0.33) | 0.67 (0.36) |
| Methyl ethyl ketone | 2.45 (1.66) | 1.49 (0.81) |
| Isopropanol | 1.16(0.78) | 1.31 (0.71) |
| Propan-1-ol | 1.48 (1.00) | 1.85 (1.00) |
| t-butanol | 1.90 (1.28) | 1.68 (0.91) |
a CarbopakC, 0.2% Carbowax (polyethylene glycol) 1500. b CarbopakB, 5% Carbowax (polyetheylene glycol) 20M.
Sometimes a rapid assessment of whether alcohol intoxication was a contributing factor in a person’s death is considered necessary. For this purpose, several on-the-spot methods of alcohol analysis are available. One such method consists of a disposable dip-stick device, which was originally developed for the analysis of alcohol in saliva specimens from living subjects. This saliva alcohol test is called QED® and stands for quantitative enzyme diagnostics. This device can be used to measure alcohol in ‘clean’ biological liquids such as vitreous humor or cere-brospinal fluid (CSF) or in blood or plasma after precipitation of proteins. The principle of the QED® analysis involves enzymatic oxidation of ethanol by alcohol dehydrogenase (ADH) and the coenzyme NAD+. The endpoint is coupled to a blue-color reaction. The result of the test is obtained after about 1 min by reading a thermometer-like scale. The length of the blue stain in the capillary tube is proportional to the concentration of alcohol in the sample analyzed.
Alternatively, a handheld instrument (Alcolmeter S-D2) intended primarily for breath-alcohol testing can be modified for headspace sampling above liquids. The Alcolmeter incorporates an electrochemical sensor for the oxidation of alcohol and about 0.50 ml of headspace vapor is aspirated from above the liquid tested (e.g. blood or urine) equilibrated in an airtight container held at constant temperature. The result of the analysis is obtained in about 30s after sampling. Although the results obtained with these quick and easy methods are less reliable than those obtained by gas chromatography, they at least indicate whether the person might have been under the influence of alcohol at the time of death.
The biological specimens received by the laboratory should be allowed to acclimatize to room temperature before aliquots are removed for analysis. Blood samples should be made homogenous and if necessary any clots homogenized to obtain a uniform sample. Otherwise, the clot can be separated by centrifugation and the clear supernatant analyzed remembering to take into account the water content of the specimen which might be different from that of whole blood. Urine, vitreous humor and CSF are usually analyzed directly without any pretreatment.
Fate of Alcohol in the Body
Traces of alcohol occur naturally in body fluids as products of metabolism and also by the microbial fermentation of sugars in the gut. However, this endogenously produced ethanol lacks any forensic significance because the concentration in peripheral venous blood from abstaining subjects determined by headspace gas chromatography are below 0.001 gl-1 (0.1 mg 100ml-1). Even in subjects suffering from various metabolic diseases such as diabetes mellitus, cirrhosis, hyperthyroidism etc., the concentrations of endogenous ethanol are similar to values observed in healthy individuals.
After drinking alcoholic beverages, the alcohol (ethanol) contained in beer, wine or spirits is diluted with the stomach contents before being absorbed and transported by the blood to all body organs and tissues. Alcohol distributes throughout the total body water without binding to plasma proteins. The solubility of ethanol in fat and bone is negligible. How fast alcohol enters the bloodstream depends on many variable factors, especially the speed of gastric emptying as controlled by the pyloric sphincter. Alcohol absorption is relatively slow through the stomach mucosa, which is less permeable to small molecules than the duodenal or jejunal mucosa. Also, the much larger absorption surface area available in the upper part of the small intestine facilitates rapid absorption of alcohol, which requires no prior digestion. Factors that delay gastric emptying such as the presence of food in the stomach before drinking, various medications, smoking, blood-sugar level and the time of daywill impacton the rate of absorption of ethanol and influence the blood-alcohol concentration reached.
After drinking small doses of alcohol (one to two drinks) some of the alcohol might become metabolized in the stomach mucosa or during the first passage of the blood through the liver. The presystemic breakdown of alcohol in the gut or the liver before reaching the systemic circulation is referred to as ‘first-pass metabolism’. The mucous membranes of the stomach contain the enzyme ADH although in much smaller amounts than in the liver where the bulk of the metabolism of ethanol occurs. Gastric ADH differs from hepatic ADH in other respects such as the optimal Km and Vmax values for oxidation of alcohol.
The blood-alcohol concentration reached after drinking alcoholic beverages depends on the amount consumed (dose), the speed of drinking, the rate of absorption from the gut and also on the person’s body weight, age and gender. Having a higher proportion of fat gives a higher BAC for the same dose of alcohol consumed because leaner individuals have more body water into which the alcohol can be diluted. Since women tend to be smaller than men and also have more fatty tissue and less body water, a given amount of alcohol in a female drinker yields a higher BAC and a correspondingly greater effect on the brain (impairment) and more damage to organs and tissues. Likewise in older individuals, who generally have more fat per kg body mass than younger people, studies have showed that higher BACs are reached in the aged.
Once absorbed from the gut, alcohol is transported via the portal vein to the liver where enzymes begin the process of breaking down the alcohol to clear it from the bloodstream. The principal alcohol-metabolizing enzyme is class I hepatic alcohol dehydrogenase (ADH), which converts ethanol into a toxic metabolite, acetaldehyde. Fortunately, this noxious substance is swiftly transformed into acetate by another hepatic enzyme called aldehyde dehydro-genase (ALDH). In this two-stage biotransformation process, the coenzyme nicotinamide adenine dinu-cleotide (NAD+) is reduced to NADH. The excess NADH in the hepatocytes during ethanol oxidation disrupts many of the normal metabolic processes that require the same coenzyme (NAD+). This leads, among other things, to reduced synthesis of glucose, increased concentration of lactate, altered fatty acid metabolism and accumulation of fat in the liver as some of the consequences. The bulk of the dose of ethanol (95-98%) is oxidized to CO2 and H2O, and the remainder (2-5%) is excreted, unchanged, in sweat, urine and expired air. A very small fraction of the alcohol ingested is converted by the liver into ethyl glucuronide and this water-soluble minor metabolite can be measured in urine specimens to confirm that a person had actually taken alcohol.
