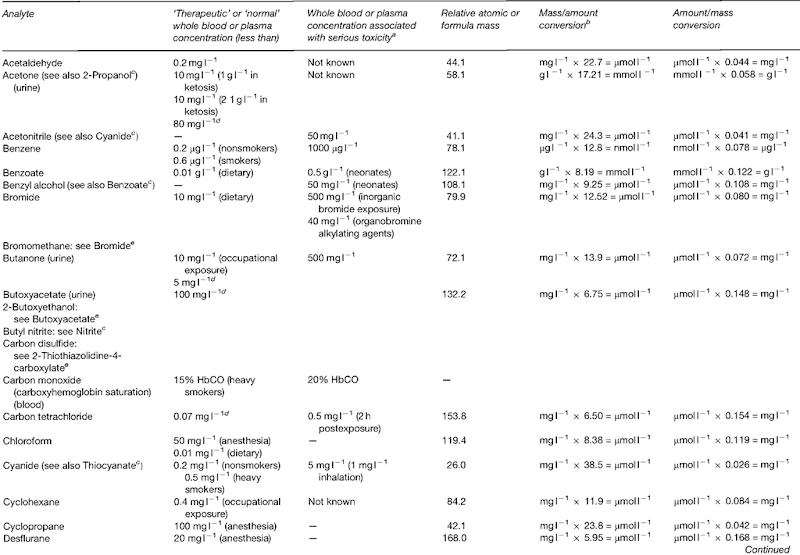
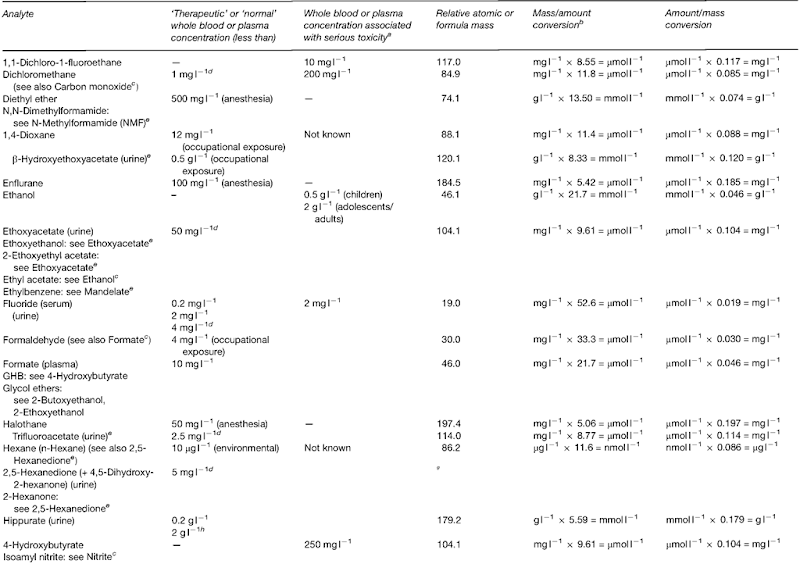
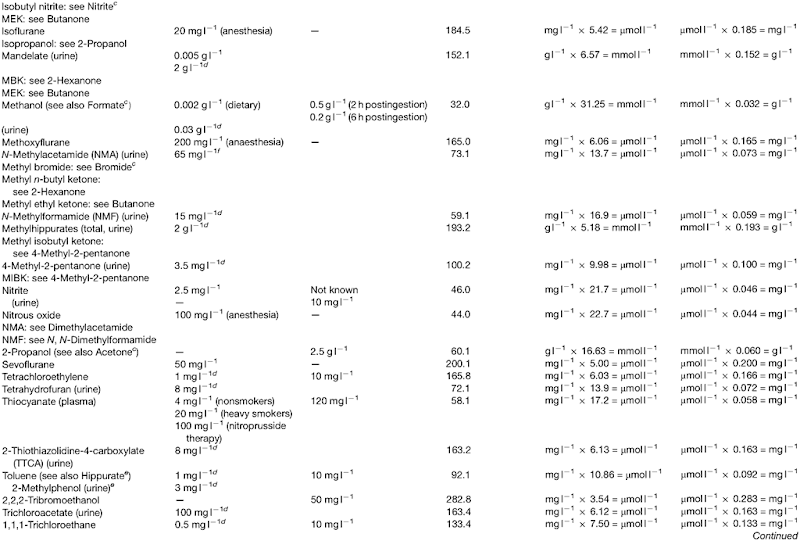
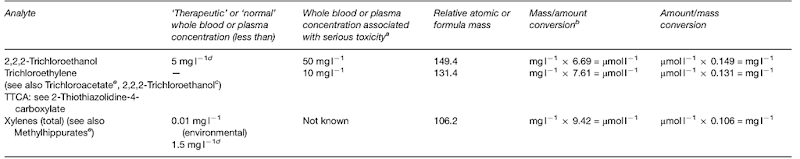
Introduction
If anesthesia is excluded, acute poisoning with volatile substances usually follows the deliberate inhalation of vapour in order to become intoxicated (‘glue sniffing’, inhalant abuse, solvent abuse, volatile substance abuse (VSA)). Solvents from adhesives, notably toluene, some correcting fluids and thinners (until recently, often 1,1,1-trichloroethane), hydrocarbons such as those found in cigarette lighter refills (usually liquefied petroleum gas (LPG)), aerosol pro-pellants, halocarbon fire extinguishers and anesthetic gases such as nitrous oxide are among the compounds/products that may be abused in this way (Tables 1 and 2).
LPG arises from the cracking of oil to make petrol (gasoline) and also in the gases trapped above oil fields. There are at least two grades of LPG available to volatile substance abusers: (1) unpurified gas intended for direct use as a fuel; and (2) purified gas intended primarily for cigarette lighter refills and as a propellant in aerosols and related products. The composition of LPG can vary depending on the source, although its major components are usually butane, isobutane and propane. Some unpurified LPGs can contain up to 40% (v/v) unsaturates (butenes and propene).
Since the mid-1970s, concern as to the consequences of the release of volatile organochlorine and organobromine compounds such as chlorofluoro-carbon (CFC) refrigerants and aerosol propellants into the atmosphere has led to the phased withdrawal of many such compounds in some countries. Deodorized LPG and dimethyl ether (DME), which is often used as a nonflammable azeotrope with a (chloro)-fluorocarbon, have already largely replaced fully halogenated CFCs as aerosol propellants in some parts of the world. In the case of refrigerants, the move is to polyfluorinated compounds such as difluoromethane, pentafluoroethane, 1,1,1,2-tetra-fluoroethane, and 1,1,1-trifluoroethane (alone or as mixtures with other fluorocarbons) and, to a much lesser extent, perfluoropropane. Aliphatic hydrocarbons are also used to some extent as refrigerants. In addition, 1,1-dichloro-1-fluoroethane has been introduced as a degreasing and foam-blowing agent, and 1,1-difluoroethane and 1,1,1,2-tetrafluoroethane are sometimes used as aerosol propellants, either alone or as mixtures with other compounds.
