Introduction
Alcoholic beverages contain besides ethanol up to 800 compounds which are responsible for their specific taste and smell. The most important compounds are the congener alcohols. These are methanol and some higher aliphatic alcohols known as fusel alcohols. In different classes of beverages, such as beer or whisky, there are characteristic qualitative and quantitative differences in the content of these alcohols. Modern analytical methods allow their detection in the blood of drunken drivers. When pharmacokinetic and metabolic peculiarities are taken into account, congener findings can be useful for the verification or refutation of allegations concerning the alcohol intake.
Congener Content of Alcoholic Beverages
Most of the flavor compounds of alcoholic beverages are present in extremely low concentrations (Table 1), but some can be found in sufficient quantities to allow detection of them in the blood of consumers. These are the congener alcohols: methanol, propanlol, butanlol, butan-2-ol, isobutanol, 2-methylbutan-l-ol and 3-methylbutan-l-ol. Methanol is a breakdown product of the vegetable pectin. The higher aliphatic alcohols are byproducts of the fermentation process and have their origin in amino acids whose amino groups are needed for the nitrogen supply of yeast. As the higher aliphatic alcohols were originally extracted from fusel oils (i.e. the third phase of the distillation procedure) they are also called fusel alcohols.
There are characteristic differences in the congener content of alcoholic beverages (Table 2). Fermented beverages such as beer, wine, rice wine etc. contain only small amounts of methanol, propan-l-ol, isobutanol and of the isoamyl alcohols. Distilled spirits can be nearly free of congener alcohols (e.g. gin, vodka), or contain the same congener alcohols as fermented beverages, but in different, sometimes very high, quantities. Other spirits, such as fruit brandies are characterized by extremely high concentrations of methanol or contain butan-2-ol as a specific component. Usually the concentrations of the congener alcohols are distributed according to Gauss’s law within the same class of beverages.
Analytical Separation of Congener Alcohols
The sensitivity of the conventional head-space gas chromatographic system is sufficient for blood ethanol determinations down to 0.0lgl_l but for the detection of congener alcohols in body fluids the limits of detection must be reduced to 0.0l mgl_l. This can only partly be managed by optimization of the analytical procedure. Additionally an improvement of the sample preparation is necessary. One possibility is to increase the sample volume from 0.2 to l ml and to add l g of potassium carbonate or sodium sulfate to the sample which causes an increased vapor pressure. This effect is more successful when handling aqueous solutions than whole blood, therefore it is necessary to subject the samples to homogenization by ultrasound and ultrafiltration. The final volume of the aqueous phase containing the congener alcohols is about 0.5 ml. As the long-chain alcohols are partly or completely bound to glururonic acid the sample should be incubated with p-glucuronidase to split the coupled compounds before salt is added.
Table 1 Flavour compounds of alcoholic beverages
| Concn. (mg l 1) | |
| Congener alcohols | < 5 000.0 |
| Aldehydes, carbon acids | <2.0 |
| Higher esters | <0.001 |
The disadvantage of this procedure is that 2 ml of substrate is necessary to analyze either the free or the coupled alcohols, and 4 ml for both when two analyses are required. When the samples are less than this, the problem can be solved by miniaturization. Since only a small fraction of the head-space vapour is injected onto the column, 2 ml vials with only 0.l ml of blood extract can be taken instead of the usual 20 ml vials. One needs only an aluminum cylinder in the shape of the orginal vial and a central bore for mounting the 2 ml vial.
The increased sensitivity of this method on the other hand has the effect that not only the congener alcohols appear in the chromatogram but also their metabolites and also some esters and endogenous compounds as well. This makes qualitative identification problematic. The problem can be solved by the introduction of a two-column technique with two different columns and a split before the columns. Because of the complicated extraction of the samples an internal standard (pentan-2-ol) should be added.
Another problem is that it takes nearly 60 min for the complete elution of all alcohols from both columns which causes broadening and diminishing of the last peaks resulting in a marked deterioration of sensitivity. This effect can be overcome by replacing the packed columns with capillaries. But the combination of capillary columns with the head-space procedure is delicate. Because of the small load capacity of these columns only short injection times up to 3 s are tolerable. Even splitless injection does not allow the volumes necessary for trace analyses to be injected. Increasing the flow rate of the carrier gas helium has no advantage because the retention times become too short for sufficient separation of all compounds. A good solution is to include a high-pressure valve in the system which is able to create a sample pressure of 450 kPa without influencing the flow rate.