Distribution of Alcohol in Body Fluids and Tissue
Alcohol and water mix together in all proportions and only a very small fraction of the total amount of alcohol absorbed into the blood penetrates into fatty tissue and bone. Accordingly, the distribution of alcohol in body fluids and tissue after diffusion equilibrium is complete follows the distribution of water in the body. Urine, vitreous humor (VH), and CSF,which consist of 98-99% w/w water, can therefore be expected to have higher concentrations of alcohol than whole blood, which is 80% w/w water on the average. Organs and tissue such as skeletal muscle, brain, liver and kidney which contain somewhat less water (75-78% w/w) will accordingly contain lower concentrations of alcohol compared with the same mass of blood. Moreover, liver and kidney have considerable enzymatic activity and the concentration of alcohol in these organs decreases for various periods of time after death owing to on-going metabolic processes.
Table 3 gives the water contents of body fluids and tissues taken at postmortem and used for analysis of alcohol. These values provide a rough estimate of the relative concentration of alcohol expected in various biofluids and tissues because the ratios of the water content should correspond to the distribution ratios of alcohol provided that diffusion equilibrium of alcohol is complete. The stage of alcohol absorption and distribution in the body and the pooling of urine in the bladder during which time the blood-alcohol concentration is changing are important to consider when urine/blood ratios and CSF/blood ratios of alcohol are interpreted.
After absorption and distribution of alcohol are complete and the concentration in blood begins to decrease, the organs and tissue such as skeletal muscles return alcohol into the venous blood and peripheral circulation. The venous blood therefore contains a somewhat higher concentration of alcohol than arterial blood when the blood-alcohol curve enters the postabsorptive stage. However, these arterial-venous differences in alcohol concentration lack significance in postmortem work.
a The ratios of alcohol concentration differ widely in any individual case depending on the concentration of alcohol present and the time after drinking when death occurred.
b Average value for specimens taken at autopsy; note that the ratio depends to a great extent on the stage of alcohol absorption and distribution in the body.
c Lumbar fluid taken during postabsorptive phase.
Table 3 Average water-content of body fluids, organs and tissue in relation to the expected concentrations of alcohol. Note that the result from analyzing an alternative biological specimen should not be used to estimate blood-alcohol concentration in any individual case
| Biological specimen | Water content (% w/w) | Alcohol ratio relative to whole blooda |
| Whole blood | 80 | 1.0 |
| Plasma/serum | 92 | 1.1-1.2 |
| Erythrocytes | 70 | 0.8-0.9 |
| Urine | 98-99 | 1.2-1.4b |
| Vitreous humor | 99 | 1.1-1.3 |
| Cerebrospinal fluid | 98-99 | 1.1-1.3c |
| Bile | 87-97 | 0.8-1.0 |
| Synovial fluid | 92-96 | 1.1-1.2 |
| Liver | 80 | 0.6-0.8 |
| Brain | 75 | 0.8-1.0 |
| Skeletal muscle | 76 | 0.8-0.9 |
| Kidney | 79 | 0.6-0.7 |
Toxicity of Alcohol
Fatalities caused by abuse of alcohol and drunkenness occur daily throughout the world and heavy drinkers and alcoholics are over-represented among individuals committing suicide. When sudden unnatural deaths are investigated, especially among males, the BACs at autopsy together with other information corroborate recent heavy drinking. Elevated blood-alcohol concentrations are the most common finding in medicolegal autopsies often in combination with narcotics or prescription drugs. Indeed, alcohol intoxication or drug-alcohol interactions should not be overlooked when sudden natural deaths are investigated and screening of all out-of-hospital deaths for elevated blood-alcohol concentration has been suggested.
Deaths attributable to alcohol impairment include all kinds of accidents and especially road-traffic fatalities, which are the leading cause of death among people aged under 35 years for drivers, passengers and pedestrians. Statistics indicate that in approximately 30-40% of fatal road-traffic accidents, especially single vehicle accidents, the driver had been drinking, and that blood-alcohol concentrations at autopsy often exceeded 150mg 100 ml”1 (1.5gl”1).
The acute toxicity of alcohol is well documented for inexperienced drinkers who consume too much too quickly, leading to gross impairment and alcoholic coma. Alcohol kills by its direct toxicological effect on the brain, e.g. depression of the respiratory center in the medulla, with paralysis of respiration. Under these circumstances the BACs at autopsy are often 300-400 mg 100 ml”1 (3-4 gl”1), or higher. In combination with other sedative drugs (e.g. barbiturates and benzodiazepines), for which the pharmacological effects are additive, death may result after drinking much smaller amounts of alcohol. Long-term abuse of alcohol eventually leads to malfunctioning of body organs and tissue necrosis. Death from liver cirrhosis is a fairly common finding in chronic alcoholics. Secondary causes of death include aspiration of vomit and suffocation while in a deep comatose drunken stupor. This mode of death is well documented in teenagers and young people unaccustomed to heavy drinking and for whatever reason have engaged in forced consumption of alcohol, such as occurs during drinking competitions.
Some people consume large quantities of alcohol daily and build-up a pronounced central nervous system tolerance, which might explain the very high blood-alcohol concentration reported in autopsy material (4-5gl”1). Death from depression of the respiratory center in the brain or a combination of gross intoxication and exposure, e.g. hypothermia is not uncommon among skid-row alcoholics. Chronic intake of alcohol results in metabolic disturbances such as hypoglycemia, lactic acidosis and ketoacidosis. These conditions are often exaggerated by poor dietary habits sometimes with fatal outcome.