It has often been stated that nail varnish or varnish remover (acetone and esters) may be abused by inhalation. However, properly documented examples of this practice have not come to light and it is probable that these compounds, although strong smelling, are too water-soluble to be intoxicants. For the same reason, acetone has never been used as an inhalational anesthetic. Diesel fuel, aviation fuel (kerosene, Avgas), white spirit, turpentine (or substitute) and paraffin are not sufficiently volatile to be abused by inhalation. Petrol (gasoline), on the other hand, is often abused, especially in less developed communities. Isobutyl and isopentyl (‘amyl’) nitrites are also inhaled in order to experience their vasodilator properties, sometimes by male homosexuals. In addition, those who ingest, or even more rarely inject, solvents or solvent-containing products, either accidentally or deliberately, and the victims of clinical, industrial and domestic accidents may be poisoned by the compounds under consideration. Finally, chloroform, diethyl ether and other volatiles are still used occasionally in the course of crimes such as rape and murder, while a further volatile compound, chloro-butanol (l,l,l-trichloro-2-methyl-2-propanol), sometimes employed as a sedative and a preservative, has been used in doping racing greyhounds.
Table 1 Some volatile substances which may be abused by inhalation
| Hydrocarbons | Pentafluoroethane (FC 125) |
| Aliphatic | Perfluoropropane (octafluoropropane, FC218) |
| Acetylene | Tetrachloroethylene (perchloroethylene) |
| Butane8 | 1,1,1,2-Tetrafluoroethane (FC 134a) |
| Isobutane (2-methylpropane)s | 1,1,1-Trichloroethane (methylchloroform, Genklene) |
| Hexanefe | 1,1,1-Trifluoroethane (FC 143a) |
| Propane8 | 1,1,2-Trichlorotrifluoroethane (FC 113) |
| Alicyclic/aromatic | Trichloroethylene (‘trike’, Trilene) |
| Cyclopropane (trimethylene) | Trichlorofluoromethane (FC 11, Freon 11) |
| Toluene (toluol, methylbenzene, phenylmethane) | |
| Xylene (xylol, dimethylbenzene)c | Oxygenated compounds and others |
| Mixed | Butanone (2-butanone, methyl ethyl ketone, MEK) |
| Petrol (gasoline)d | Butyl nitritef |
| Petroleum ethers6 | Enflurane [(R,S)-2-chloro-1,1,2-trifluoroethyl difluoromethyl ether] |
| Halogenated | Ethyl acetate |
| Bromochlorodifluoromethane (BCF, FC 12B1) | Desflurane [(R,S)-difluoromethyl 1,2,2,2-tetrafluoroethyl ether] |
| Carbon tetrachloride (tetrachloromethane) | Diethyl ether (ethoxyethane) |
| Chlorodifluoromethane (FC 22, Freon 22) | Dimethyl ether (DME, methoxymethane) |
| Chloroform (trichloromethane) | Isobutyl nitrite (‘butyl nitrite’)f |
| Dichlorodifluoromethane (FC 12, Freon 12) | Isoflurane [(R,S)-1-chloro-2,2,2-trifluoroethyl difluoromethyl ether] |
| 1,1-Dichloro-1-fluoroethane(FC 141b, Genetron 141b) | Isopentyl nitrite (3-methylbutan-1-ol, isoamyl nitrite, ‘amyl nitrite’)f> 9 |
| Dichloromethane (methylene chloride) | Methoxyflurane (2,2-dichloro-1,1-difluoroethyl methyl ether) |
| 1,2-Dichloropropane (propylene dichloride) | Methyl acetate |
| 1,1-Difluoroethane (FC 152a) | Methyl isobutyl ketone (MIBK, isopropyl acetone, 4-methyl-2-pentanone) |
| Difluoromethane (FC 32) | Methyl tert.-butyl ether (MTBE) |
| Ethyl chloride (monochloroethane) | Nitrous oxide (dinitrogen monoxide, ‘laughing gas’) |
| Halothane [(R,S)-2-bromo-2-chloro-1,1,1- | Sevoflurane [fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl ether] |
| trifluoroethane] | Xenon |
Diagnosis of Acute Poisoning with Volatile Substances
VSA should be suspected in children and adolescents with ‘drunken’ behavior, unexplained listlessness, anorexia and moodiness. The hair, breath and clothing may smell of solvent, and empty adhesive tubes or other containers, potato crisp bags, cigarette lighter refills or aerosol spray cans are often found. The smell of solvent on the breath is related to the dose and duration of exposure and may last for many hours. The so-called ‘glue-sniffer’s rash’ (perioral eczema) is probably caused by repeated contact with glue in a plastic or other bag held to the face. Although primarily a phenomenon of adolescence, it must be remembered that adults, especially those such as dentists, operating theatre personnel, and medical and dental students, with ready occupational access to abusable volatile compounds, also indulge in VSA. In the late 1970s, for example, it was estimated that some 1-1.6% of US dentists were abusing nitrous oxide.
The major risk from VSA is the possibility of sudden death. There have been at least 1596 (1394 male) VSA-related deaths (‘sudden sniffing deaths’) in the UK in the period 1971-1996 (Fig. 1), some 60% in adolescents aged 14-18 years. There have also been many reports of VSA-related sudden deaths in other parts of the world. Some deaths occur from ‘indirect’causes such as inhalation of vomit (14.8% of UK VSA-related deaths), asphyxia associated with the use of a plastic bag to contain the vapor being inhaled (11.5%), and trauma (11.7%). However, most UK deaths (53.3%) were attributed to ‘direct toxicity’. These effects could include anoxia, respiratory depression or vagal stimulation, all leading to cardiac arrest. Alternatively, direct cardiotoxicity is a possibility. Whatever the precise sequence of events leading to death, it is clear that ‘direct toxicity’is reported more commonly in deaths due to abuse of fuel gases, aerosol propellants and halogenated solvents than in deaths due to abuse of solvents from adhesives (Fig. 2).
