Introduction
Forensic toxicology concerns the analysis of biological specimens (fluids and tissues) for the presence and, often, the concentration of drugs and poisons. The results of the analyses must be correlated with the circumstances of the case to determine what role, if any, the detected substances played. This correlative function is commonly called interpretation. This article will examine the three major subspecialties of forensic toxicology and the various factors that enter into the interpretation process in each.
The forensic toxicology laboratory exists for the sole purpose of providing interpretable analytical data. Therefore, the analytical strategy is designed with anticipation of the need for later interpretation. The most appropriate specimens should be analyzed by sensitive, specific and quantitatively accurate and precise techniques to yield reliable data upon which to base opinions. The toxicologist must be absolutely certain that the analytical data are accurate. Furthermore, the samples must be properly preserved and clearly traceable to the subject of the investigation by an unbroken chain of custody, and handled and stored with a level of security sufficient to preclude tampering.
The choice of specimen(s) and the scope of analysis are determined largely by the purpose of the investigation. Modern forensic toxicology can be divided into three major categories: forensic urine drug testing (FUDT), human performance toxicology and postmortem toxicology. FUDT seeks evidence of illegal drug use by current or prospective employees; human performance toxicology attempts to determine whether the subject was impaired or intoxicated at some specific time by analyzing specimen(s) collected later; and postmortem toxicology investigates the role of drugs and poisons in causing or contributing to the subject’s death. Each presents specific requirements and challenges for the interpreting toxicologist.
Forensic Urine Drug Testing
FUDT, also known as workplace drug testing, has as its goal the minimization of drug abuse in the workplace. This goal is accomplished by intimidating potential drug users through fear of detection and by elimination of drug users from the workforce through treatment or discharge. Since most employees are not suspected of any wrongdoing, the sampling process is designed to be minimally intrusive. Urine is usually the specimen of choice, although hair and sweat are undergoing evaluation as potential alternative specimens. To control the cost of analysis, FUDT programs restrict the scope of analysis to the drugs deemed most dangerous by virtue of their addictive nature, illegality, abuse liability or potential harm to the employees’health or productivity. The most commonly included drugs are methamphetamine, amphetamine, cocaine, marijuana, opiate narcotics (morphine, codeine, heroin) and phencyclidine (PCP). Other drugs such as lysergic acid diethylamide (LSD) and methylenedioxymethamphetamine (MDMA; ‘ecstasy’) may be added if their use is perceived to be common.
In workplace drug testing, the only issue is whether or not the subject of the test illegally used a controlled drug. The drug testing is usually done on urine. If a drug or drug metabolite(s) is conclusively identified, in a properly collected sample, then the person who provided the urine must have ingested the drug. However, legitimate questions may be raised about whether ingestion was intentional or unknowing. Such questions are often referred to a physician trained in interpretation of drug testing reports, a medical review officer (MRO), but they may also be asked of the toxicologist. The toxicolo-gist or the MRO may be able to comment on the reasonableness of arguments offered in defense of one who tested positive for a drug. Above all, unbiased interpretation must include consideration of alternative explanations for apparently incriminating findings.
First, the toxicologist or MRO must be aware of circumstances that can produce positive tests in a person who has not abused the drug (Table 1). For example, research has shown that passive exposure to marijuana smoke does not yield urinary concentrations of A9-tetrahydrocannabinol (THC) metabolites above 40ngml_1. However, taking dietary supplements containing hemp seed oil or eating hemp seed confections can cause readings over 200ngml-1. Over-the-counter diet products containing phenylpropanolamine may have traces of amphetamine, produced as a byproduct of manufacture. If a trace of amphetamine is found with a high concentration of phenylpropanolamine, ingestion of the amphetamine may have been inadvertent. Poppy seeds contain traces of morphine sufficient to cause a positive drug test if poppy seed pastries are eaten. Quite high urine morphine concentrations have been obtained in controlled experiments in which human volunteers provided urine samples for opiate analysis after consuming poppy seed pastries. Testing for the alkaloid, thebaine, derived from poppy seeds, may helpto distinguish food from drug. Several pharmaceuticals contain, or are metabolized to, the ‘l’ isomer of methamphetamine (i.e. l-deoxyephedrine), rather than the controlled ‘d’ isomer. Routinely applied confirmatory tests can not distinguish between optical isomers, but chiral separation columns or derivatives can. Such definitive testing may be required if someone challenges a positive urine test for metham-phetamine. Another commonly reported explanation for positive urine drug test results is the subject’s unknowing consumption of a food or beverage containing a drug that someone else added surreptitiously (e.g. marijuana brownies). The testing methods are sufficiently sensitive to detect excretion products from doses of drug that may be too small to produce observable symptoms. The possible motivation for someone to administer the drug should be considered, as well as the opportunity to do so without detection.