Many attempts have been made to correlate blood-alcohol concentration with degree of impairment, but the association is weak and only general guidelines can be given, in part owing to the development of functional tolerance. The blood-alcohol concentration necessary to cause death varies widely but is often quoted as being 350-450 mgdl” 1 although many exceptions exist and some people attempt to drive a motor vehicle after drinking to reach these levels.
Sampling Considerations
Obtaining representative samples is a critical element in all analytical work. The late W.J. Youden of the National Bureau of Standards, Washington, DC is quoted as saying: ‘It is common knowledge that the result of an analysis is no better than the sample used for analysis. The qualifying phrase on the sample as received should be included in a report.’
Analytical chemists and the methods they use are carefully scrutinized regarding precision, accuracy and selectivity of the results, but less attention is usually given to the way the sample was obtained and its condition on arrival at the laboratory. Indeed, the variance and uncertainty associated with sampling often dominates the total variability of the analytical method. For this reason, autopsy specimens should be taken in accordance with a standard protocol and all materials carefully labelled including identification of the deceased, the date and time of autopsy and the type and amount of specimen collected. These aspects of the sampling procedure become important if a second autopsy has to be performed, and new specimens are taken for toxicological analysis. The size, shape, and composition of the containers used to hold the body fluids should be appropriate for the kind of specimen collected. The containers (previously unused) are generally made of glass or polypropylene with tight fitting screw tops. This reduces the likelihood of contamination of specimens and losses of sample occurring owing to a leaky container or adsorption or reaction of the analyte with the container material. The mode of transport, storage and overall security of the materials sent for analysis often needs to be documented in medicolegal casework whenever the results of alcohol analysis are challenged in court.
The cleanliness of the sampling equipment (sterile syringes and needles) and any transfer lines also warrant attention to avoid contamination of specimens with extraneous solvents used in the autopsy room. For volatile substances, like ethanol, use of airtight containers with small air-spaces helps to minimize losses by evaporation. Whenever possible the biological specimens should be stored in the cold (4°C) before shipment to the laboratory. Finally a system should be devised to permit the definitive identification of samples received by the laboratory including the kind of specimen, the site of sampling blood, the volume of specimen obtained, and not least the person responsible for inspecting the specimen. All this falls under the rubric ‘chain-of-custody’ which becomes critical whenever the integrity of the sample has to be defended in court.
Examples of the biological specimens taken at autopsy and subsequently used for alcohol analysis are presented in Table 4. The rank order of preference is femoral venous blood, bladder urine or vitreous humor, and cerebrospinal fluid or various combinations of these depending on availability, e.g. blood and urine or blood and vitreous humor.
Blood Samples
The concentration of alcohol in a sample of postmortem blood can provide useful information about the BAC at the time of death and within limits the amount of alcohol the person might have consumed.
Table 4 Biological specimens available for analysis of alcohol and sometimes taken during postmortem examination
| • | Whole blood | • | Bile |
| Heart (cardiac) | • | Stomach contents | |
| Cubital vein | • | Synovial fluid | |
| Jugular vein | • | Bone marrow | |
| Femoral vein8 | • | Organs and tissue | |
| Intercranial blood | Skeletal muscle | ||
| • | Urine8 | Brain | |
| • | Vitreous humor8 | Liver | |
| • | Cerebrospinal fluid | Kidney |
The effects of alcohol and other psychoactive substances on performance and behavior tend to be correlated with the concentrations infiltrating the brain and the person’s BAC provides the best indirect estimate of central nervous system (CNS) exposure to the drug. Tabulations of therapeutic, toxic and fatal concentrations of drugs are available for comparison to help with interpretation of the concentrations determined at autopsy.
The kind and quality of the blood sample available at autopsy depends to a large extent on the condition of the corpse and in particular the existence of severe trauma and whether any evidence of decomposition exists. Taking relevant samples is fundamental to allow correct interpretation of the analytical results. Blood (~50ml) should be taken from an extremity, such as an undamaged femoral vein in the leg or a jugular vein in the neck and the specimens should be obtained early during the autopsy and before evisceration. A recommended practice is to clamp the femoral vein and then withdraw a suitable blood sample with a sterile syringe and wide bore needle. Blood samples intended for alcohol analysis should not be taken from the pericardium, abdominal or thoracic cavities because at these anatomical locations there is an increased risk of contamination by alcohol spreading from the gut. Although heart blood is occasionally used as a specimen for postmortem alcohol analysis, this is not recommended owing to the risk of contamination with alcohol diffusing from the stomach or upper airways.
Blood specimens submitted for alcohol analysis are often clotted and completely hemolyzed, and occasionally, also diluted with other biological fluids. Making a blind-stick through the chest enhances the risk of contamination of samples with ethanol that might have diffused from the stomach into the pleural and pericardial spaces. This problem is especially acute if the stomach has ruptured as often happens in multitrauma deaths, e.g. fall from a high building, motor-vehicle accident or plane crash. The postmortem diffusion of alcohol and other drugs is a recurring issue in postmortem toxicology when the analytical results are interpreted. The lungs, cardiac blood and abdominal spaces might also be contaminated with alcohol if the deceased aspirated vomit when high concentrations of alcohol remained in the stomach.
The sampling tubes and containers used to collect and store postmortem specimens for alcohol analysis must contain sufficient sodium or potassium fluoride so that the final concentration of preservative is approximately 2%, wlw. The fluoride ion is a potent enzyme inhibitor and helps to prevent glycolysis and any production of alcohol by fermentation
if viable yeasts or other microorganisms are available to ferment glucose to alcohol.
Obtaining intracranial blood from a subdural or subarachnoid hematoma is one useful strategy to investigate whether the person had been drinking alcohol before receiving a blow to the head, fracture of the skull and cerebral hemorrhage. If the victim survives several hours after the trauma the concentration of alcohol in peripheral venous blood might have decreased to zero owing to metabolism occurring in the liver. Because circulation in a cerebral blood clot is diminished or nonexistent the concentration of alcohol present should reflect the concentration in peripheral blood at an earlier point in time. Thus, by comparing the alcohol concentration in subdural blood with, for example, femoral venous blood gives a clue about the person’s BAC at the time of the accident. However, low concentrations of alcohol in intracranial blood clots might have been produced by microbial activity, which underscores the need for obtaining supporting evidence of alcohol consumption such as the analysis of urine or vitreous humor. It is not easy to introduce fluoride ions into a blood clot.