Table 2 Some products which may be abused by inhalation
| Product | Major volatile components |
| Adhesives | |
| Balsa wood cement | Ethyl acetate |
| Contact adhesives | Butanone, hexane, toluene and esters |
| Cycle tyre repair cement | Toluene and xylenes |
| Polyvinylchloride (PVC) cement | Acetone, butanone, cyclohexanone, trichloroethylene |
| Woodworking adhesives | Xylenes |
| Aerosols | |
| Air freshener | LPG, DME and/or fluorocarbonsa |
| Deodorants, antiperspirants | LPG, DME and/or fluorocarbonsa |
| Fly spray | LPG, DME and/or fluorocarbonsa |
| Hair lacquer | LPG, DME and/or fluorocarbonsa |
| Paint | LPG, DME and/or fluorocarbonsa and esters |
| Anesthetics/analgesics | |
| Inhalational | Nitrous oxide, cyclopropane, diethyl ether, halothane, enflurane, desflurane, |
| isoflurane, methoxyflurane, sevoflurane, xenon | |
| Topical | Ethyl chloride, fluorocarbonsa |
| Dust removers (‘air brushes’) | DME, fluorocarbonsa |
| Commercial dry cleaning and degreasing agents | Dchloromethane, FC 113, FC 141b, methanol, 1,1,1-trichloroethane, |
| tetrachloroethylene, toluene, trichloroethylene (nowvery rarely carbon | |
| tetrachloride, 1,2-dichloropropane) | |
| Domestic spot removers and dry cleaners | Dichloromethane, 1,1,1-trichloroethane, tetrachloroethylene, |
| trichloroethylene | |
| Fire extinguishers | BCF, FC 11, FC 12 |
| Fuel gases | |
| Cigarette lighter refills | LPG |
| ‘Butane’ | LPG |
| ‘Propane’ | Propane and butanes |
| Paints/paint thinners | Acetone, butanone, esters, hexane, toluene, trichloroethylene, xylenes |
| Paint stripper | Dichloromethane, methanol, toluene |
| Racing fuel super-charge tanks | Nitrous oxide |
| ‘Room odorizer’ | Isobutyl nitrite |
| Surgical plaster/chewing gum remover | 1,1,1-Trichloroethane, trichloroethylene |
| Typewriter correction fluids/thinners (some) | 1,1,1-Trichloroethane |
| Whipped cream dispensers | Nitrous oxide |

Figure 1 UK VSA-related deaths, 1971-1996 (n = 1596).
The analytical toxicology laboratory may be asked to perform analyses for solvents and other volatile compounds in biological samples and related specimens to:
• assist in the diagnosis of acute poisoning;
• confirm a suspicion of chronic VSA in the face of denial from the patient and/or a caretaker;
• aid the investigation of deaths where poisoning by volatile compounds is a possibility, including deaths associated with anesthesia;
• aid investigation of rape or other assault, or other offence such as driving a motor vehicle or operating machinery, which may have been committed under the influence of volatile substances;
• aid investigation of incidents such as rape or other assault in which volatile substances may have been administered to the victim;
• help investigate fire or explosion where VSA might have been a contributory factor; and
• assess occupational or environmental exposure to anesthetic or solvent vapor. However, other techniques such as ambient air monitoring or, in a few instances, the measurement of urinary metabolite excretion may be more appropriate in this latter context.
The laboratory analysis of volatile substances presents particular problems. Firstly, many of the compounds of interest occur commonly in laboratories and this necessitates special precautions against contamination and interference. Secondly, collection, storage and transport of biological samples must be controlled as far as practicable in order to minimize loss of analyte: quantitative work is futile if very volatile compounds such as propane are encountered unless special precautions are taken to prevent the loss of analyte from the sample prior to the analysis. Thirdly, many compounds of interest are excreted unchanged via the lungs and thus blood (and/or other tissues in fatalities), and not urine, is usually the sample of choice. Finally, the interpretation of results can be difficult, especially if legitimate exposure to solvent vapor is a possibility.
A diagnosis of VSA should be based on a combination of circumstantial, clinical and analytical evidence rather than on any one factor alone. It is especially important to consider all circumstantial evidence in cases of possible VSA-related sudden death, as suicide or even homicide cannot be excluded simply on the basis of the toxicological examination. There have been a number of reports of the use of inhalational anesthetics for suicidal purposes, for example, and there has been one example in the UK of a serial murderer whose victims were thought initially to have died as a result of VSA.
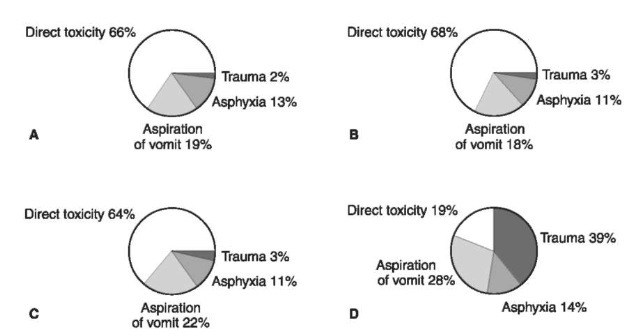
Figure 2 UK VSA-related deaths by mechanism and by type of product abused. (Source Taylor ef a/., 1998.) (A) Aerosol propellants; (B) fuel gases; (C) chlorinated and other solvents; (D) solvents from adhesives.
Analytical Methods
The analysis of biological samples for solvents and other volatiles which may be abused by inhalation has similarities to the analysis of methanol, ethanol and 2-propanol. However, poisoning with these latter compounds is normally the result of ingestion or occupational exposure to vapour. Gas chromatography (GC) with flame ionization and/or electron capture detection (FID and/or ECD) is widely used in the analysis of volatiles in blood and other biological specimens which may be obtained without using special apparatus such as breath-collection tubes. Nitrous oxide and most halogenated compounds respond on the ECD, although the thermal conductivity detector (TCD) may be used as an alternative if nitrous oxide poisoning is suspected. Although not reported as yet, abuse of xenon is a possibility. GC methods for this element would require TCD or mass spectrometry (MS). Direct MS of expired air can also detect many compounds several days postexposure. However, at present the use of this technique is limited by the need to take breath directly from the patient. Vapour-phase infrared (IR) spectrophotometry may be useful in the analysis of abused products or ambient atmospheres.
