Forensic Hair Comparison
Hairs, whether human or from a wild or domesticated animal, are often found associated with personal injury accidents and criminal activity. The determination of the source of a hair is often of interest to investigators and the principal means for determining its origin is by microscopical comparison. A comparison of all types of hairs is possible; although head and pubic hairs are most often encountered in criminal investigations, others can be compared using the same techniques.
History of forensic hair microscopy
Four centuries of microscopical study have described the morphological structure of hair, how populations of people differ, and how individuals differ (inter-individual variation), and what variation can be expected within an individual (intraindividual variation). In the seventeenth and eighteenth centuries, hair was a ready-made specimen for Robert Hooke (1635-1703), Antony van Leeuwenhoek (1632-1723), and Henry Baker (1698-1774). In the nineteenth century, there were scattered reports of medical and forensic investigations into the use of hair as evidence. In the twentieth century, anthropologists studied hair as a means to learning more about human variation, and forensic scientists applied microscopy to the identification and association of hair and used it as evidence. Hair is now well established as one of the many types of useful trace evidence and there is no reason to think that its use will not continue.
Morphological features for comparison
Forensic hair comparison is a comparative biological discipline grounded in microscopy, biology, anatomy, histology, and anthropology. In addition to biological processes, subsequent treatment of the hair and accidental changes due to the individual’s environment create extra dimensions useful for comparison. Therefore, from the beginning, forensic hair examiners have concentrated on certain microscopic features, characteristics and traits for both human and animal hairs that have been shown, through collaborative research and collective experience, to be useful for comparison because these characteristics effectively distinguish one individual from others and one population from another. For human hairs, these characteristics can be broadly grouped into color, structure and treatment. Hair color, shaft, medulla and scales characterize animal hairs.
Color The color of the hair depends on its pigment, surface, transparency and reflectivity. A range of colors in the exemplar is common and a direct comparison is necessary between the questioned hair and the known hair in order to detect subtle differences. The amount and distribution of pigment within the cortex should be examined at high magnification (200-600 x ). Color is probably the most useful characteristic for comparison.
Structure The diameter (Table 1), medullation, cross-section, cortical fusi and spatial configuration reflect the hair’s structure. The diameter can be measured and the medullation can be classified according to various schemes. The spatial configuration refers to the appearance of the hair as it lies, unconfined, on a surface and depends on the cross-sectional shape.
Treatment The tips, roots, color and length are all subject to treatment. Cut tips may be freshly cut, split, frayed or worn and the angle of cut may be significant. The shape of the root may not only indicate the method of hair removal, but abnormalities may provide valuable comparative data.
Bleached or dyed hair can usually be identified by a distinct demarcation near the proximal end between the treated and untreated portion of the shaft. The treated hair shaft sometimes shows signs of chemical wear; the cuticle is often damaged and cortical cells, separating under the chemical assault, may be distinct.
Microscopy Light microscopy
Light microscopy is the use of any one or a combination of microscopical techniques including stereo, polarized, fluorescence and comparison microscopy.
As many comparisons may be solved quickly and inexpensively by light microscopy, it is the beginning point in nearly all forensic trace evidence analysis. In those cases where other techniques are indicated, light microscopy is used to prepare samples for further examination.
Illumination Good resolving power and optimum specimen contrast are prerequisites for forensic light microscopy. Though the optics (ocular, objectives and substage condenser) may be suitable, proper adjustment of the illumination is of paramount importance in order to see the details necessary for an adequate hair comparison.
Kohler illumination (August Kohler, 1866-1948) is the most useful illumination, and may be obtained with any lamp not fitted with ground glass. Diffuse illumination, so called because a ground glass is positioned between the lamp filament and the microscope condenser, seems easier to set up and gives illumination of high quality although of somewhat lower intensity. The illuminator and lamp filament need no adjustments, although a field diaphragm is helpful. Both types of illumination require that the
Table 1 Caucasian head hair diameter. Human hair shaft diameter measurements illustrate the range of values for many hair characteristics. These values were obtained from a random population of 50 individuals. The diameter distributions were often bimodal, probably reflecting the hair cross-sectional shape; and, although they overlapped, the distributions were unique for each individual. When many characteristics, each with their own distributions of values, are combined into a multidimensional space, the uniqueness of a representative sample of hairs from an individual can be imagined microscope substage condenser be focused on the specimen to give a full illuminating cone and allow the substage iris to be focused on the objective back focal plane. Defenders of the diffuser say they can obtain illumination in the field of view comparable to and, in fact, indistinguishable from Kohler. This may be true, but the only way to be sure the diffuse illumination is optimized for the hair comparison is to proceed through the steps necessary to achieve true Kohler illumination. If desired, the ground glass can be replaced in the light path.