If necessary, the blood-alcohol concentration determined at autopsy can be translated into the amount of alcohol in the body at the time of death. With additional information (e.g. gender, body weight, time of starting to drink) the total quantity of alcohol consumed can also be estimated although the results are subject to wide variations. The scientific basis for making these blood-alcohol calculations, which almost always involves use of the Widmark equation, is presented elsewhere.
Vitreous Humor
Vitreous humor or fluid is the transparent gelatinous material filling the eyeball just behind the lens. This watery fluid makes an ideal specimen for forensic analysis of alcohol because of the isolated location of the sampling site, that is, the remoteness of the eyes from the gut, thus minimizing the risk of contamination with microorganisms or diffusion of alcohol from the stomach. The sampling and analysis of alcohol in vitreous humor (VH) is therefore highly recommended as a complement to taking blood samples for toxicological analysis and specimens of VH can be obtained without making a full autopsy. Comparing the concentration of alcohol in VH with the blood-alcohol concentration allows a check on whether postmortem synthesis of alcohol in the blood samples needs to be considered. Good agreement has been observed for the concentrations of alcohol determined in VH retrieved from both eyeballs.
Experience has shown that VH is more resistant to putrefactive changes than peripheral blood samples especially in highly traumatic deaths, e.g. aircraft accidents. When there is extensive trauma to the body, the spread of bacteria from the alimentary canal to various parts of the vascular system is much more likely. Under these circumstances, sampling and analysis of VH becomes virtually essential to allow making a reliable interpretation of the prevailing blood-alcohol concentration at the time of death. Moreover, it remains feasible to sample VH for analysis of alcohol when the corpse has become moderately decomposed. Finding a negative concentration of alcohol in VH and an elevated BAC strongly suggests that alcohol has been produced in the blood after death.
The concentration of alcohol in VH should exceed that of the femoral venous blood as there is roughly 10-20% more water in the eye fluid. The VH/BAC ratio depends to some extent on the time after drinking when death ensued, that is, on the stage of absorption and distribution of alcohol in the body. During or shortly after the end of drinking, one might expect the VH/BAC ratio of alcohol to be less than or close to unity whereas in the postabsorptive stage of alcohol pharmacokinetics when equilibration of alcohol in all body fluids is complete, the VH/BAC ratio should be about 1.2:1.
Figure 2 shows a scatter plot of the concentrations of alcohol in VH and in femoral venous blood in samples from 56 autopsies. The correlation coefficient was high (r = 0.98) although the scatter of the points around the regression line as reflected by the standard error estimate (syx) was large, being 0.23 mgl-1 so that 95% of cases should be expected to fall within + 0.46mgl-1 (2 x syx). It is obvious, therefore, that estimating blood-alcohol concentration indirectly from analysis of VH is rather uncertain if 95% limits of agreement are used, and even more uncertain if 99% confidence is required. The negligible intercept (0.01 gl-1) indicates rapid equilibration of alcohol between blood and eye fluids with no pooling of the alcohol and a minimal concentration-time lag. The regression coefficient of 1.19 indicates that VH-alcohol is 19% higher than the corresponding blood alcohol concentration in this material.

Figure 2 Scatter plot showing the relationship between the concentration of alcohol in femoral venous blood and vitreous humor in 56 autopsy cases
The proper role of VH as a biological specimen for alcohol analysis in postmortem toxicology is to compare results with the BAC and thus to corroborate the presence of alcohol in the body at the time of death. Without the analysis of VH, the blood-alcohol concentration alone, especially if this is low (<0.5gl-1 or <50mg 100ml-1), makes the conclusion that a person was drinking before death highly contentious. VH is also the most useful specimen for alcohol analysis whenever embalmed bodies are examined. Embalming fluids contain, among other things, formalin and also various alcohols (mainly methanol).
Urine Samples
The sampling and analysis of bladder urine aids in the interpreting of BAC determined at autopsy in the same way as VH. The total volume of urine in the bladder should be measured and an aliquot (1050ml) taken for toxicological analysis. The container into which the urine specimen is placed should be prepared in advance with sodium or potassium fluoride so that the final concentration is about 2%, wlv, taking care that the salt dissolves in the urine by mixing thoroughly. Sodium fluoride is less soluble in water than potassium fluoride.
Drugs and their metabolites are excreted in the urine and are present in higher concentrations than in the peripheral blood. The normal rate of urine production is 1mlmin-1 (60 ml h-1), but diuresis is enhanced after drinking alcohol especially when the BAC curve is rising. Studies have shown that the mean urinelblood ratio of alcohol increases from 1.25:1 to 1.65:1 as the volume of urine in the bladder at autopsy increases from < 5 ml to 200-400 ml. Higher urine-to-blood alcohol concentration (UACl BAC) ratios tend to be associated with low concentrations of alcohol, because like percentages the value of a ratio depends on the size of the denominator. At low BAC (<0.5gl-1 or 50mg 100ml-1), abnormally high UAClBAC ratios are generally obtained, in part because of the properties of ratio variables.
The UAClBAC ratio might give a hint about the status of alcohol absorption at the time of death, which could have forensic significance when fatal accidents are investigated. Figure 3 shows a plot of urine alcohol and blood alcohol in 21 subjects who first emptied their bladders and then drank a moderate dose of alcohol in the morning without having eaten breakfast. Note that UAC is less than BAC during the first 1-2 h after drinking and higher than BAC during the remainder of the postabsorptive phase 2-7 h after drinking. Finding a UAClBAC ratio of 1.1 or less suggests that absorption and distribution of alcohol was incomplete and that the person might therefore have consumed alcohol within 2 h of death. In contrast, a UAClBAC ratio of 1.3 or more indicates that the alcohol was already equilibrated in body fluids and tissues and that consumption had probably ended several hours before death. Abnormally high or low UAClBAC ratios are sometimes encountered in postmortem work for the reasons given in Table 5.