If the analyte is very volatile (e.g. propane or butane) and a quantitative analysis is required, a blood sample should be collected directly into the headspace vial in which the analysis will be carried out. Many other volatile compounds are relatively stable in blood and other tissues if simple precautions are taken. In the case of blood, the container used for the sample should be glass, preferably with a cap lined with metal foil; greater losses may occur if plastic containers are used. The tube should be as full as possible and should only be opened when required for analysis, and then only when cold (4°C). If the sample volume is limited it is advisable to select the container to match the volume of blood so that there is minimal headspace. An anticoagulant (sodium ethylenediamine tetra-acetate (EDTA) or lithium heparin) should be used. Specimen storage between —5 and 4°C is recommended and 1% (w/v) sodium fluoride should be added to minimize esterase and other enzymic activity. Tissues (approximately 10 g each of brain, lung, liver, kidney and subcutaneous fat) should also be obtained, if a necropsy is to be performed, in addition to standard toxicological specimens if available (femoral blood,urine, stomach contents, vitreous humor). Tissues should be stored before analysis in the same way as blood. No preservative should be added. Products thought to have been abused or otherwise implicated in the incident (and stomach contents if in-gestion is suspected) should be packed, transported and stored entirely separately from (other) biological specimens to avoid crosscontamination. Investigation of deaths occurring during or shortly after anesthesia should include the analysis of the inhalation anesthetic(s) used in order to exclude an administration error.
Gas chromatography
A summary of GC methods for volatile compounds in biological specimens published since 1989 is given in Table 3. Packed columns, for example 2 m x 2 mm internal diameter (i.d.) 0.3% (w/w) Carbowax 20M on Carbopack C programmed from 35 to 175°C, have been used extensively in conjunction with head-space sample preparation. On-column septum injections of up to 400 ul headspace can be performed and good sensitivity (of the order of 0.1mgl —1 or better using 200 ul of sample) can be obtained. Disadvantages include the poor resolution of some very volatile substances, a long total analysis time, and variation in the peak shape given by alcohols between different batches of column packing. Porous layer open tubular (PLOT) columns give good retention, and thus resolution, of compounds with similar relative formula mass, but peak shapes of polar compounds are poor and it is difficult to screen for compounds of widely different volatility in one analysis.
Bonded-phase wide-bore capillary columns permit relatively large volume septum injections and can offer advantages of improved efficiency, reproducibility and reliability. A 60 m x 0.53 mm i.d. fused silica capillary coated with the dimethylpolysiloxane SPB-1 (5 um film thickness) programmed from 40 to 200°C offers many advantages over packed column and PLOT systems. Improved resolution of very volatile compounds is obtained and, even with an initial temperature of 40°C, the total analysis time can be reduced to 26 min, and good peak shapes can be obtained even for alcohols (Fig. 3). Moreover, split-less septum injections of up to 300 ul headspace can be performed with no noticeable effect on column efficiency; hence, sensitivity is as least as good as that attainable with a packed column.
The use of a capillary column together with two different detectors (FID and ECD) confers a high degree of selectivity, particularly for low formula mass compounds where there are very few alternative structures. If more rigorous identification is required,GC combined with MS or Fourier transform IR spectrometry (FTIR) may be used. However, GC-MS can be difficult when the fragments produced are less than m/z 40, particularly if the instrument is used for other purposes as well as solvent analyses. In particular, the available sensitivity and spectra of the low molecular weight alkanes renders them very difficult to confirm by GC-MS. Inertial spray MS allows introduction of biological fluids directly into the MS without prior chromatographic analysis and has been used in the analysis of halothane in blood during anesthesia.
Table 3 Summary of gas chromatographic methods for volatile compounds in biological fluids published 1989-1997
| Application | Sample | Extraction8 | GC column and/or conditions | Detection | LoDfe |
| Anesthetics | Blood (0.6 ml) | HS | 15% Apiezon L, 80/100 mesh Chromosorb W, 2 m x 4 mm i.d., 130°C | FID | |
| Toxicology | Blood, tissue | HS | DB-1701,40m x 0.25mm i.d., 1.0 urn film, 30-120°C | MS (ion trap) | |
| Toxicology | Blood, tissue (1 g) | HS | 15% Carbowax 1500, 80/100 mesh Chromosorb W NAW, 1.8 m x 0.32 mm i.d., 70°C |
FID | |
| Toxicology | Blood, tissue | HS | 30% Carbowax 20 M, 60/80 mesh Chromosorb W AW-DMCS, 2m, 70°C |
FID | |
| Anesthetics | Blood (1 ml) | HS | Porapak S, 80/100 mesh, 1.9m x 2mm i.d., 165°C | FID | |
| Environmental | Blood (10ml) | PT | DB-624, 30m x 0.32mm i.d., 1.8 um film, 0-190°C | MS | 0.05 ugr1c |
| Toxicology | Blood (0.2 ml), tissue | HS | SPB-1, 60 m x 0.32 mm i.d., 5 um film, 40-200°C | FID, ECD | |
| Toxicology | Blood (1 ml), tissue (1 g) |
HS | 0.2% Carbowax 1500, 80/100 mesh CarbopakC, 1.8 m, 100°C | FID | |
| Toxicology | Blood (1.5ml) | HS | DB-1, 30m x 0.25mm i.d., 1.0um film, 40-250°C | MS (ion trap) | |
| Workplace | Urine (0.5-1 ml) | HS | Porapak Q, 80-100 mesh, | MS | 0.7 ugr1d |
| monitoring | 2m x 1.8mm i.d., 100°C HP-5, 25m x 0.2mm i.d., 25°C | MS | 0.1 ugr1 | ||
| Toxicology | Blood, CSF (1 ml) | HS | Porapak Q, 80/100 mesh, 1m x 2.5mm i.d., 50-170°C | MS | 0.02 mgl-1e |
| Workplace | Urine (50ml) | HS | DB-5, 30m x 0.25mm i.d., 40°C | MS | |
| monitoring | |||||
| Toxicology | Blood, plasma, serum (1-3 ul) | PH PH PH | GS-Q, 30m x 0.53mm i.d., 70- 230°C (screening) Porapak Q, 1 m x 2.6mm i.d., 180°C (screening) DB-17, 15 m x 0.53 mm i.d., 1.0 um film, 50°C (quantification) |
MS FID MS | |
| Anesthetics | Blood (3 nl) | PH | GS-Q, 30m x 0.53mm i.d., 160°C | MS | 0.2mgr1 |
| Blood (0.5ml) | HS | DB-1, 30m x 0.53mm i.d., 5um film, 60°C | MS | ||
| Toxicology | Blood (0.5-5 ml) | PT | PoraPLOT Q, 25 m x 0.32 mm i.d., 10 um film, 30-250°C | FTIR, FID | 0.05 mgr1e |
| Toxicology | Blood, brain (35 mg) | HS-FE | DB-1, 30m x 0.25mm i.d., 1.0um film, 40-250°C | MS (ion trap) |
GC-FTIR is generally more appropriate than GC-MS in the analysis of volatiles, but sensitivity is poor, particularly when compared with the ECD. In addition, interference, particularly from water and carbon dioxide in the case of biological specimens, can be troublesome. ‘Purge and trap’and multiple headspace extraction offer ways of increasing sensitivity and, although not needed for most clinical and forensic applications, have been used either in conjunction with GC-FTIR, or in occupational/environmental monitoring. Pulse heating has also been employed in the analysis of volatiles in biological specimens (Table 3). Advantages of this latter technique include use of a small sample volume (0.5-5 ul), short extraction time and lack of matrix effects.