| Measurement | Size (nm) |
| Diameter range | 13-132 |
| Most common range | 39-111 |
| Average range | 79.4 |
| Grand mean | 70 |
| Smallest mean diameter | 44.8 |
| Largest mean diameter | 90 |
| Range of mean diameter | 45.2 |
| Smallest mode | 39 |
| Largest mode | 104 |
| Mode range | 65 |
In any case, Kohler illumination is not difficult for any microscope with a lamp that can be focused and centered. First, focus on a preparation using the high dry objective and be sure the rotating stage, if present, is centered with respect to the microscope’s optical axis. Close the field diaphram and focus its image in the field of view by racking the substage condenser up or down. Center the focused, field diaphragm image by centering the substage condenser. Next, simply remove the ocular and look down the bodytube to see the objective back focal plane, and focus an image of the lamp filament in the objective back focal plane by moving the lamp bulb fixture along the lamp axis relative to the lamp condenser. Lateral movement of the lamp bulb itself centers the filament. Replace the ocular and, looking again at the preparation, adjust the substage iris to give optimum contrast and resolution. Each compound microscope needs to be adjusted similarly.
Calibration If linear measurements are to be made with the light microscope, the eyepiece micrometer must be calibrated for each objective. Likewise, the microscopes on each side of the comparison microscope must be matched to provide the same magnification and color balance for each objective.
The eyepiece micrometer is calibrated by comparing the ocular scale to be calibrated to a second scale on a stage micrometer slide having known dimension. The two scales are each carefully focused, the ocular scale by rotating the focusing top lens of the eyepiece and the stage scale by focusing the microscope itself. The scale images are then arranged parallel to each other and the size of each ocular division is determined by comparing with the length of the stage micrometer.
Color balance on both sides of the comparison microscope can be obtained with proper illumination, adjustment of the rheostat, new lamps, color-balancing filters or by illuminating both microscopes with a bifurcated fiber optic from a single lamp.
Cuticular scales The microscopical comparison of cuticular details illustrates the limits of a metrical approach to hair comparison and the effect of mounting media. The scale patterns of hairs are useful for distinguishing between broad classes of animals, and they provide some value for individualizing human hair.
Scales can be seen when mounted dry between glass slides; but, if required, better delineation of the cu-ticular surface is possible by preparing a clear cast, using fingernail polish for example. The scale casts are then viewed with a microscope with transmitted light. When hairs are mounted in a liquid with a refractive index near that of the hair (1.52-1.54), the scales disappear (unless stained) and cannot be used for identification or comparison.
The cuticular surface can be characterized by counting the number of scales per unit length or calculating a scale index defined as the ratio of the scale length to the hair shaft diameter. Scales can best be viewed with the aid of scanning electron microscopy because the small details are in three dimensions on the surface. Possibly, when more objective research data are compiled on the morphology of cu-ticular features using scanning electron microscopy and image analysis, their significance to species identification and hair comparisons will be enhanced.
Mountants Hairs can be viewed dry, simply mounted between glass slides; but, for a detailed comparison of the internal structures, hairs must be cleared with a liquid somewhat closer to the refractive index of the hair. The brand of mountant is not particularly important, but the refractive index and ease of use are (Table 2). Hairs mounted in water («=1.33), for example, are too contrasty for an effective comparison of internal structures. There is too great a difference between the refractive index of the hair and the refractive index of the mounting medium. The moun-tant should have a refractive index near that of the hair cuticle and cortex, somewhere between 1.52 and 1.54. Mountants can either be volatile solvents, natural and synthetic oils, semisolid resins hardened by evaporation, thermoplastic solids or plastics cured by UV radiation.
Relatively permanent mounting media, such as Permount (Fisher Scientific Co.) or Cargille Melt-mount 1.539, are frequently used in the United States to protect the integrity of the hair while at the same time clearing it sufficiently for microscopical comparison. If necessary, an easy way to remove the hairs from a semipermanent preparation is to first freeze the slide and then simply pick off the cover slip and the hair of interest. Sometimes the preparation in Permount needs to be soaked for some time in xylene or a Meltmount preparation can be warmed on a hot plate to remove the cover slip and hair.