The best results can be obtained with a cry of ocusing technique. Behind the injector is installed a neutral l m capillary precolumn with CP-Sil-5 linked over a l:l split to the two separating fused silica capillaries with CP-Sil-l9 and CP-Wax-52, respectively. The precolumn is inserted in a Teflon tube through which refrigerated gaseous nitrogen is passed in the opposite direction to the carrier gas flow. The end of the cool trap opens into the oven near the injector. Refrigeration is managed by passing gaseous nitrogen through a cooling coil inside a Dewar container with liquid nitrogen. The valve can be controlled to open at the beginning of the pressurizing phase and close at the end of the injection phase. With a pressurizing time of 2.2 min and an injection time of 0.l min the temperature at the outlet of the cool trap is about — 60°C. Sample temperature is 80°C with a thermostatting time of 32 min. The method works with a temperature program: 45°C isothermal for ll minutes, l0°C min—l to l00°C, 5 minutes isothermal, then 30°Cmin—l to l20°C and 7.8 min isothermal. All fusel alcohols are eluted from both columns within 30 min. For the detection two FIDs (250°C) are necessary. An example of a resulting chromato-gram is shown in Fig. 1.
Table 2 Congener content (mg I 1) of alcoholic beverages
Toxicity of Congener Alcohols
Richardson’s law, formulated in the middle of the nineteenth century, implies that the toxicity of alcohols increases with the length of the carbon chain. Side chains as well as hydroxyl groups in a secondary position on the other hand seem to diminish toxicity. These observations provoked some speculation as to whether the acute or the postacute effects of alcoholic beverages may at least partly be due to the congener alcohols. Acute toxicity of various beverages has been little studied but the results support the conclusion that compounds other than ethanol have little effect. Reports of less serious intoxication after beer than after brandy or vodka in similar quantities seem to be mainly explained by absorption effects.

Figure 1 Gas chromatogram of a calibration standard. Capillary column CP-Sil-19, 50 m, FID. From left to right: methanol, ethanol, propan-2-ol, propan-1-ol, ethylmethylketone, butan-2-ol, isobutanol, butan-1-ol, pentan-2-ol (inner standard), 3-methyl-butan-1-ol, 2-methyl-butan-1-ol. Concentrations: methanol 4mgl—1, ethanol 1 mg l—1, pentan-2-ol 2 mgl—1, others 1 mgl—1
On the other hand marked hangover symptoms were observed in the post-ethanol phase after consumption of congener-rich beverages. The fact that, during this hangover phase, neither ethanol nor other aliphatic alcohols could be traced supports the hypothesis that the pharmacologically active compounds in this regard are not the alcohols themselves, but their metabolites, particularly aldehydes.
Recent studies confirm the hypothesis that the accumulation of methanol by exogenous supply, endogenous sources or both, caused by a competitive inhibition with ethanol leads during the postalcoholic phase to a production ofthe pharmacologically highly active metabolite formaldehyde, when the inhibition of alcohol dehydrogenase by ethanol has ceased. This would explain the heavier hangover symptoms after the consumption of congener-rich beverages, which usually also contain higher amounts of methanol, and also the well-known phenomenon that hangover symptoms can be diminished by new intake of alcoholic beverages.
Following this hypothesis that apart from acetaldehyde formaldehyde in particular could possibly be responsible for severe damage after heavy and chronic drinking, the intensive studies about the mutagenic, carcinogenic and teratogenic effects of alcoholic beverages could be seen in quite a new light. The high incidence of increased risk of cancer of the upper digestive tract after long-term consumption of home-distilled alcoholic beverages observed in specific geographic regions across the world has mainly been related to acetaldehyde; but possibly it can also be attributed to formaldehyde. In blood and in cell cultures formaldehyde showed strong mutagenic and carcinogenic properties which were not shown by the corresponding alcohols and higher aldehydes. The same circumstances might be valid in cases of fetal alcohol syndrome or alcoholembryopathia which according to some authors are caused by the primary metabolite of ethanol, acetaldehyde.
Pharmacokinetics and Metabolism
It has been demonstrated that congener alcohols are resistant to storage alterations in blood samples and can be distinguished from putrefactive products. It therefore seemed to be of forensic importance to look at the congener content of blood samples of drunken drivers. Analytical data may possibly be helpful for the evaluation of allegations concerning the alcohol intake. The question was whether there is a close correlation between the consumed amount of a congener alcohol, and the resulting blood level. Initially it was shown that there were characteristic relations, but these could not be described according to Wid-mark’s laws. This observation made extensive experiments necessary to elucidate the pharmacokinetics and metabolism of the congener alcohols.