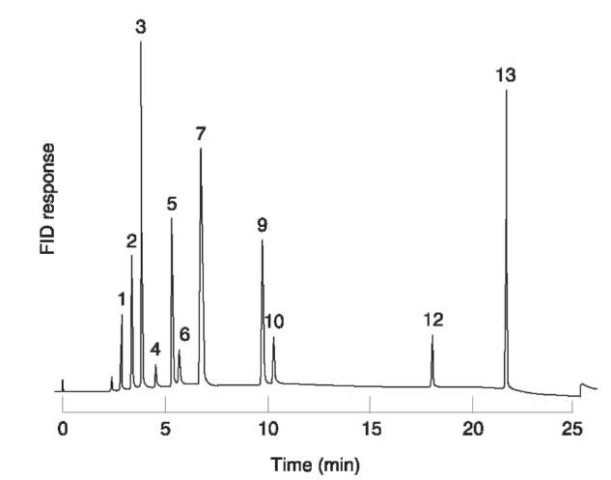
Figure 3 Analysis of a whole blood sample obtained post mortem from a patient who had inhaled the contents of a cigarette lighter refill. Sample preparation: internal standard solution (ethylbenzene and 1,1,2-trichloroethane (approximately 25 and 10mgl-1, respectively) in outdated blood-bank whole blood) (200 ul) incubated (65°C, 15min) with specimen (200 ul) in a sealed 10ml glass septum vial. Chromatographic conditions: column: 60m x 0.53mm i.d. SPB-1 (5um film). Oven temperature: 40°C (6min), then to 80°Cat5°Cmin-1, then to 200°Cat 10°Cmin-1 Injection: 300 ul headspace. 1, Propane; 2, iso-butane; 3, butane; 4, ethanol; 5, acetone; 6, 2-propanol; 7, 2-methyl-2-propanol; 9, butanone; 10, 2-butanol; 12, 1,1,2-tri-chloroethane (IS); 13, ethylbenzene (IS).
Pharmacokinetics and the Interpretation of Results
Knowledge of the pharmacokinetics of volatile compounds is important in understanding the rate of onset, the intensity and the duration of intoxication with these substances. The UK maximum exposure limit (MEL) or occupational exposure standard (OES) (Table 4) provide information on the relative toxicities of different compounds after chronic exposure to relatively low concentrations of vapor. Inhaled compounds may rapidly attain high concentrations in well-perfused organs (brain, heart),whereas concentrations in muscle and adipose tissue may be very low. Should death occur, this situation is ‘frozen’to an extent, but, if exposure continues, the compound will accumulate in less accessible (poorly perfused) tissues, only to be slowly released once exposure ceases. Thus, the plasma concentrations of some compounds may fall monoexponentially, while others may exhibit two (or more) separate rates of decline (half-lives).
The solubility of a volatile compound in blood is an important influence on the rate of absorption, tissue distribution and elimination of the compound. The partition coefficients of a number of compounds between air, blood and various tissues have been measured in vitro using animal tissues, and some in vivo distribution data have been obtained from postmortem tissue measurements in humans (Table 4). However, these latter data must be used with caution, as there are many difficulties inherent in such measurements (sampling variations, analyte stability, external calibration, etc.). Published data on the elimination half-lives of volatile substances (Table 4) are not easily comparable, either because too few samples were taken or the analytical methods used did not have sufficient sensitivity to measure the final half-life accurately.
Metabolism of volatile substances
Exogenous compounds may be metabolized in a number of ways, a frequent result being the production of metabolites of greater polarity (water solubility) and thus lower volatility than the parent compound. The pharmacological activity and phar-macokinetics of any metabolite(s) often differ from those of the parent compound(s). After ingestion, extensive hepatic metabolism can reduce systemic availability (‘first-pass’metabolism). Many volatile substances, including butane, dimethyl ether, most fluorocarbon refrigerants/aerosol propellants, isobu-tane, nitrous oxide, propane, tetrachloroethylene and 1,1,1-trichloroethane, are largely eliminated unchanged in exhaled air. Others are partly eliminated in exhaled air and also metabolized in the liver and elsewhere, the metabolites being eliminated in exhaled air or in urine (Table 5), or incorporated into intermediary metabolism.
Interpretation of qualitative results
The likelihood of detecting exposure to volatile substances by headspace GC of blood is influenced by the dose and duration of exposure, the time of sampling in relation to the time elapsed since exposure, and the precautions taken in the collection and storage of the specimen. In a suspected VSA- or anesthetic-related fatality, analysis of tissues (especially fatty tissues such as brain) may prove useful, as high concentrations of volatile compounds may be present even if very little is detectable in blood.