Table 1 Innocent causes of positive urine drug tests.
| Drug | Source |
| Marijuana | Hemp seed oil or hemp seed |
| confections | |
| Cocaine | Coca tea, matte de coca, may be |
| imported from South America and | |
| hasbeen available in health food | |
| stores in the past | |
| Morphine, heroin, | Poppy seed pastry – contains |
| codeine | morphine and codeine |
| Amphetamine | Some phenylpropanolamine |
| productsasa byproduct of | |
| manufacture | |
| Metabolism of medications, e.g. | |
| clobenzorex | |
| Methamphetamine | Nasal inhalers: /-deoxyephedrine= |
| methamphetamine; metabolism of | |
| medications, e.g. | |
| deprenyl=selegiline |
Women sometimes allege that they were exposed to a drug such as cocaine through transfer of semen from a drug-using sex partner; however, the amount of cocaine in semen of a user has been measured and is insufficient to produce a positive urine test if ingested. Some people have suggested that their cocaine-positive drug test resulted from handling contaminated money. The amount of cocaine found in US currency is too small to cause a positive test by dermal absorption, but a single cocaine particle falling into the sample container from the subject’s hand could produce a positive result. To prevent contamination, test subjects are often required to wash their hands before providing a urine sample.
Human Performance Toxicology
Human performance toxicology deals with the effects of alcohol and other intoxicating substances on a subject’s ability to drive a motor vehicle or engage in other potentially hazardous activities. When impairment, rather than use, is the issue, more information is needed for interpretation. Presence of drug or metabolites in urine is useless for demonstrating impairment. At best, finding an intoxicating substance in urine can explain the source of obvious symptoms of impairment. The concentration of drug and its active metabolites(s) in blood is important, but other factors must also be considered. Individual variability and tolerance can affect the degree of impairment at any given blood drug concentration. Combinations of drugs can interact to increase or decrease impairment. For most drugs, a relationship between drug concentration and performance impairment has not been established. Therefore, evidence for interpretation should include the subject’s blood concentrations of all drugs capable of causing impairment, as well as evidence that the subject was impaired. The toxicologist familiar with the behavioral effects of drugs may be able only to offer an opinion that the observed symptoms are consistent, or not consistent, with the effects of the detected drugs. However, to infer that because a drug was present, regardless of concentration, the subject must have been impaired without having evidence of symptoms of intoxication is speculation, not objective interpretation.
Drug recognition programs
Drug recognition (DR) programs, such as that sponsored by the US National Highway Traffic Safety Administration, can provide valuable documentation of intoxication. In DR programs, trained observers examine subjects suspected of being intoxicated, and record their observations in a standardized format. The examination includes physiological symptoms (e.g. pulse, respiration, pupil responses and others), physical tests of coordination and balance, and tests of mental agility, memory and perception. Finally, an algorithmic approach is applied to the observations to place symptom patterns into eight categories: not impaired, or impaired by sedative, stimulant, narcotic, hallucinogen, marijuana, PCP-like or volatile inhalant substances. If the subject is examined while symptoms are present, the documentary evidence can be evaluated in conjunction with results of toxico-logical analyses. The forensic toxicologist may then be able to offer opinions on the cause-and-effect relationshipof the observed behavior or symptoms and the detected intoxicants. Research has shown accuracy rates in the range of 50-80% for DR examinations, depending upon the drug category and the examiner’s training and experience. Therefore, DR reports must always be confirmed by laboratory analysis before any punitive action, such as conviction for driving while intoxicated, can be justified.
Postmortem Toxicology
Because of their variety and complexity, postmortem toxicological investigations provide the greatest challenges for the interpreting toxicologist. The questions that may be raised in a death investigation may be the same as those involved in workplace drug testing or human performance toxicology, but often the postmortem toxicologist must deal with far more complicated issues. To do so requires a familiarity with principles of pharmacology, physiology, biochemistry and anatomy, but also an awareness of pathological changes associated with toxicity and postmortem changes affecting drug concentrations. Knowledge of factors such as the behavioral effects of drugs and the lifestyle of drug-using subcultures can help in understanding and explaining some aspects of a case.