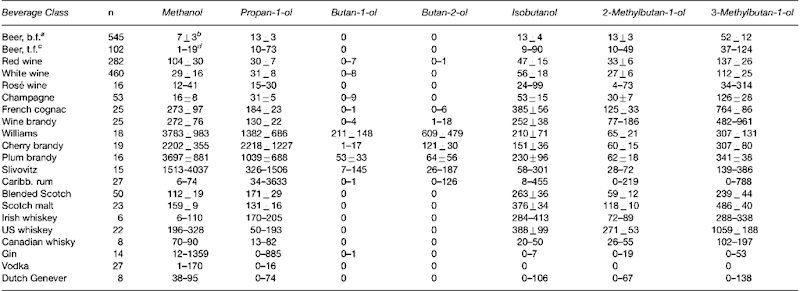
Information about the pharmacokinetics of the congener alcohols is mostly derived from drinking experiments with healthy volunteers. After drinking, the alcohols were rapidly absorbed through the gastrointestinal mucosa and distributed throughout the body. Methanol, propan-l-ol, butan-2-ol and ethanol are only soluble in body water. Isobutanol and the isoamyl alcohols have a much greater distribution volume because they are also soluble in lipids (Fig. 2). The fusel alcohols (not methanol) were partly or completely conjugated with glucuronic acid, this effect being more significant the longer the carbon chain of the alcohol (Fig. 3) and accompanied by an increase in the water solubility. However, only 5-10% of the consumed congener alcohols were excreted in free or conjugated form in the urine. This means that their elimination is mainly due to metabolism.
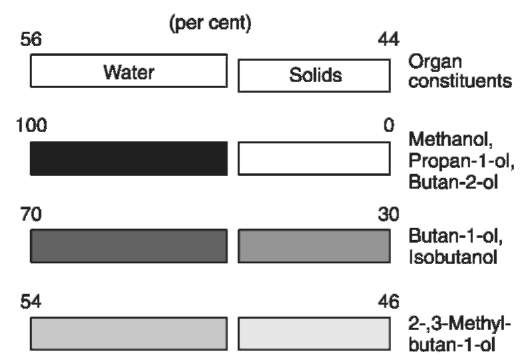
Figure 2 Solubility of congener alcohols.
Figure 3 Conjugation rate of congener alcohols with glucuronic acid.
Normally the metabolism of congener alcohols as of ethanol is nearly exclusively catalyzed by liver ADH. Because of this competition inhibitory influences can be expected. There are two controversial effects, which complicate this intricate matter. On the one hand the affinity of ADH to aliphatic alcohols increases with the carbon-chain length; the oxidation of a short molecule will be (partly) inhibited by a higher alcohol. On the other hand the high concentration of ethanol in comparison to the very low congener-alcohol levels is of importance.
Both effects could be demonstrated. In general, the longer the carbon chain the faster the congener alcohol is eliminated from the blood. But the elimination speed is also influenced by the actual ethanol concentration. Very low concentrations (below 0.5gkg-1) have nearly no inhibitory effect, which results in a rapid elimination of all fusel alcohols. In consequence these alcohols can only be detected when significant ethanol levels are present. However, only such levels are of forensic interest. With higher ethanol levels the elimination kinetics of all fusel alcohols follows a first-order reaction in contrast to the zero-order reaction of ethanol detected by Widmark. Examples are given in Fig. 4.
The elimination kinetics of methanol is completely different. During the first two or three hours after cessation of drinking slight increases can be seen followed by a more or less constant plateau phase. Elimination only starts when the blood ethanol concentration decreased to about 0.4gkg_1. The reason for this is the twofold handicap resulting from the lower ADH affinity and the great concentration differences.
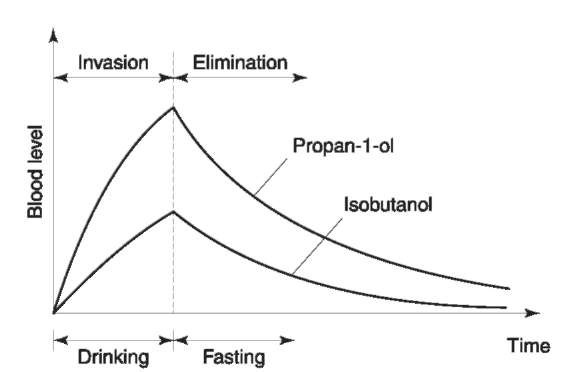
Figure 4 Invasion-elimination kinetics of propan-1-ol and isobutanol, schematized.