Table 2 Mountants for microscopic hair comparison. The choice of mountant depends on what image is necessary for the analysis. If surface features are to be examined, a mountant with very low refractive index is used, like air. If internal details are to be examined, a mountant with a refractive index near that of the cuticle is chosen. Whether the hair must be used for another purpose or preserved indefinitely mounted on a slide determines if a liquid (solvent, oil or thermoplastic mountant) or plastic is used. The type and refractive index of several possible media are listed
| Type | Name/brand | Refractive index |
| Dry | Air | 1.00 |
| Solvent | Water | 1.33 |
| Toluene | 1.49 | |
| Xylene | 1.50 | |
| Orthodichlorobenzene | 1.54 | |
| Bromoform | 1.60 | |
| Oils | Glycerine | 1.47 |
| Mineral oil | 1.47 | |
| Immersion oil | 1.515 | |
| Cedar wood oil | 1.52 | |
| D C 710 silicone oil | 1.53 | |
| Clove oil | 1.53 | |
| Cargille Liquids | 1.46-1.64 | |
| Semisolids | Protexx | 1.49 |
| Permount | 1.52 | |
| Canada Balsam | 1.54 | |
| Histoclad | 1.55 | |
| Thermoplastic | Cargille Meltmounts | 1.539-1.704 |
| Aroclor 5442 | 1.660 | |
| UV plastics | Plastic Mount | 1.49 |
| Norland optical #65 | 1.52 | |
| Norland optical #68 | 1.54 | |
| Norland optical #60 | 1.56 |
Stereomicroscopy The stereomicroscope, also known as a dissecting microscope, is the starting point for virtually every microscopical analysis. At low magnifications of approximately 5x to 50 x, the color, texture, structure and treatment of a sample may be observed and, in some cases, a complete identification can often be gained. Different colored backgrounds and different light sources should be used during initial comparisons of hairs. A black background often affects the appearance of the medulla and cortical fusi, for example. Stereomicroscopy is also used to prepare samples for the microscopical comparisons to follow.
Polarized light microscopy A polarized light microscope is based on a compound microscope and is the most useful of all light microscopy techniques, particularly for the identification and comparison of hairs, fibers and the small particles that might be adhering to them. Direct observation of hairs at magnifications of 40 x to 600 x provides morphological information and further manipulation of light provides optical information. Nearly every sample for analysis finds its way to the polarizing light microscope.
Because hair is long compared to its thickness, it is, in some ways, a difficult subject for thorough microscopical investigation. The study of important morphological details necessitates relatively high magnifications, and at these magnifications the depth of focus is less than the diameter of the hair, and the field of view limits observation to a short section of the shaft at any one time.
Fortunately, hair is anisotropic, and since birefringence increases with the degree of keratinization, the cortex is more birefringent than the medulla or cuticle. When a hair is examined between crossed polars, the background appears dark and the hair, if properly oriented, appears bright due to interference colors. The greater the birefringence or the longer the optical path length (thickness), the greater the ultimate phase differences and the more retardation, interference and orders of color. Therefore, portions of the hair, differing in either thickness or birefringence, appear in different colors, sometimes called optical staining.
In the examination of an unmedullated hair shaft, these colors indicate thickness. The optical path-length through the narrower edges of a hair with an oval-shaped section is less than the pathlength through the thicker center. These color bands are especially useful to see structural variations, nodules, twists, constrictions or bandings.
The medulla, too, can be studied with the aid of the polarizing microscope. Under ordinary illumination, an air-filled medulla is a cylinder of low refractive index in a medium of higher refractive index and becomes a dispersing lens, so that the medulla appears deceptively black. Under the polarizing microscope, the medulla remains an isotropic inclusion in a birefringent matrix, and the retardation of polarized light passing through the portion of the hair containing the medulla is less than the retardation in surrounding parts. Hence the medulla appears in a different color from the surrounding cortex, and pigment granules (if present) remain dark, due to their specific absorption. In this way, the pigment content of the medulla is easily differentiated from empty space; or medullae, which are difficult to see in nearly opaque hairs, can sometimes be found.