In postmortem toxicology, interpretation is a thought process that begins with the initial case review and guides the analytical strategy that culminates with the toxicologist forming opinions regarding the involvement of drugs or other substances in the death of an individual. The first question that must be addressed is what, if anything, was ingested by the deceased? The answer lies in the outcome of a thorough toxicological analysis that is capable of detecting relevant (i.e. therapeutic or subtherapeutic) concentrations of any substance that could realistically be present. The extent of the search is governed, in part, by the other aspects of the investigation. If there is a strong suspicion of a drug or poison then the toxicologist must search more diligently than if there is no such suspicion. A negative result must be interpreted from an understanding of the sensitivity of the analytical method. It implies that the analyte targeted by the test was not present, or if present, was at a concentration below the detection limit. If methods of sufficient sensitivity are used, negative findings mean that either nothing was ingested or something outside the scope of analysis was involved. Knowledge of the chemical properties of various drugs and poisons and of the capabilities and limitations of available analytical techniques are essential to the determination of whether the analysis is capable of detecting all targeted substances.
When drugs or other toxic substances are detected in body fluids or tissues, the toxicologist must gather additional data in an effort to determine what role, if any, the detected substances played in the events leading to death. In some cases where the cause of death is manifestly obvious, such as accidental, the result of assault or self-inflicted trauma, the issue becomes one of the impairing or intoxicating effects of drugs, including alcohol (behavioral toxicity). Substances lacking psychoactivity are usually incidental to the investigation, unless they could have affected survivability. In cases of death attributable to natural disease, the toxicologist is concerned with substances that can exacerbate pre-existing illness. Examples include cocaine and other sympathomimetic agents with heart disease or berry aneurysms, and nonselec-tive p-adrenergic blocking agents in patients with asthma. Other potential issues for the toxicologist include adverse interactions of therapeutic drugs and unsuspected intentional overdose in an otherwise seriously ill person. In some cases, such as death from epileptic seizures, it may be important to document whether the deceased was compliant with life-sustaining therapy.
When a toxic mechanism of death is suspected from the findings of the investigation and autopsy, the toxicologist is asked to identify the offending substance(s) and, insofar as possible, to determine the route and mode of entry. Was ingestion accidental or intentional? Was enough drug or poison present to cause death, and was it ingested acutely or cumulatively? If multiple substances are detected, do they possess additive or synergistic toxic activity?
Drug interactions
If multiple substances are identified, some may be unrelated to the cause of death, whereas others may have interacted, resulting in fatal consequences. Drugs with similar toxic mechanisms, such as respiratory depression or cardiovascular stimulation or depression, can be expected to contribute additively or synergistically to the net toxic effect. For example, ethanol, benzodiazepines and opiates all have respiratory depressant activities at toxic concentrations. In combination they can produce serious or fatal respiratory depression, even when the individual drugs are present in subtoxic concentrations. The calcium channel blocker, verapamil, and p-adrenergic antagonists, such as propranolol or timolol, may both be prescribed for migraine headaches or to lower blood pressure. When combined, verapamil and beta block-ers can sometimes lead to fatal cardiogenic shock and can even cause the heart to stopbeating. It is sometimes apparent that one drug exerted the predominant effect, without which the death would not have occurred. This is often the case with heroin toxicity. Other drugs, such as alcohol, benzodiazepines, cocaine or other opiates, if present, would be expected to contribute to toxicity, but usually would not be lethal without the addition of heroin.