Table 4 Physical properties and pharmacokinetic data of some volatile compounds
| Compound | MEL/OES | Vapour pressure | Inhaled dose | Proportion absorbed dose (%) | Half-life | Braimblood | Partition coefficient | |
| (mgm~3f | (20 °C, mmHgf | absorbed (%) | Eliminated unchanged | Metabolized | distribution ratio (deaths) | (blood-.gas) (37 °C) | ||
| Acetone | 1810 | 183 | — | — | — | 3-5d | — | 243-300 |
| Benzene | 16 | 75 | 46 | 12 | 80 | 9-24 | 3-6 | 6-9 |
| Butane | 1750s | (1554) | 30-45 | - | - | - | - | - |
| Isobutane | 1750s | (2282) | - | - | - | - | - | - |
| Butanone | 600 | 75 | 70 | 99+ | 0.1 | 0.5 | - | 116 |
| Carbon disulfide | 32 | 294 | 40 | <30 | 50-90 | < 1 | - | 2.4 |
| Carbon tetrachloride | 13 | 90 | - | 50? | 50? | 48 | - | 1.6 |
| Chlorodifluoromethane | 3590 | (6701) | - | - | - | - | 1.9 | - |
| Chloroform | 9.9 | 157 | - | 20-70 (8 h) | >30 | - | 4 | CO |
| Cyclopropane | - | (4701) | - | 99 | 0.5 | - | 1.5-3.6 | 0.55 |
| Desflurane | - | 669 | - | - | 0.02 | - | 1.29f | 0.42 |
| Dichlorodifluoromethane | 5030 | (3639) | 35 | 99 | < 0.2 | - | 1.4 | 0.15 |
| Dichloromethane | 350 | 350 | - | 50? | <40 | 0.7 | 0.5-1 | 5-10 |
| Diethyl ether | 1230 | 438 | - | >90 | - | - | 1.1 | 12 |
| Enflurane | 383 | 172 | 90+ | >80 (5 days) | 2.5 | 36 | 1.4s | 1.9 |
| Ethyl acetate | 1460 | 72 | - | - | - | - | - | - |
| Halothane | 82 | 244 | 90+ | 60-80 (24 h) | <20 | 2-3 | 2-3 | 2.57 |
| Hexane | 72 | 122 | - | - | - | - | - | - |
| Isoflurane | 383 | 240 | - | - | 0.2 | - | 1.57f | 1.38 |
| Methoxyflurane | - | 23 | - | 19 (10 days) | >44 | - | 2-3 | 13 |
| Methyl isobutyl ketone | 208 | 15 | - | - | - | - | - | - |
| Nitrous oxide | 183 | (39800) | - | >99 | - | - | 1.1 | 0.47 |
| Propane | 1750s | (6269) | - | - | - | - | - | - |
| Sevoflurane | - | 157 | - | - | 3 | 20 | 1.7′ | 0.68 |
| Styrene | 430 | 4 | - | 1-2 | >95 | 13 | - | 32 |
| Tetrachloroethylene | 345 | 14 | 60+ | >90 | 1-2 | 72 | 9-15 | 9-19 |
| Toluene | 50 | 22 | 53 | <20 | 80 | 7.5 | 1-2 | 8-16 |
| 1,1,1 -Trichloroethane | 1110 | 98 | - | 60-80 (1 week) | 2 | 10-12 | 2 | 1-3 |
| Trichloroethylene | 550 | 58 | 50-65 | 16 | >80 | 30-38 | 2 | 9.0 |
| Trichlorofluoromethane | 5710 | 667 | 92 | 89 | < 0.2 | 1.5 | 2.5 | 0.87 |
| ‘Xylene’ | 441 | 6 | 64 | 5 | >90 | 20-30 | - | 42.1 |
Table 5 Summary of the metabolism of some solvents and other volatile substances
| Compound | Principal metabolites (% absorbed dose) | Notes |
| Acetone | 2-Propanol (minor) and intermediary | Endogenous compound produced in large amounts in |
| metabolites (largely excreted unchanged | diabetic or fasting ketoacidosis; also the major | |
| at higher concentrations) | metabolite of 2-propanol in man | |
| Acetonitrile | Inorganic cyanide (at least 12%) thence to | Cyanide/thiocyanate may accumulate during chronic |
| thiocyanate | exposure | |
| Benzene | Phenol (51-87%), catechol (6%), | Excreted in urine as sulfate and glucuronide conjugates. |
| hydroquinone (2%), trans,trans-muconic | Urinary phenol excretion has been used to indicate | |
| acid | exposure but is variable and subject to interference | |
| Bromomethane | Inorganic bromide | Serum bromide has been used to monitor exposure, |
| although the concentrations associated with toxicity | ||
| are much lower than when bromide itself given orally | ||
| Butanone | 3-Hydroxybutanone (0.1%) | 3-Hydroxybutanone excreted in urine. Most of an |
| absorbed dose of butanone excreted unchanged in | ||
| exhaled air | ||
| Carbon disulfide | 2-Mercapto-2-thiazolin-5-one, 2- | 2-Mercapto-2-thiazolin-5-one glycine conjugate and |
| thiothiazolidine-4-carboxylic acid | TCCA glutathione conjugate of carbon disulphide. | |
| (TCCA), thiourea, inorganic sulfate and | Urinary TCCA excretion reliable indicator of exposure | |
| others | ||
| Carbon tetrachloride | Chloroform, carbon dioxide, | Trichloromethyl free radical (reactive intermediate) |
| hexachloroethane and others | probably responsible for hepatorenal toxicity | |
| Chloroform | Carbon dioxide (up to 50%), diglutathionyl | Phosgene (reactive intermediate) depletes glutathione |
| dithiocarbonate | and is probably responsible for hepatorenal toxicity | |
| Cyclohexanone | Cyclohexanol, trans-1,2-cyclohexanediol, | Metabolites excreted mainly as glucuronides in adults |
| trans-1,4-cyclohexanediol | ||
| Desflurane | Trifluoroacetic acid (< 0.02%), inorganic | - |
| fluoride | ||
| Dichloromethane | Carbon monoxide (+35%) | CO blood half-life 13 h breathing air (atmospheric |
| pressure) (CO half-life 5 h after inhalation of CO itself). | ||
| Blood carboxyhemoglobin measurement useful | ||
| indicator of chronic exposure | ||
| Dimethylsulfoxide | Dimethylsulfide (3%), dimethylsulfone (18- | After oral/dermal administration, dimethyl sulfide |
| 22%) | excreted in exhaled air and dimethylsulfone in urine | |
| Dioxane | p-Hydroxyethoxyacetic acid (HEAA) | HEAA excreted in urine |
| Enflurane | Difluoromethoxydifluoroacetic acid | - |
| (>2.5%), inorganic fluoride | ||
| Ethyl acetate | Ethanol, acetic acid | Rapid reaction catalyzed by plasma esterases |
| Ethylbenzene | Methylphenylcarbinol (5%), mandelic acid | Methylphenylcarbinol excreted in urine as conjugate, |
| (64%), phenylglyoxylic acid (25%) | others as free acids. Mandelic acid excretion has been | |
| used to monitor ethylbenzene exposure | ||
| Halothane | 2-Chloro-1,1,1-trifluoroethane, 2-chloro- | The formation of reactive metabolites may be important |
| 1,1-difluoroethylene, trifluoroacetic acid, | in the etiology of the hepatotoxicity (‘halothane | |