Fluorescence microscopy Through the use of special attachments to a compound light microscope, the fluorescent nature of hairs may be determined, allowing hairs which are similar in normal or polarized light, but which have different fluorescence, to be distinguished. Fluorescence is most often used to compare dyed fibers, but on occasion treatments or contamination of the hair may be detected with this technique. Because fluorescence is so valuable for fiber comparison, many comparison microscopes are equipped with the appropriate attachments so they are readily available for hair comparisons. Special stains may be used to treat materials on the surface to facilitate their detection by fluorescence microscopy. The color and intensity of fluorescence at different excitation wavelengths may provide important information for both the characterization and comparison of hairs.
Comparison microscopy Skillful microscopical technique when hairs are viewed simultaneously side-by-side on a comparison microscope furnishes the forensic hair examiner with a highly discriminating means to compare hair. The stereomicroscope allows gross observations of the hairs including trace evidence on the hair’s surface. The wide range of traits in a large hair sample can be rapidly reviewed using the lower magnification and ample working space. The compound or polarizing microscope with its higher magnification and resolution delineates the hair’s finer structural characteristics. The pigment, scale structure, cortical fusi and medulla can be scrutinized using the polarizing microscope and its accessories. Using a calibrated ocular micrometer, the necessary measurements can be made.
The final phase of a detailed microscopic comparison is a side-by-side examination of the known and questioned hair using a transmitted light comparison microscope where each of the microscopic features are juxtaposed and compared. An ideal comparison microscope would be of good quality with attachments for both polarized light and fluorescence microscopy.
Scanning electron microscopy
Scanning electron microscopy is valuable because it can image small structures with greater depth of field than can a light microscope, but it cannot see below the surface of the hair; therefore, for the purpose of hair comparison it is less reliable than the light microscope. The other reason scanning electron microscopy and elemental analysis are unreliable, either to prove identity or to eliminate a possible source, is that the techniques do not normally take into account variation along the length of the hair or variation from one hair to the next in the sample. The only reasonable way to compare hairs by electron microscopy is to make numerous measurements and observe numerous images at numerous points along numerous hairs from the root to the tip; therefore, it is generally unsuitable for making hair comparisons. Infrared microspectroscopy
A Fourier transform infrared spectrometer attached to a light microscope allows analysis of samples down to about 10 um in size. On occasion, a microscopical examination of either questioned or known hairs will reveal adherent material on the surface which will sometimes be useful for comparison. Infrared micro-spectroscopy can be used in addition to polarized light microscopy, fluorescence, and scanning electron microscopy for identification of these microtraces.
Visible light microspectrophotometry
Although color is very important to hair comparisons and with a stereo- or comparison microscope many different hues can be distinguished, microspectro-photometry of these differences has not been very successful. It seems that hair pigments, which are essentially all brown, produce flat and featureless absorption spectra in the visible region, 400700 nm. A good way to define hair color has not yet been developed and it remains quite subjective.
Comparison of Hairs
Microscopical technique
The microscopy of hair is relatively simple. Using your hands and eyes and good microscopes, the hair is approached like a crime scene being investigated with the spiral technique. You survey the scene and begin a slow careful approach circling around in an ever-decreasing spiral until you have viewed all that there is to see. Look at the gross anatomy: how the hair sits on the surface, its shape, color, curl, length, etc. Next, circle around and get a little closer and look at the surface: microtraces might be there, the scales will be of interest including their size, shapes, and weathering along the length of the hair. Finally, peer inside, using a mountant near the refractive index of the cuticle and keratinous fibrils of the shaft, looking for: pigment particles, cortical fusi, ovoid bodies, the medulla, inner cuticular margin, etc. If you are looking at animal hairs look for: shaft shapes and sizes, uncut tips, color banding, cuticular patterns, unusual medullae, and root structures. If you must compare hairs to determine if they could have originated from the same individual, look at all these features again and determine whether they are similarly evident, in every detail, in both hairs.
Exemplars
Before a comparison can be attempted, the portions of the body from which the hair originated must be determined. No comparison can be made without obtaining an exemplar from the homologous somatic region. Scalp hairs must be compared with scalp hairs, beard hair with beard hair, mane hair with mane hair, and dorsal guard hair with dorsal guard hair, etc. No attempt should ever be made to draw conclusions about pubic hairs based on observations of head hairs, for example.