Figure 4 shows a scatter plot of UAC and BAC in 56 postmortem samples. The individual values are highly correlated (r = 0.94) but the scatter around the regression line was considerable (syx = 0.48). The individual UAC-BAC fluctuations were so wide that attempts to estimate BAC indirectly from analysis of UAC cannot be recommended. At zero BAC the mean UAC (y intercept) was 0.25gl-1, which indicates a pooling of urine in the bladder during which time the BAC continues to decrease owing to metabolism of alcohol in the liver. Besides, this constant bias of 0.25gl-1, the UAC showed a proportional bias of 15% compared with BAC as indicated by the regression coefficient of 1.15.
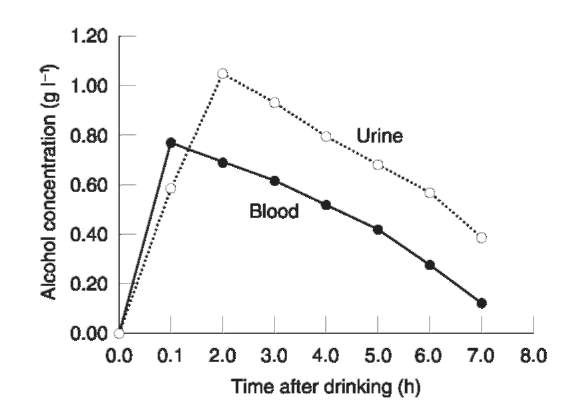
Figure 3 Mean concentration-time profiles of alcohol in urine and blood after 21 subjects dranka moderate dose of alcohol on an empty stomach
Table 5 Interpreting urine/blood ratios of alcohol in autopsy specimens
• Appreciable concentration of alcohol in urine with low or zero concentration in blood (<0.1 g l”1 or < 10 mg 100 ml”1). This suggests elimination of alcohol from the bloodstream owing to metabolism with a subsequent pooling of urine in the bladder. These situations are encountered when death is caused by a blow to the head resulting in cranial fracture, cerebral hemorrhage, and when the victim survived the traumatic injuries for several hours.
• Another explanation for elevated UAC and low or zero BAC might be if alcohol was synthesized in the urine owing to infection with bacteria and/or yeast and if the specimen contained sugar, e.g. in a person with diabetes.
• Zero or very low concentration of ethanol in urine (<0.1 g l”1 or <10mg 100ml”1) and appreciable concentration of alcohol in blood samples. These situations suggest microbial synthesis of ethanol in blood, which is often seen after traumatic injuries and decomposition of the body. An alternative explanation is death occurring immediately after drinking a large amount of alcohol with the bladder containing an alcohol-free pool of urine.
• Abnormally high urine/blood ratios of alcohol are seen when low concentrations of alcohol are present in body fluids or when the bladder contains unusually large volumes of urine (>300ml).
The main advantage of urine over blood is that invasion of the bladder by microbes and yeasts appears to be less of a problem and except in those cases with a history of diabetes, urine does not normally contain any significant amounts of sugar for alcohol fermentation. The combination of sugar, viable yeast or other microorganisms, and optimal time and temperature conditions leads to the formation of alcohol in urine within about 24-36 h after death; one molecule of glucose produces two molecules of ethanol and carbon dioxide during the fermentation process. A glycosuria of 500 mg 100 ml”1 could result in a UAC of 250mg 100 ml”1 on complete fermentation by a yeast such as Candida albicans. The concentration of alcohol measured in urine should not be used to estimate the concentration of alcohol in the body at the time of death.

Figure 4 Scatter plot showing the relationships between the concentration of alcohol in femoral venous blood and bladder urine in 56 autopsy cases
In aviation fatalities where polytraumatic deaths are the rule rather than the exception, drawing correct conclusions about alcohol consumption from analysis of a single specimen of blood or tissue is very difficult. Rupturing of the stomach and bursting of the bladder are commonly seen in victims of plane crashes making it difficult to obtain urine for toxico-logical analysis. In aircraft accidents, there is always a bigger risk for postmortem synthesis of alcohol because of the abdominal trauma and microorganisms from the gut spread more quickly throughout the body. Table 6 lists some of the problems associated with the analysis and interpretation of alcohol concentrations in postmortem specimens.
Table 6 Some factors that need to be considered when interpreting results of alcohol analysis in postmortem specimens
• Specificity of the analytical method used and whether other volatile substances could interfere with the analysis of alcohol.
• Water content of the biological specimens analyzed.
• Variations in alcohol concentration depending on sampling site in the vascular system.
• Variations in alcohol concentration depending on stage of alcohol pharmacokinetics when death occurred.
• Postmortem diffusion of alcohol from the stomach.
• Postmortem synthesis of alcohol by the action of a host of possible microorganisms acting on glucose, amino acids and other substrates.
• Postmortem losses by evaporation or degradation of ethanol by various microorganisms.
• Contamination of specimens with extraneous solvents during emergency service treatment in the hospital or the mortuary or the laboratory.
• Embalmed bodies and the kind of embalming fluid used and whether any alcohol present might have contaminated the material analyzed?
Cerebrospinal Fluid (CSF)
The CSF is mainly composed of water (97-99%) and the concentration of alcohol reaching CSF indicates the concentration that has passed through the brain giving an indication of pharmacological exposure. CSF is obtained either by lumbar puncture at the base of the spine with a hypodermic syringe and wide-gauge needle or by withdrawing cisternal fluid by puncturing the back of the neck. CSF is a clean biological specimen well suited for the analysis of alcohol and other drugs. The CSF is relatively protected from fermentation processes and is sufficiently isolated so that diffusion of alcohol from the stomach is not a major problem. However, CSF might be contaminated with blood when death is caused by a blow to the head.