| inorganic bromide and others | hepatitis’) which may occur in patients re-exposed to | |
| halothane or similar compounds | ||
| Hexane | 2-Hexanol, 2-hexanone, 2,5-hexanedione | Hexan-2-ol excreted in urine as glucuronide. |
| 2,5-Hexanedione thought to cause neurotoxicity. | ||
| Methyl butyl ketone also neurotoxic and also | ||
| metabolized to 2,5-hexanedione | ||
| Isobutyl nitrite | 2-Methyl-1-propanol (99%+), inorganic | Parent compound not normally detectable in blood. |
| nitrite | Blood methemoglobin can be used to monitor | |
| exposure | ||
| Isoflurane | Trifluoroacetic acid (< 0.2%), inorganic | - |
| chloride, inorganic fluoride | ||
| Isopentyl nitrite | 3-Methyl-1 -butanol (99%+), inorganic nitrite | Parent compound not normally detectable in blood. |
| Blood methemoglobin can be used to monitor | ||
| exposure | ||
| Methanol | Formaldehyde (up to 60%), formic acid | Urinary formic acid excretion has been advocated for |
| monitoring methanol exposure | ||
| Methoxyflurane | Methoxydifluoroacetic acid, dichloroacetic | Abuse has been manifested as chronic fluoride |
| acid, oxalic acid, inorganic fluoride | poisoning (fluorosis) | |
| 2-Propanol | Acetone (80-90%) thence others | 2-Propanol blood half-life + 2 h, acetone half-life + 22 h. |
Table 5 (continued)
| Compound | Principal metabolites (% absorbed dose) | Notes |
| Sevoflurane | 1,1,1,3,3,3-Hexafluoropropanol (<3%), | 1,1,1,3,3,3-Hexafluoropropanol excreted as glucuronide |
| inorganic fluoride | in urine | |
| Styrene | Mandelic acid (85%) and phenylglyoxylic | Urinary mandelic acid excretion indicates exposure. |
| acid (10%); hippuric acid may be minor | Ethanol inhibits mandelic acid excretion | |
| metabolite | ||
| Tetrachloroethylene | Trichloroacetic acid (< 3%) | Urinary trichloroacetic acid excretion serves only as qualitative index of exposure |
| Toluene | Benzoic acid (80%) and ortho-, meta- and | Benzoic acid largely conjugated with glycine giving |
| para-cresol (1%) | hippuric acid which is excreted in urine (blood half-life 2-3 h). Not ideal index of exposure since there are other (dietary) sources of benzoic acid | |
| 1,1,1-Trichloroethane | 2,2,2-Trichloroethanol (2%) and | Urinary metabolites serve as qualitative index of |
| trichloroacetic acid (0.5%) | exposure only (compare tetrachloroethylene) | |
| Trichloroethylene | 2,2,2-Trichloroethanol (45%) and | Trichloroethanol (glucuronide) and trichloroacetic acid |
| trichloroacetic acid (32%) | excreted in urine (blood half-lives about 12 and 100 h respectively). Trichloroacetic acid excretion can indicate exposure | |
| Xylenes | Methylbenzoic acids (95%) and xylenols | Methylbenzoic acids conjugated with glycine and urinary |
| (2%) | methylhippuric acid excretion used as index of exposure – no dietary sources of methylbenzoates. |
Analysis of metabolites in urine may extend the time in which exposure may be detected but, of the compounds commonly abused, only toluene, the xylenes and some chlorinated solvents, notably tri-chloroethylene, have suitable metabolites (Table 5). Chronic petrol ‘sniffing’has been diagnosed by the measurement of blood lead concentrations or detection of aromatic components such as toluene and ethylbenzene. Abuse of the fluorinated anesthetic methoxyflurane has been detected by measuring serum and urine fluoride ion concentrations. With some petrols and other complex mixtures such as petroleum ethers (Table 1), however, the blood concentrations of the individual components are often below the limit of detection of headspace GC methods even after significant inhalational exposure.
Detection of a volatile compound in blood does not always indicate VSA or occupational/environmental exposure to solvent vapor. Acetone and some of its homologs may occur in high concentrations in ketotic patients. Large amounts of acetone and butanone may also occur in blood and urine from children with acetoacetylcoenzyme A thiolase deficiency, for example, and may indicate the diagnosis. In addition,acetone is the major metabolite of 2-propanol in humans (Table 5). Conversely, 2-propanol has been found in blood from ketotic patients. Other ketones may also give rise to alcohols in vivo. Cyclohexanol, for example, is the principal metabolite of cyclo-hexanone in humans (Table 5). Other volatile compounds, such as halothane or chlorobutanol, may be used in therapy or inadvertently added to the sample as a preservative. When interpreting the results of qualitative analyses it is important to remember that some compounds often occur in association one with another (Table 6).
Use of aerosol disinfectant preparations when collecting specimens may contaminate the sample if an aerosol propellant is used. Contamination of blood samples with ethanol or 2-propanol may also occur if an alcohol-soaked swab is used to cleanse skin prior to venepuncture. Gross contamination with technical xylene (a mixture of o-, m- and p-xylene together with ethylbenzene) has been found in blood collected into Sarstedt Monovette serum gel blood collection tubes; contamination with toluene (up to 22mgl_1), 1-butanol, ethylbenzene and xylene has been found in more recent batches of these same tubes. Contamination with 1-butanol or 2-methyl-2-propanol occurs commonly in blood collected into tubes coated with EDTA. Care should be taken when handling frozen tissue prior to analysis as any compounds present in ambient air may condense on the cold surface and give rise to false positives. Processing blank frozen tissue can control for this possibility.