Comparison process
Samples received for examination are initially inspected without the aid of any microscope, simply by viewing how they rest on a surface or holding them up to the light. They are next examined with a stereomicroscope noting color in reflected light, length, curliness, or anything of apparent significance. Usually at that time, hairs that appear to be representative of each sample as a whole or represent specific hairs for comparison are selected for additional study and mounted for microscopical analysis by polarized light microscopy, fluorescence microscopy and possibly by scanning electron microscopy or infrared microspectroscopy of adherent residues.
If the need for a detailed comparison is apparent, hairs from each exemplar are examined one at a time and thoroughly described with regard to all of the observable characteristics. The variation shown along each hair shaft is noted individually for each characteristic, and the process is repeated for each hair examined. A list of characteristics and traits can be used to assist with a systematic recording of features and their variations (Tables 3-5; Figs 1-11). A description of hair from each individual which expresses the range of traits observed for each characteristic and any significant special features or uncommon traits can be recorded.
The questioned hairs are likewise scrutinized and characterized microscopically. Consideration must be given to the origin of the evidence hair in deciding how to treat these samples. If several hairs were collected together from a source that makes it clear that they share a common origin, they may be treated as one sample and evaluated as a group in the same manner as the exemplars. If individual evidence hairs were collected from different sources, there is no justification for an assumption that they share a common origin. They must, therefore, be treated as several discreet samples, perhaps limited to a single hair each.
Finally, the selected hairs from the exemplar are compared with each questioned hair using a comparison microscope. The side-by-side analysis will either confirm or disprove the apparent similarities between known and questioned hair. The direct comparison is also a time to search for areas that show the same traits at corresponding points along the length of the hair shaft.
In other words, when the initial examination indicates a similar range of values for each characteristic between the known and questioned hair, it must now be determined whether the same individual traits for each characteristic can be juxtaposed at corresponding points along the questioned and known hair. If two samples of hair have a similar range of like features demonstrated during the initial separate microscopical comparisons using a stereomicroscope and polarizing microscope, and selected hairs are found to be alike for all or nearly all characteristics and traits in the side-by-side comparison using a comparison microscope, then a conclusion that the hairs are similar in all respects (a match) is justified and the samples could have shared a common origin.
Analysis
When comparing hairs, all characteristics of both the known and unknown specimens must be considered. A single significant difference between the two is a strong indication of two sources. Several repeated, fundamental dissimilarities establish with certainty that two specimens are not from a single individual.
Individualization rests therefore not only on a similar combination of identifying traits, a condition which always must be fulfilled, but also on a coexistent lack of any dissimilarities between the questioned and known hairs.
No two specimens of hair, even if from the same spot on the body, are identical in every detail since variation is an integral part of natural growth. The amount and kind of variation differs. With some it is slight and occurs only in subtle details; with others it covers a rather wide range. Variation does not preclude identification of the hair. In fact, variation around the basic qualities of the hair forms an additional factor, which serves to individualize hair.
Each characteristic may vary in any or all of the following ways. They may show variation at different points along a single hair shaft, between different hairs of the same individual, or between the hairs of different individuals. Hair of two different individuals may be entirely dissimilar or they may be very much alike but not identical. Individualities, many of them inconspicuous details, distinguish hair samples that appear very much alike. Everyone seems to appreciate that two specimens are not from the same individual when there are a vast number of differences, but only a few fundamental dissimilarities may not seem to lead to so positive a conclusion; nevertheless, they do. If two specimens are from a single source, then no fundamental differences should exist. Conversely, if there is any basic dissimilarity which cannot be accounted for, then the two specimens must have been obtained from different sources. Hair comparison involves the discovery and study of all identifying characteristics; the differentiation between those which are typical and those which are abnormal or represent the unusual; recognition of those which are disguised or have been deliberately and consciously changed; and determination of the normal amount of variation common to the particular sample. See Table 6 for summary of fundamental concepts of a microscopical hair comparison.