Figure 5 shows an example of the pharmacokinetic profiles of alcohol in venous blood and CSF obtained by lumbar puncture at various time intervals after subjects drank a moderate dose of alcohol in 5 min. The concentration-time profiles of alcohol in blood and CSF are shifted in time. This is particularly apparent for CSF drawn from the lumbar region but less so for cisternal fluid. This creates a pooling of alcohol in the lumbar fluid so the concentration of alcohol in lumbar CSF does not reflect the BAC at the time of death.
The concentration of alcohol in CSF clearly lags behind the BAC during absorption and distribution of alcohol and the CSF/BAC ratios are less than unity. After 60-90 min when BAC is decreasing, the CSF/ BAC ratios are now 1.2-1.35 times the coexisting venous BAC. The CSF/BAC ratio of alcohol should be about 1.2:1 on the basis of differences in water content and complete equilibration in the body. In practice, the CSF/BAC ratio for lumbar fluid often exceeds 1.3:1 or 1.4:1 in the postabsorptive phase owing to the time lag and the fact that BAC is continuously decreasing. Note that the time lag for cisternal fluid is less than for lumbar fluid because of the slow movement of CSF down the spinal subar-achnoid space. The concentration-time course of cisternal CSF therefore runs closer to BAC and the CSF/BAC ratio in the postabsorptive phase is accordingly less than for lumbar CSF fluid.

Figure 5 Mean concentration-time profiles of ethanol in cerebrospinal fluid (CSF) obtained by lumbar puncture and venous blood in nine subjects after ingestion of a moderate dose of alcohol
Other Specimens
The first choice of body fluids for postmortem alcohol analysis are femoral venous blood, bladder urine and vitreous humor (Table 4). When these are not available other biological specimens or tissues are desirable and occasionally submitted for analysis of alcohol. These might include bile from the gall bladder, synovial fluid from the knee joint, marrow from the bones, as well as stomach contents. Tissue such as liver, brain, kidney and skeletal muscle have also served as specimens for analysis of alcohol in postmortem toxicology. In tissues capable of metabolizing ethanol, e.g. liver and kidney, a progressive decrease in the concentration of alcohol has been observed for various periods of time after death. The presence of oxygen in any surviving tissue and availability of NAD+ are sufficient conditions for continued enzymatic oxidation.
Skeletal muscle contains glycogen which is converted into glucose after death providing an abundance of substrate for microbial synthesis of alcohol. Several reports indicate that alcohol is generated in muscle tissue within the first few days after death or during the time specimens are sent by mail to the laboratory for analysis. Alcohol is not evenly distributed in the brain owing to an uneven distribution of water so the results depend on which brain region was analyzed. In forensic practice, a useful strategy when dealing with tissue samples would be to prepare a homogenate of the material intended for analysis immediately after completion of the autopsy and making sure to add about 2% NaF as a preservative before sending the specimens for toxicological analysis.
It is important to consider the water content of organs and tissue when the results from analyzing unconventional specimens are interpreted. Water content is easily determined by drying a sample of tissue or fluid to reach a constant weight. Studies have shown that the water content of fresh whole blood is about 80 + 5%, w/w, but in postmortem blood the water content is much more variable and tends to decrease with increasing time after death. Postmortem blood has a greater proportion of erythrocytes compared with fresh blood and the amount of water in postmortem specimens might vary from 60% to 90%, wlw, depending on various circumstances. Some investigators have recommended that blood and tissue water is determined by freeze drying or desiccation along with the blood-ethanol concentration. A correction could then be applied to the measured BAC for the diminished amount of water in postmortem specimens. Making these allowances for water-content have been advocated and applied by forensic pathologists in Germany when results of postmortem alcohol analysis are interpreted. However, there are so many other uncertainties when interpreting results of postmortem alcohol analysis that making a correction for water content might not be so important.
Stability of Alcohol in Postmortem Blood
The stability of alcohol in blood after death is another problem faced when the analytical results are evaluated and a judgment is made about a person’s state of inebriation at the time of death. Comparing alcohol concentration in venous blood obtained shortly after death, e.g. at the scene of a road traffic accident with femoral venous BAC obtained at autopsy has shown that both increases and decreases in the concentration of alcohol can occur. However, the results of these studies are confounded by inherent site-to-site variations in concentrations of alcohol, any life-saving medication administered and the possibility of postmortem diffusion taking place prior to autopsy.
The mechanism of alcohol loss from tissues and body fluids after death might involve evaporation, enzymatic breakdown or microbiological degradation. A host of different microorganisms can utilize alcohol as a substrate. Loss of alcohol can occur in the interval between death and performing the postmortem examination, and from the time of autopsy to making the toxicological analysis. Whenever possible, postmortem blood specimens should be stored at low temperature to minimize the decrease in BAC during long periods of storage. But even with blood specimens from living subjects taken in sterile 10 ml Vacutainer tubes containing 1% NaF as preservative and kept at 4°C for several months, the concentration of alcohol decreased at a rate of 0.03 gl-1 per month (3 mg 100 ml-1) on average. None of the bloods from living subjects showed increases in the concentration of alcohol and the rate of alcohol loss did not depend on the BAC present initially.
Postmortem Synthesis of Alcohol
Distinguishing antemortem ingestion of alcohol from postmortem synthesis has always been and still is a major dilemma for the forensic pathologist and toxicologist. Deciding whether alcohol was produced after death, e.g. by the action of microorganisms (bacteria or yeasts) on glucose or other substrates, is not easy. The opportunity for postmortem synthesis of alcohol exists from the moment of death to the time of the autopsy. After biological specimens are sampled, any further production of alcohol can be blocked by keeping the specimens cold (4°C) and treating them with ~2% sodium or potassium fluoride. However, to be effective as a preservative, the fluoride must be dissolved in the blood and this is not always easy to guarantee when specimens are clotted, e.g. intracranial hematoma.