The special pharmacokinetics of methanol conditioned by competitive inhibition causes accumulation of methanol once continuous consumption of alcoholic beverages is practiced as long as the blood ethanol level remains above 0.4 g kg-1. In its most extreme form this behavior will be found among chronic alcoholics. The question is whether high blood methanol concentrations indicate chronic abuse. Analysis of blood samples from intoxicated chronic alcoholics were compared with those from drinking experiments with healthy volunteers. Most volunteers had methanol levels of less than 3 mgl-1, some had levels up to 5mgl-1 and very few had higher levels (Fig. 5). The highest was 6.2mgl-1.It is remarkable that more than 60% of the chronic alcoholics had values above 5 mg l- 1 and nearly 40% exceeded 10mgl-1 (Fig. 5). The highest value was more than 120mgl-1.
This observation confirmed the hypothesis, that blood methanol concentrations above 10 mgl-1 indicate chronic abuse. Even levels above 5 mgl-1 can be found nearly exclusively in alcoholics and should cause suspicion. However, the drinking behavior and the kind of beverages have a significant influence on the resulting methanol level.
Until recently it was not possible to define clear pharmacokinetic formulas for the invasion and elimination of all congener alcohols. Instead, correlation formulas for the calculation of some blood congener concentrations from drinking amounts were derived from drinking experiments (Table 3). The formulas are fixed on certain times after the end of drinking. For times in between intrapolation is necessary. Careful extrapolation is possible. Drinking time should not be less than one or more than three hours.
Figure 5 Distribution (percent) of methanol concentrations (mgl-1) in blood samples of healthy subjects (A, n=720), chronic alcoholics (B, n=110), drivers with blood-alcohol concentrations below 2gkg-1 (C, n=1000) and drivers with blood-alcohol concentrations above 2.5gkg-1(D, n=1000).
Forensic Significance of Congener Analysis
The reason to start congener analyses in the late 1970s was to contribute to an everyday problem which seemed to be a specific German problem because of legal peculiarities. It deals with the so-called hit-and-run delinquency. In Germany many drunken drivers prefer to leave the scene of an accident even when the other party needs help. The fear of disqualification from driving is greater than that of punishment for hit-and-run driving. When caught one or two hours later with alcohol in their blood many of these drivers claim to have been sober at the time of the accident and only started to drink after the accident, because of excitement.
Table 3 Calculation of expected blood-congener concentrations from drinking amounts + standard deviation 30 (C30), 90 (C90) and 150 (C150) min after drinking end

This behavior is so characteristic that a special expression was born in the German language (‘Nach-trunk’) which has no counterpart in English; perhaps ‘postconsumption’. There was no method for the verification or refutation of such allegations. This is exactly the field where congener analysis can contribute to a clear decision. To demonstrate how such analytical results can be used for checking the truth of a claim it seems appropriate to present typical examples of actual forensic cases.
Example 1 A driver obviously did not perceive that a traffic light turned to red and crashed into a halting car. He drove back, made a U-turn and left the scene. The police were called, got the car number from the other driver involved and obtained the name and address of the car owner from the registration files. The address was checked at regular intervals but nobody opened after ringing. There was no damaged car in the street. Only four hours after the accident was the door opened. The car owner pretended to be totally surprised. He smelled of alcohol but alleged that he had drunk a lot of whiskey after midnight in his apartment. When questioned about the accident he claimed to have been at home all the time. His car must have been stolen. As a precaution a blood sample was taken which revealed a blood alcohol concentration of 1.12gkg-1.
The car was found one day later in a backyard not far from the apartment. On the steering wheel only clear fingerprints of the owner were traced. The owner was confronted with this finding and confessed that he was the driver. He stuck to his claim concerning the alcohol intake after the accident and added that he did not drink before. As according to Widmark’s formula the alleged volume of 200 ml US whiskey could only produce a blood alcohol concentration of 0.330.68 gkg-1 after 3.5 h of elimination, this could not be true. However, the postconsumption could not be excluded. Therefore, the respective concentration of maximally 1.03 gkg-1 had to be subtracted. Recalculation resulted in an actual blood alcohol concentration of 0.49 – 0.89 gkg-1 for the time of the accident. As the lower value is below the legal limit the driver would have been cleared of suspicion.