Table 6 Associated volatile compounds
| Compound | Associated compound(s) |
| Acetone | Butanone and higher ketones in ketoacidosis, 2-propanol (metabolite, rare) |
| BCF | FC 11 |
| Butane | Butanone (metabolitea), isobutane, 2-butanol (metabolitea), propane |
| Cyclohexanone | Cyclohexanol (metabolite) |
| Dimethyl ether | FC 22 or other fluorocarbon |
| Ethanol | Propanols and higher alcohols if bacterial fermentation has occurred; methanol or other volatile poisons if |
| denatured alcohol has been consumed | |
| Ethyl acetateb | Ethanol (metabolite) |
| Ethylbenzene | (see Xylenes below) |
| FC 11 | BCF, FC 12 |
| FC 12 | FC 11 |
| FC 22 | Dimethyl ether |
| Halothane | 2-Chloro-1,1-difluoroethylene, 2-chloro-1,1,1-trifluoroethane (metabolitesa) |
| Isobutane | Butane, 2-methyl-2-propanol (metabolitea), propane |
| Isobutyl nitriteb | 2-Methyl-1-propanol (degradation product) |
| Isopentyl nitriteb | 3-Methyl-1-butanol (degradation product) |
| Methyl acetateb | Methanol (metabolite) |
| Propane | Butane, isobutane, 2-propanol (metabolitea) |
| 2-Propanol | Acetone (metabolite) |
| 1,1,1-Trichloroethane | Isopropyl nitrate (stabilizer3) |
| 2,2,2-Trichloroethanol | Trichloroethylene (also metabolite of chloral hydrate, dichloralphenazone and triclofos) |
| Trichloroethylene | 2,2,2-Trichloroethanol (metabolite), chloroform [possibly from thermal degradation of trichloroacetic acid |
| (metabolite) in vitro] | |
| Xylenes | ortho-, meta- and para-Xylene occur together in technical xylene, m-xylene predominating. Ethylbenzene |
| also contaminant in technical xylene |
The interpretation of case data involving chloroform is particularly difficult, especially as this compound is still sometimes used in the course of crimes such as rape and murder. In addition to sometimes being present in drinking water at low concentrations, chloroform is found in a variety of medicinal preparations, in cigarette smoke, soft drinks, margarines, and in swimming pools if a chlorination plant is in operation. A further possible source of chloroform on headspace GC is from thermal decomposition of trichloroacetic acid. Trichloroacetic acid is a metabolite of several compounds, including the solvent tri-chloroethylene (Table 5) and the drugs chloral hydrate, dichloralphenazone and triclofos. Trichloro-acetic acid has a half-life in blood of 3-5 days and thus may be detected for a relatively long time after exposure to, or ingestion of, a precursor. Trichloro-acetic acid plasma concentrations of up to 40mgl —1 have been reported after occupational exposure to trichloroethylene vapor.
In 25 Caucasian adult women in Florida, USA, over a period of 6 months average plasma chloroform concentrations were generally less than 25 ugl —1 but in two subjects plasma chloroform concentrations of 2.9 and 4.0 mgl-1, respectively, were found during routine sampling. All subjects were carefully screened to exclude occupational and recreational exposure to chloroform and other compounds that could give rise to chloroform on headspace GC. At the other extreme, postmortem blood chloroform concentrations in fatalities involving this agent have been reported as 10-50mgl_1.
It is well known that ethanol may be both produced and metabolized by microbial action in biological specimens. Small amounts of hexanal may arise from degradation of fatty acids in blood on long-term storage, even at — 5to — 20°C. Hexanal is resolved from toluene on the SPB-1 capillary GC system discussed above, but resolution may be lost if an isothermal quantitative analysis is performed. Interference from hexanal is only likely to be important, however, if very low concentrations of toluene (0.1 mgl —1 or less) are to be measured.
In some deaths attributed to the abuse of LPG, only butane, isobutane and propane are detected on head-space GC of postmortem samples. In other cases these three compounds are present, but in addition 2-pro-panol, acetone, 2-methyl-2-propanol, 2-butanol and/or butanone are present (Fig. 3). By analogy with the metabolism of hexane (Table 5), these latter compounds probably arise from the metabolism of the butanes and propane (Fig. 4).
The alkyl nitrites which can be abused by inhalation (isobutyl nitrite, isopentyl nitrite) are a special case in that: (1) they are extremely unstable and break down rapidly in vivo to the corresponding alcohols (Fig. 5); and (2) usually also contain other isomers (butyl nitrite, pentyl nitrite). Any products submitted for analysis will usually contain the corresponding alcohols as well as the nitrites.
Interpretation of quantitative results
Data to aid the interpretation of quantitative results in individual cases for a range of solvents, metabolites, etc. are given in Table 7. There may be a big overlap in the blood concentrations of volatile compounds attained after workplace exposure and as a result of deliberate inhalation of vapor. In the occupational setting, blood toluene concentrations after exposure to up to 127 p.p.m. toluene (UK occupational exposure limit at the time was 100 p.p.m.) for 8h ranged between 0.4 and 6.7mgl-1. After brief exposure only signs of moderate intoxication (e.g. slurred speech, unsteady movements) have been associated with blood toluene concentrations as high as 30 mgl-1. Blood toluene concentrations in samples from 132 patients who were thought to have engaged in VSA ranged from 0.2 to 70mgl-1, and were above 5 mgl-1 in 22 of 25 deaths. On the other hand, 13 patients with blood toluene concentrations greater than 10mgl-1 were either asymptomatic or only mildly intoxicated (headache, nausea, vomiting and/or drowsiness), although these manifestations of toxicity can lead to ‘indirect’acute VSA-related death, as discussed above. Aside from individual differences in tolerance and possible loss of toluene from the sample prior to analysis, sample contamination, etc., the lack of a strong correlation between blood concentrations and clinical features of poisoning is probably due to rapid initial tissue distribution and elimination.

Figure 4 Summary of the metabolism of butane, isobutane and propane.

Figure 5 Breakdown of alkyl nitrites in humans. Note: pentyl = amyl.
Some 80% of a dose of toluene is converted to hippuric acid (Table 5), which is excreted in urine. Similarly, more than 90% of a dose of xylene is metabolized to methylhippuric (toluric) acids. The principal isomer found in urine is 3-methylhippurate, as m-xylene is the principal component of technical grade xylene (Table 1). Methylhippurates are not normal urinary constituents, but hippuric acid may arise from the metabolism of benzoates in foods and medicines, and thus caution is needed in the interpretation of results. Hippurate and methylhippurate excretion is often expressed as a ratio to creatinine, as this obviates the need for 24 h urine collections. Occupational exposure to toluene can give rise to ratios of up to 1 g hippurate per gram creatinine, or more; in patients suspected of VSA a ratio of more than 1 g hippurate per gram creatinine strongly suggests, but does not prove, toluene exposure. Measurement of urinary o-cresol has been proposed as an alternative means of monitoring toluene exposure selectively, particularly in occupational circumstances, but the assay procedure is relatively complex and is thus not widely used.
After exposure to 350 p.p.m. 1,1,1-trichloroethane (UK maximum exposure limit at the time) for 1 h, the mean blood 1,1,1-trichloroethane concentration was 2.6mgl-1. Blood 1,1,1-trichloroethane concentrations ranged from 0.1 to 60mgl-1 in samples from 66 patients suspected of VSA, 29 of whom died. There was a broad relationship between blood 1,1,1-trichloroethane concentration and the severity of poisoning, but as in the case of toluene there was a big overlap between the blood concentrations encountered in fatalities and those attained after occupational exposure.