Whenever gross decomposition and putrefaction of tissue has occurred, the question of postmortem synthesis of ethanol becomes particularly difficult. Factors to consider include the time since death to autopsy, indoor or outdoor conditions, bodies recovered from water or flames, temperature and humidity of ambient air, adequate ventilation, dehydration of the body etc. Many of these factors are listed in Table 7. Evidence of mummification, skin slippage, bloating, purging, discoloration, maggots, and bad-smelling corpses give strong indications of decomposition and putrefaction making it all the more likely that any alcohol present was produced after death. Addition of a preservative and enzyme inhibitor such as sodium or potassium fluoride after sampling will not help if alcohol has already been produced.
Bodies recovered from water and incinerated cadavers present special problems when the results of forensic alcohol analysis are interpreted. Both losses and increases in the concentration of alcohol are possible when a corpse is submerged in water for extended periods or burnt. Loss of alcohol from body fluids and tissue occur by dilution owing to high solubility in water as time after death increases. Environmental factors (temperature, salt or fresh water), long time lapses and extensive trauma to the body as well as lipolysis are other examples of complicating factors. An abundance of microorganisms present in the water might accelerate the production of ethanol after death.
Table 7 Factors to consider when judging the significance of postmortem synthesis of alcohol
• Time between death and making the postmortem examination
• Condition and location of the body
Indoors or outdoors Time of year
Temperature and humidity of the environment
Extent of traumatic injuries
Abdominal trauma, e.g. ruptured stomach
Incinerated body
Body recovered from water
Extent of decomposition/putrefaction
• Preservative (NaF) present in the blood samples at appropriate concentration (~2%)
• Number and kind of yeast or microorganisms present
• Availability of substrate for ethanol production: glucose, glycerol, lactate, ribose, various amino acids
The products of putrefaction besides ethanol include other alcohols, e.g. propan-1-ol, butan-1-ol, amyl alcohol, acetaldehyde, various fatty acids and their esters along with amines and other nitrogenous low-molecular-weight species including a number of gases, e.g. H2S, NH3,CH4 and SO2. Finding other peaks on the gas chromatogram besides ethanol and the internal standard confirms the presence of other volatile substances such as putrefaction products. Some investigators have suggested that identifying the presence of higher alcohols, e.g. propan-1-ol and butan-1-ol in the blood along with ethanol might be a useful indicator or marker for postmortem synthesis of ethanol. The concentrations of these other primary alcohols are usually much less than that of ethanol. Because propan-1-ol might be produced in decomposing tissue and typically in corpses retrieved from water, some toxicologists recommend that t-butanol is more appropriate as an internal standard for GC analysis. However, making duplicate determinations using both these internal standards and finding good agreement between the results speaks against the presence of propan-1-ol and thus any significant postmortem synthesis of ethanol. Table 8 gives examples of various strategies available for judging whether alcohol determined in postmortem blood was produced after death by microbial activity.
A multitude of microorganisms are capable of producing alcohol from endogenous and exogenous substrates. The main candidate is glucose, which increases in concentration after death, especially in the liver and skeletal muscles. If necessary, the specimens of blood or tissue can be cultured and tests made for the presence of alcohol-producing species according to classical microbiological techniques. Low concentrations of ethanol (<20-30mg 100ml-1) are more likely to be produced in postmortem blood than high alcohol concentrations (>200mg 100 ml-1).
Postmortem Diffusion
Postmortem diffusion relates to the movement or redistribution of alcohol andlor other drugs or their metabolites from one part of the body to another after death. The stomach, portal vein and liver are the main sites from which alcohol can diffuse into the peri-cardial and pleural fluid, and less readily into the chambers of the intact heart and extremities. These processes proceed for various periods of time after death. The more distant the tissue or fluid from the gut the lower the risk of diffusion artifacts occurring. Many factors influence postmortem diffusion including the physicochemical properties of the drug, tissue pH and the way the body was moved and handled after death. Whenever large quantities of alcohol remain unabsorbed in the stomach, postmortem diffusion needs to be considered when analytical results are interpreted. Furthermore, aspiration of vomit or gastroesophageal reflux of stomach contents still containing a high concentration of alcohol will promote diffusion of alcohol into the upper airways and lungs.
Table 8 Methods used to judge whether postmortem synthesis of alcohol might have occurred
• Compare and contrast the concentrations of alcohol in blood taken from different parts of the vascular system (heart, femoral, cubital and jugular vein) look for wide discrepancies in results.
• Divide the blood sample into two portions one with preservative (2% NaF) and one portion without NaF and compare results of alcohol analysis.
• Make an initial analysis on blood without preservative then keep the specimen at room temperature in an airtight container for a few days before reanalysis. An appreciable increase or decrease in the concentration of alcohol suggests activity of microorganisms.
• Analyze and compare alcohol in femoral venous blood, bladder urine, vitreous humor and/or cerebrospinal fluid and lookfor abnormal concentration ratios.
• Culture the postmortem blood specimens and test the ability of any microorganisms present to produce ethanol by incubation with a suitable substrate.
• Identify the presence of other volatile substances in the blood specimen such as propan-1-ol, or butan-1-ol. These higher alcohols are produced in decomposing tissue and can therefore serve as indicators of postmortem synthesis of ethanol.
• Classify the extent of decomposition and putrefaction of the body from its appearance and distinctive color and smell.
• Determine the concentration of serotonin metabolites, 5-hydroxytryptophol to 5-hydroxyindoleacetic acid in urine. Ratios of 5HTOL/5HIAA above 15 indicate ingestion of alcohol some time before death.
• Consider the drinking habits of the deceased person and trace events 24 hours prior to death – was there any evidence of alcohol consumption?