Postmortem changes affecting toxicology
During life, drugs are differentially concentrated in the various organs to levels that may be orders of magnitude higher than that in blood. In general, the higher the volume of blood into which a dose of the drug appears to distribute (volume of distribution), the greater is the difference in concentrations between blood and tissues. The liver, heart and lungs are among the tissues known to sequester various drugs. After death, the physiological and chemical conditions that maintain the differential begin to degrade and the drugs diffuse out of tissues into blood. Therefore, blood collected from the vena cava is likely to contain excess drug released by the liver, as well as any drug freshly absorbed from the gastrointestinal tract. Heart blood, ‘chest cavity blood’ or aorta blood may contain drug released by the heart tissue, lungs and liver and, potentially, may be contaminated by stomach contents. A therapeutic concentration of drug can quickly rise into the ‘toxic’ or ‘lethal’ range on tables derived from clinical observations of living subjects. Large differences in drug concentrations are often found when blood from these visceral sites is compared with blood from a peripheral site, such as femoral or subclavian vein (Table 2). It is now recognized that analysis of peripheral blood yields more consistent and more reliable quantitative data. However, the toxicologist can not simply compare a drug’s concentration in blood with published clinical data of therapeutic, toxic and lethal concentrations. To do so would probably lead to erroneous conclusions. Postmortem drug concentrations should be compared with postmortem data on the same site from cases of known drug toxicity and from cases where toxicity was not involved. Such an approach will often help to distinguish elevated from normal drug levels. Analysis of tissue such as liver frequently reveals extremely high concentrations of many drugs in overdose cases. Likewise, measuring the total amount of unabsorbed drug remaining in the stomach after death can support the conclusion of toxicity if a quantity representing more than a normal dose is found. Finding a trace of drug in the stomach does not prove an oral route of administration, because most drugs diffuse from the blood into the stomach. The same is true when cocaine is detected in a sample obtained by swabbing the nasal passages. A small amount of cocaine will pass from blood to the nasal secretions regardless of the route of administration. Only if a relatively large amount of cocaine is found can one conclude that it was taken intranasally.
Table 2 Drugs implicated in postmortem redistribution.
| Alprazolam | Imipramine |
| Amantadine | Lidocaine |
| Amitriptyline | Maprotiline |
| Amobarbital | Meperidine |
| Amoxapine | Mesoridazine |
| Amphetamine | Methadone |
| Antipyrine | Methamphetamine |
| Brompheniramine | Metoprolol |
| Butalbital | Nordiazepam |
| Caffeine | Nortriptyline |
| Chlordiazepoxide | Pentobarbital |
| Chlorpheniramine | Phencyclidine |
| Chlorpromazine | Phenobarbital |
| Cocaine | Phentermine |
| Codeine | Promethazine |
| Desipramine | Propoxyphene |
| Dextromethorphan | Secobarbital |
| Diazepam | Temazepam |
| Digoxin | Thioridazine |
| Diltiazem | Trazodone |
| Diphenhydramine | Triazolam |
| Doxepin | Trichloroethanol |
| Doxylamine | Trifluoroperazine |
| Ethclorvynol | Trimipramine |
| Fluoxetine |
While postmortem redistribution may elevate drug concentrations in blood, some drugs continue to be metabolized after death. Enzymes, especially esterases, that do not require oxygen or consume energy, remain active and hydrolyze susceptible drugs, including cocaine, heroin and a heroin metabolite, 6-monoacetylmorphine. The net effect of the competing processes, redistribution and metabolism, on drug concentrations in postmortem specimens is unpredictable.
Ethyl alcohol can be produced by fermentative microorganisms during putrefaction so that ethanol concentrations in specimens from a decomposing body are not representative of the blood alcohol concentration at the time of death. In many cases where ethanol is found in a decomposed body, it may be said that the deceased probably consumed some alcohol before death, but when ethanol concentrations are lower than 0.1 gdl-1 in a severely putrefied body, the alcohol may have been entirely produced by postmortem fermentation. Even higher values of postmortem ethanol production (over 0.2gdl_1) have been reported.
Putrefaction can also affect interpretation of carbon monoxide measurements in postmortem blood. The percent of total hemoglobin combined with carbon monoxide (i.e. carboxyhemoglobin) is usually measured spectrophotometrically. A decomposition product, sulfmethemoglobin, has an absorption spectrum that overlaps that of carboxyhemoglobin. Putrefaction will elevate the carbon monoxide reading with most methods, but may depress it with others. Therefore, the toxicologist must understand the effects of sulfmethemoglobin on the method whose result is being interpreted to know whether the reading is artifactually high or low in a decomposed sample.
Correlative information
The presumption of toxicity must be further evaluated, taking other physical and factual evidence into consideration. The terminal events, medical and social history, and autopsy findings must be correlated with drug concentration data. Tolerance from chronic use of a drug such as morphine can raise the threshold for therapeutic effect into a range that would be lethal to a nontolerant individual. Pulmonary edema and congestion revealed by autopsy can represent respiratory depression and lend support to a suspicion of drug toxicity. Even though many pathological changes in the body are not specifically associated with drug toxicity, their presence is supportive, whereas their absence would indicate that drug toxicity was not responsible for the death. Some diagnoses, such as sudden infant death syndrome (SIDS), and some cardiac deaths are justified only after other causes, including poisoning, are excluded.