Table 3 Human hair characteristics and traits
| Feature Characteristic | Trait | Feature | Characteristic | Trait |
| Color Hue | Colorless | Inner cuticle margin | Distinct | |
| Blond | Smooth | |||
| Red | Cracked | |||
| Auburn brown | Indistinct | |||
| Golden brown | Cortical texture | Coarse | ||
| Ashen brown | Uniform | |||
| Black | Ovoid bodies | Size | ||
| Pigment density | Absent | Shape | ||
| Light | Color | |||
| Medium | Distribution | |||
| Heavy | Abundance | |||
| Opaque | Cortical fusi | Size | ||
| Pigment distribution | Uniform | Shape | ||
| Peripheral | Distribution | |||
| One-sided | Abundance | |||
| Random | Medulla | Continuous | ||
| Central/medial | Interrupted | |||
| Cuticle pigment | Fragmental | |||
| Banded | Opaque | |||
| Pigment aggregation | Streaked | Translucent | ||
| Clumped | Relative width | |||
| Patchy | Amorphous | |||
| Pigment aggregate size | Large | Patterned | ||
| Medium | Absent | |||
| Small | Treatment | Root present | Telogen | |
| Pigment shape | Round | Catagen | ||
| Oval | Anagen | |||
| Oblong | Sheathed | |||
| Pigment size | Large | Follicular tag | ||
| Medium | Post-mortem band | |||
| Small | Root absent | Cut | ||
| Artificial treatment | Dyed | Broken | ||
| Bleached | Decomposed | |||
| Other | Crushed | |||
| Structure Length | Distal ends | Tapered | ||
| Diameter | Rounded | |||
| Curvature | Abraded | |||
| Form | Straight | Square | ||
| Wavy | Angle | |||
| Curly | Frayed | |||
| Twisted | Split | |||
| Tightly coiled | Crushed | |||
| Crimped | Broken | |||
| Cross-sectional shape | Round | Cut | ||
| Oval | Singed | |||
| Triangular | Alterations | Straightening | ||
| Flattened | Waving | |||
| Pear-shaped | Curling | |||
| Shaft configurations | Buckling | Artifacts | Lice | |
| Convoluting | Mold/fungus | |||
| Shouldering | Bite marks | |||
| Undulating | Debris | |||
| Splitting | Blood | |||
| Cuticular thickness | Thin | Abnormalities | Pili annulati | |
| Medium | Trichoschisis | |||
| Thick | Monilethrix | |||
| Outer cuticle margin | Smooth | Trichorrhexis nodosa | ||
| Flattened | Trichorrhexis invaginati | |||
| Serrated | Pili torti | |||
| Cracked | Trichonodosis | |||
| Looped | Cartilage hair hypoplasia |
Table 4 Animal hair characteristics and traits
| Feature | Characteristic | Trait |
| Color | Color | Bicolored Multicolored Banded Unbanded |
| Color band configuration | Single (one light band) Triple (two light and one dark band) 5-banded (three light and two dark bands) |
|
| Color band location | Upper shield Central shield Lower shield Lower shaft Upper shaft | |
| Shaft | Undulations | |
| Basal configuration | Wine-glass shaped Slightly tapered | |
| Length | ||
| Diameter | ||
| Spatulate shield | In upper shaft In middle shaft | |
| Flattened shaft | In lower shaft | |
| Stricture | Lower shaft Subshield | |
| Cross-sectional shape and appearance | Circular medium medulla Circular large medulla Oval large medulla Oval medium medulla Oval medulla absent Eye-shaped Oblonglarge medulla Oblongmedium medulla Cigar-shaped Concavoconvex divided medulla Concavoconvex bilobed medulla Concavoconvex large medulla Reniform Dumbbell shaped |
|
| Medulla | Medulla | Opaque Infiltrated Width Medullar index |
| Medulla type | Absent Simple unbroken amorphous Unbroken cellular Unbroken vacuolated Unbroken with cortical intrusions Unbroken lattice Narrow medulla lattice Wide medulla lattice Narrow aeriform lattice Wide aeriform lattice Uniserial ladder Multiserial ladder Interrupted Fragmental Globular Stellate Intruding |
|
| Scales | Scale pattern | Simple coronal Regular petal Broad petal Diamond petal |
Table 4 continued
| Feature | Characteristic | Trait |
| Narrow diamond petal | ||
| Irregular petal | ||
| Single chevron | ||
| Double chevron | ||
| Regular mosaic | ||
| Irregular waved mosaic | ||
| Flattened irregular mosaic | ||
| Regular wave | ||
| Irregular wave | ||
| Streaked wave | ||
| Pectinate | ||
| Shape | Spatulate | |
| Flattened and tapered | ||
| Round to oval | ||
| Scale margin | Smooth | |
| Crenate | ||
| Rippled | ||
| Scalloped | ||
| Dentate |
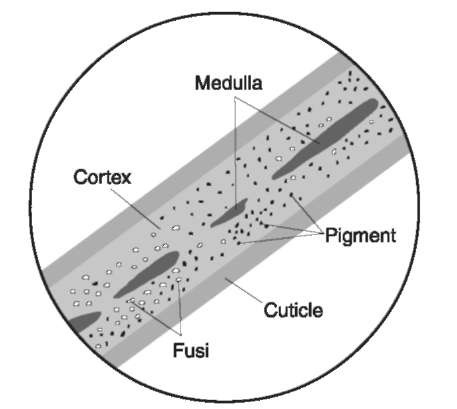
Figure 1 Hair shaft. The hair shaft of human hair as viewed microscopically in a mountant. All hairs, whether human or domesticated or wild animal, have the same structure which includes a cuticle, cortex, pigment, fusi, and medulla.