Experiments in which high concentrations of alcohol (5% v/v or 40% v/v) were instilled into the stomachs of cadavers showed conclusively that alcohol spreads to many other sampling sites. However, the results from carefully sampling blood from the femoral vein were not compromised. The number of people who die with high concentrations of alcohol in their stomachs has not been well established although it is generally thought to be rather few. Finding a stomach-fluid alcohol concentration over 500 mg dl”1 (5 gl”1) and a considerably lower BAC, or UAC gives a strong indication of recent intake of alcohol before death.
Markers of Postmortem Synthesis of Alcohol
Methods are needed to decide whether a positive blood-alcohol concentration at autopsy actually reflects antemortem ingestion or microbial synthesis after death. Table 8 lists some of the current approaches to this problem although none are perfect and several should perhaps be used in combination.
A fairly new approach to this problem takes advantage of the metabolic interaction between ethanol and serotonin. When ethanol is being oxidized in the body, the coenzyme NAD+ is converted into its reduced form NADH and the NADH/NAD+ ratio in blood and tissue increases appreciably. This altered redox state in the liver disrupts many of the normal metabolic processes, including the metabolism of serotonin. The enzyme aldehyde dehydrogenase (ALDH), which is needed for the oxidation of acet-aldehyde derived from ethanol, is also diverted from normal enzymatic pathways.
The biogenic amine serotonin (5-hydroxytrypta-mine) is deaminated by monoamine oxidase to produce an intermediate aldehyde, which is normally oxidized by ALDH to give an acid metabolite 5-hydroxyindoleacetic acid (5HIAA), which is excreted in urine. At the same time, a small fraction of the biogenic aldehyde is reduced to an alcohol metabolite 5-hydroxytryptophol (5HTOL) by the action of ADH or aldehyde reductase. Studies have shown that the oxidative pathway, which normally dominates, switches to the reductive pathway during the metabolism of ethanol in part because of the availability of excess NADH and also the fact that ALDH is engaged in the oxidation of acetaldehyde into acetate. What all this means is that the ratio of 5HTOL/HIAA in urine, which is normally < 15 pmol nmol” 1, increases in a dose-dependent manner during the time that alcohol is being metabolized. In postmortem toxicology, finding an elevated blood-alcohol concentration and a urinary 5HTOL/5HIAA ratio of less than 15 pmol nmol”1 suggests that postmortem synthesis of ethanol has occurred in the blood samples. An elevated 5HTOL/5HIAA ratio in urine indicates that the person had been metabolizing alcohol before death.
Concluding Remarks
Many factors need to be considered when the results of analyzing alcohol in postmortem materials are interpreted for medicolegal purposes. Relating the measured postmortem BAC with an equivalent ante-mortem BAC and making a statement about whether a person had ingested alcohol before death is fraught with pitfalls. Translating the BAC into the amount of alcohol consumed is also subject to considerable uncertainty. The condition of the cadaver and especially any advanced state of decomposition/putrefaction may make the analysis and interpretation ofBAC pointless. Under these conditions some or all of the alcohol might have been produced after death. Finding a postmortem BAC of 200 mg 100 ml” 1 or more suggests that some alcohol at least was ingested prior to death even if the body was appreciably decomposed.
If emergency medical treatment was administered prior to death, e.g. infusion of solutions such as mannitol which causes an osmotic diuresis and helps to reduce swelling in the brain, or if intravenous fluids were given to counteract shock, this should be documented and considered when the results of alcohol analyses are interpreted. Such intensive care treatments can alter the concentrations of alcohol in the various body compartments and complicate interpretation of toxicological results. Moreover, mannitol is a sugar-alcohol and a good substrate for microbial synthesis of ethanol. The time delay between death and autopsy, and from autopsy to performing the laboratory analysis and the condition of the specimens on arrival (color, smell, viscosity, presence of clots) are also important to consider. Care is needed to ensure that the biological specimens were not contaminated by extraneous solvents at the place where the person died or when any emergency medical treatment was administered before death or at autopsy or in the laboratory where the specimens were analyzed.
Although blood from a femoral vein has become the preferred specimen for toxicological analysis of alcohol and other drugs, this is not always possible owing to severe injuries to the body. Scooping a specimen of ‘blood’ from the liquid pooled in the chest cavity is not good practice owing to the risk for internal or external contamination. These questionable procedures might falsify the results of alcohol analysis. Taking multiple specimens including blood, urine, vitreous humor or CSF provides additional information and gives a clearer picture about the person’s BAC at the time of death. The concentrations of alcohol measured in urine, VH or CSF should not be routinely translated into the coexisting BAC owing to the wide individual variations in body fluidlblood ratios of alcohol. However, population average conversion factors are available and these might be considered if and when a conservative estimate of the coexisting BAC is required by analyzing one of the other body fluids or tissue in Table 3.
Much thought is needed when the results of postmortem blood-alcohol analysis are reported to the police because of the stigma attached to any suggestion that a deceased person was under the influence of alcohol. This becomes particularly important whenever fatal accidents on the roads or in the workplace are investigated and responsibility for the accident and insurance claims are made. Much debate and litigation erupts when claims of negligence are brought especially in mass transportation fatalities, which underscores the need to corroborate an elevated postmortem BAC by analyzing alternative biological specimens such as urine or vitreous humor or both. Finding a low blood-alcohol concentration <0.5gl-1 (<50mg 100 ml-1) without any supporting evidence (e.g. positive urine or VH) or ancillary information concerning the person’s drinking habits does not prove conclusively that the person had been drinking alcohol before death. The case history and the circumstances of the death including tracing important events by interviewing friends or relatives of the victim or by reading police records can sometimes provide useful hints about what to expect from the toxicological alcohol analysis.
In cases of extreme trauma when fragmented bodies are recovered (e.g. as might occur in aircraft accidents), when putrefaction is advanced, and when the body is burnt or recovered from water, an unequivocal answer about antemortem ingestion of alcohol from the analysis of postmortem blood specimens or tissue is probably not realistic. To paraphrase Professor Derrick J. Pounder ‘Dead sober or dead drunk? Maybe hard to determine’