Observations from the death scene, such as a body posture that restricts respiration or a plastic bag over the head, can explain why some people die with drug concentrations that, while elevated, would not normally be expected to cause death. Drug intoxication in these cases would be one factor that prevents the victim from restoring normal respiratory exchange. Elevated body temperature measured at a death scene can be related to a disturbance in thermoregulatory homeostasis that is often associated with toxicity due to cocaine, amphetamines, neuroleptics and some other drugs.
Witnesses to a death can sometimes provide valuable information about the behavior of the deceased and the terminal symptoms. Reports of bizarre and violent behavior followed by sudden death are evidence of excited delirium, which may be attributable to cocaine or amphetamines if supported by analytical documentation of their presence. Concentrations are usually below the range considered to be lethal, so the behavioral evidence may be critical in arriving at a correct conclusion. Drug paraphernalia found at a death scene raises or supports a suspicion of drug toxicity, which can be confirmed by autopsy and toxicological analysis.
When a hospitalized victim of trauma or poisoning dies in spite of treatment, medical records documenting the progression of symptoms can sometimes be correlated with postmortem toxicology data. The mechanism of toxicity of the drugs or poison, such as the electrocardiographic abnormalities caused by tricyclic antidepressants, should be manifest in the reported symptoms and observed physical findings. This becomes especially important when life support efforts delay the terminal event, allowing the responsible toxin to be eliminated to a point that postmortem concentrations are too low to be detected, or, if detected, to be considered toxic without the additional supporting evidence. In some jurisdictions, the postmortem toxicology laboratory obtains specimens of blood, urine and stomach contents collected soon after the patient’s arrival at the hospital. These ante-mortem specimens sometimes yield the only reliable evidence of the deceased’s state of intoxication at the time of admission. For example, drugs administered by physicians, including morphine, haloperidol, lignocaine (lidocaine), atropine and others, will not usually be found in admission specimens, whereas drugs taken prior to hospitalization will.
Drug metabolism (biotransformation)
Whenever a foreign substance is introduced into the body, drug-metabolizing enzymes act upon it to accelerate its elimination. Cytochromes of the P450-class, esterases and other hydrolases introduce polar functional groups, such as hydroxyl, carboxyl or amino, and conjugating enzymes, including glucuro-nyl transferases and sulfotransferases, link the polar functional groups to highly water-soluble polar molecules, glucuronic acid or sulfate, in order to form water-soluble conjugates that are readily excreted.
Biotransformation of drugs and poisons is a factor that is frequently considered in the interpretation process. Drug metabolites are often pharmacologically inactive, but some retain activity or possess activity different from the parent drug. In some cases such as aromatic amines (2-naphthyl amine, 4-aminobiphenyl), halogenated hydrocarbons (chloroform, carbon tetrachloride) and acetaminophen, metabolism yields toxic products. Some biotransfor-mation reactions are subject to induction or inhibition by certain drugs so that the rate of metabolism of other drugs or poisons is increased or decreased. Thus, if an inhibitor of a specific enzyme is present, concentrations of that enzyme’s substrate can rise to toxic levels, or an inducer can cause accumulation of a toxic metabolite or can lower a drug’s concentration to an ineffective level. An awareness of metabolic interactions and biotransformations can helpthe toxicologist distinguish between intentional and accidental intoxication.
The postmortem toxicology laboratory often quantifies both parent drug and its principal metabolite(s). The ratio of parent drug to metabolite concentrations can indicate whether ingestion was chronic or acute. Metabolites of many drugs accumulate in the body with chronic administration. Conversely, an acute overdose may yield only a small amount of metabolite before death occurs. Furthermore, drugs such as propoxyphene and meperidine that are converted to toxic metabolites may produce toxicity by virtue of accumulation of the metabolite during chronic administration.
Pharmacokinetics
The science of pharmacokinetics describes the time course of drug action in terms of the processes of absorption, distribution and elimination. The onset of drug action is determined, first, by the rate of distribution to the site of action. The action is terminated by the elimination of the drug via metabolism and excretion.