This was why a congener analysis of the stored blood sample was ordered by the court. Table 4 demonstrates how the expert opinion was elaborated. Comparison of the actual congener concentrations of the blood sample with the expected values calculated with the correlation formulas of Table 3 shows that the real concentrations of methanol and propan-1-ol are higher than expected. As already known from the ethanol calculation (cf. above) the driver must have drunk also before the accident. This consumption can be responsible for the relatively high concentrations of these two congeners, because methanol is not metabolized as long as the blood ethanol concentration exceeds 0.4 g kg-1 and propan-l-ol is eliminated much slower than the other fusel alcohols. On the other hand the actual concentrations of isobutanol and 3-methylbutan-l-ol are less than expected. The differences exceed 6 standard deviations altogether. This means that the claim can be excluded almost certainly. The court accepted this opinion and sentenced the accused.
Table 4 Comparison of analytical results and expected congener concentrations calculated from a postconsumption claim
| Given times Preconsumption: Postconsumption: |
no statement 01.00-03.00 h | Accident: 00.30 h Blood sample: 04.30h | |
| Body data Weight: 80 kg |
Height: 180 cm | /-Amed to be taken | |
| Drinking amounts claimed Preconsumption: Postconsumption: |
no alcoholic drinks US-Whiskey ‘Jim Beam’, 200ml | ||
| Congener content of ‘Jim Beam’ (mg l-1) Methanol: 306 Propan-1-ol: 50 | Isobutanol: 3-Methylbutan-1-ol: | 365 1320 | |
| Blood-congener concentrations (mg l 1) Methanol: 2.40 Propan-1-ol: 0.25 |
Isobutanol: 3-Methylbutan-1-ol: | 0.04 0.10 | |
| Expected concentrations (mg l-1) (cf. Table 3) Methanol: 1.05 (s = 0.44) Propan-1-ol: 0.12 (s = 0.07) | Isobutanol: 3-Methylbutan-1-ol: | 0.31 (s = 0.09) 0.25 (s = 0.04) | |
Example 2 A quarter past midnight a man left a bar and started his car. When leaving the car park he scraped another car just entering the car park. He left his car and looked at the damage. When the other driver went to the bar in order to call the police, he left the scene with his car. The police searched for the damaged car. About two hours later the car was found in a suburban street. The driver was inside his car. He told the police that he had drunk only half a liter of beer during the last two hours before midnight in the bar mentioned. Because of the accident he was so frightened that he drove away randomly, stopped somewhere, opened the trunk and pulled five bottles of beer out of a crate and emptied them because of his excitement. The police examined the trunk. There was a crate containing 20 empty bottles of beer. Later on the police questioned the barkeeper, who stated that the driver had drunk 2.5 liters of beer during his stay in the bar. However, there remained doubts, because the other party of the accident was the son of the barkeeper.
The blood sample of the driver contained 1.21 g kg-1 alcohol (and a second one taken 45 min later 1.13 gkg-1). As a preconsumption of 2.5 liters of beer (instead of only half a liter as claimed by the driver) and an additional postconsumption of 2.5 liters would have produced a much higher blood ethanol concentration, either the statement of the barkeeper or the claim of the driver must be false. Calculating both alternatives with Widmark’s formula did not allow differentiation. This was the reason for the court to order a congener analysis of the stored blood sample. The results are presented in Table 5. Comparison of the analytical results with the expected concentrations shows that the detected fusel alcohol concentrations are lower than those expected after the alleged postconsumption. The differences amount to 10 standard deviations altogether. On the contrary, there is a good correspondence with the expected fusel-alcohol concentrations calculated from the testimony. Therefore, the claim of the driver was disproved almost certainly, whereas the statement of the barkeeper could be confirmed. The driver was convicted by the court.