Although the concentration of drug in the postmortem blood, tissues or urine cannot usually be related to pharmacokinetic parameters determined in living humans, the clinical data are still valuable to the postmortem toxicologist. For example, a drug’s elimination rate can usually be expressed in terms of its half-life (ti elim.), which is the length of time required for the concentration to decline to half of its initial value in a process following first-order kinetics (i.e. the rate of elimination is proportional to drug concentration at any time point). Approximately 99% of the drug will be eliminated by 6-7 half-lives after its distribution is complete. Usually absorption and distribution occur much faster than elimination. Therefore, if a drug is detected in post-mortem blood, it was probably taken within 6-7 half-lives prior to death.
The distribution pattern between blood and other biofluids and tissues can also be useful for interpretation. Probably the drug whose pharmacokinetic behavior is best understood is ethanol. In postmortem toxicology laboratories, ethanol is usually measured in different body fluids from the same individual to facilitate interpretation. At or near equilibrium, the concentration of ethanol in vitreous humor (eye fluid) is usually about 15-20% higher than that in whole blood. If both fluids are analyzed and blood has higher ethanol concentration than vitreous, then by the pharmacokinetics of ethanol (Fig. 1), the individual was in the absorption phase, or the sample was contaminated with ethanol from stomach contents or putrefaction. Conversely, if the vitreous ethanol concentration is much higher than the blood, the blood sample may have been diluted, and the toxicologist should consider the site of collection and whether intravenous fluids were administered prior to death. To resolve discrepancies, other samples may be analyzed, including blood from another site, bile, brain tissue, urine and stomach contents. A pattern should emerge from the data to explain the pharmacokinetic state at the time of death.
Behavioral toxicity
Psychoactive drugs, both licit and illicit, can cause alterations in perception, judgment, coordination, mood and information processing. In cases involving violence or accidental trauma, when such drugs are detected in the body of the deceased, questions inevitably arise regarding the role of the drug(s) in precipitating the fatal event. The issues are similar to those in human performance toxicology, except that specimens are usually collected postmortem rather than from a living subject. Therefore, drug concentrations must be considered in light of postmortem changes, and impairment or intoxication must be deduced from factual evidence (eyewitnesses) and physical evidence available at the scene where the fatal incident occurred. For example, a motor vehicle crash investigation may discover evidence of driver error, and later toxicological analysis reveals elevated blood alcohol or a high concentration of a drug with the potential to cause impairment. Similarly, when a person becomes excited, violent and irrational, then disrobes, breaks objects, struggles with police and suddenly becomes unresponsive and dies, a finding of even a few hundred nanograms per milliliter of cocaine in the deceased’s blood suggests that the death can be attributed to cocaine-induced excited delirium. This opinion is supported by a documented high postmortem body temperature and a postmortem blood benzoylecgonine concentration of several thousand nanograms per millimeter.
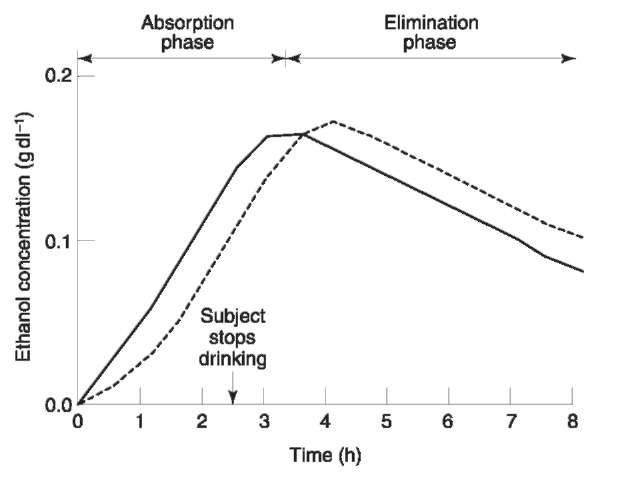
Figure 1 Hypothetical pharmacokinetic curvesfor ethyl alcohol in blood (—) and vitreoushumor (—). Note that the vitreousalcohol concentration islower than that in the blood during the absorption phase, but it exceeds the blood alcohol concentration during elimination.
Because of the many factors affecting measured postmortem drug concentrations, such data can rarely serve as the sole basis for interpretation. They must be correlated with evidence from the autopsy and with information gathered by police, and other investigators. Conversely, inferences and suspicions derived from autopsy and investigation must be refined and confirmed thorough toxicological studies. Only then can reliable opinions be formulated regarding the role of drugs or poisons in a death investigation.