Table 5 Comparison of analytical results and expected congener concentrations calculated from a postconsumption claim and a testimony, respectively.
| Given times Preconsumption: Postconsumption: |
22.00-24.00h 00.30-02.00h | Accident: 00.15 h Blood sample: 02.30h | ||
| Body data Weight: 75 kg |
Height: 176 cm | ^Amed to be taken | ||
| Drinking amounts claimed Preconsumption: Postconsumption: |
Beck’s beer, 500 ml Beck’s beer, 2500 ml | |||
| Drinking amount according to testimony Preconsumption: |
Beck’s beer, 2500ml | |||
| Congener content of ‘Beck’s Beer’ (mg l-1) Methanol: 9 Propan-1-ol: 9 | Isobutanol: 3-Methylbutan-1-ol: | 9 62 |
||
| Blood-congener concentrations (mg l-1) Methanol: 0.50 Propan-1-ol: 0.21 | Isobutanol: 3-Methylbutan-1-ol: | 0.06 0.05 | ||
| Expected concentrations according to claim (mg l 1) Methanol: 0.41 (s = 0.58) Isobutanol: Propan-1-ol: 0.35 (s = 0.05) 3-Methylbutan-1-ol: |
0.19 (s 0.35 (s |
= 0.11) = 0.05) |
||
| Expected concentrations according to testimony (mgl-1) Methanol: 0.57 (s = 0.28) Isobutanol: Propan-1-ol: 0.22 (s = 0.12) 3-Methylbutan-1-ol: |
0.07 (s 0.07 (s | = 0.04) = 0.06) | ||
These examples show the information that is necessary for meaningful congener analysis: times of pre-and postconsumption, body data, amount and type of consumed beverages. If the brands of the beverages involved are known, they should be included in the analytical procedure. If only the types are known, their congener contents as listed in Table 2 can be used. There are, however, other limitations. The amounts of beverages consumed should exceed 1 l of beer, 0.51 of wine and 60 ml of spirits. Spirits such as vodka or gin that are (nearly) congener-free are problematic, unless it is claimed that only such drinks were consumed and the blood sample contains congeners. The consumption time should be between 1 and 3 h and the time between end of drinking and blood sampling below 3 h. When these limitations became known to the public it was feared that the defense tactics would be altered, but so far they have not been.
As demonstrated most alcoholic beverages contain the same congener alcohols, but in different amounts. As the blood concentrations and their relations to each other are changed during elimination there is no obvious correspondence between the congener spectrum of a blood sample and the beverage consumed. This means that it is not possible to determine the type of beverage from a blood-congener analysis. Only if butan-2-ol is found can one conclude that a respective spirit must have been consumed. The congener spectrum of a urine sample corresponds much better to that of the beverage consumed. Such, additional urine samples can be useful on special occasions. However, there is no clear correlation between drinking amounts and urine concentrations.
The congener method is widely accepted by the German courts. Congener analyses are offered by nearly all German institutes of forensic medicine. In the Duesseldorf institute about 100 expert opinions based on this method are written each year. In around 75% of the cases the claim is almost certainly excluded, in around 14% the allegation is valued as improbable and in 10% as irrefutable. In the remainig cases the claim is confirmed. Astonishingly the opinions are also very often accepted by the accused. Often they make a confession during the court procedure. The author has been contacted more than once by an accused after conviction and assured that the conclusions exactly matched the real circumstances.
Although the congener method was presented more than once to the international public during the first years there was little interest. However, during the mid 1980s colleagues from northern and eastern Europe, and later on also from some western countries asked for cooperation. In 1987 an international workshop under the auspices of the International Committee on Alcohol, Drugs and Traffic Safety was held in Duesseldorf. It was found that the hit-and-run problem is not specific to Germany, which means that the method could also be advantageous in other legal systems.
Congener (especially methanol) findings can also be useful for the diagnosis of chronic alcohol abuse. As there are no analytical difficulties it has much to recommend it, especially for epidemiological studies. As an example the following observations may be mentioned. From forensic blood samples those with blood ethanol concentrations below 2.0 g kg-1 and those with levels above 2.5gkg-1 were separated. The reason for this selection was the assumption that drivers with ethanol levels below 2.0gkg-1 may nearly represent the normal population. Levels above 2.5gkg-1 will only seldom be reached by normal persons and this was expected to contain a good numbers of alcoholics.
The findings confirmed this assumption. Concerning the methanol levels in the group of drivers with ethanol concentrations below 2.0gkg-1 there were striking similarities to the nondrinker group of the above mentioned experiments (Fig. 5). Even more interesting was the comparison of the chronic alcoholics with the drivers with ethanol concentrations above 2.5gkg-1 (Fig. 5). There was also a conspicuous correspondence indicating that at least 40% of these drivers must have alcohol problems, possibly even more than 70%.