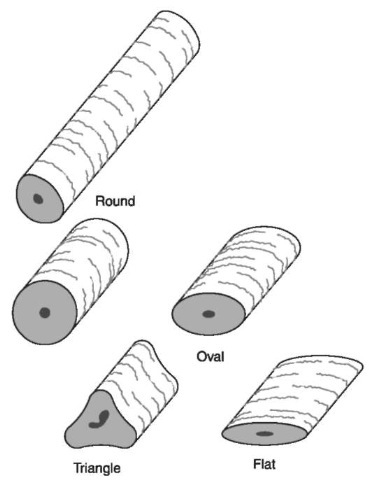
Figure 2 Cross-sectional shapes. Microscopically, hairs often appear as two-dimensional objects, but they all have a third dimension represented by a cross-sectional shape. Human hairs range in shape from round to flat. Beard hairs are often triangular in cross section.
Quality Assurance
When a questioned hair is said to be consistent with a known source, there are two possibilities. Either the hair actually originated from that source or there was a coincidental match. Since it is possible for two different individuals to have hairs that are microscopically indistinguishable, it is known that coincidental matches can occur in forensic hair comparisons.
Table 5 Terminology for microscopic hair comparisons
| Term | Definition |
| Central region | Toward the core area of the hair shaft, near where the medulla is found |
| Characteristic | Any feature which may be useful for identification and/or comparison |
| Comparison | The process of examiningtwo or more hairs for the purpose of either identifyingthem as having |
| come from the same class of hairs or attemptingto associate them with or disassociate them from | |
| a given individual | |
| Competency test | Tests given at the end of the training period, successful completion of which demonstrates an |
| examiner’s ability to perform forensic hair examinations in casework | |
| Dissimilar | Significant unexplained differences between questioned and known hairs |
| Distal end | The end of the hair distant from the root |
| Feature | Any morphological part of a hair that reflects the hair’s appearance, texture, or microscopic |
| characteristics and traits | |
| Identification | The process of determining that a given hair belongs to or came from a defined class of hairs (e.g. |
| species, somatic or racial origin) | |
| Individualization | The process of determiningthat a given hair came from one particular (individual) source to the |
| exclusion of all other similar sources | |
| Known | A sample taken as representative of a particular body area of a specific individual |
| Limited sample | A sample of hairs known not to be of sufficient number or quality to represent all possible |
| characteristics or traits | |
| Match | The association of known and questioned hairs by showingthat the hairs are similar in all respects |
| Medial region | The portion of the hair intermediate between the proximal and distal ends |
| Peer review | A confirmation process where a technical peer (colleague) independently reviews the conclusions |
| and supportingdata from the hair examiner before it is reported | |
| Peripheral region | Toward the outermost areas of the hair, including the cuticle and the cortex distant from the medulla |
| and near the cuticle | |
| Practical examination | Practice tests given during the training period |
| Proficiency test | A quality assurance measure used to monitor performance and identify areas where improvement |
| may be needed | |
| Proximal end | The end of the hair nearest the root |
| Questioned | A sample of unknown origin collected for the purpose of identification and/or comparison with a |
| known sample | |
| Range | The complete set of values or traits exhibited by the hair of one individual with regard to a specific |
| characteristic | |
| Representative sample | A sample of hairs that is expected, from a statistical standpoint, to represent the range of values or traits |
| Sample | One or more hairs used for identification, comparison or reference |
| Similar | A conclusion when the combination of microscopic characteristics, values, and traits are exhibited |
| by the known and the questioned hair sample with no significant unexplained differences; or when | |
| the known and questioned hairs are microscopically indistinguishable | |
| Trait/value | The qualitative or quantitative assessment of a particular variable characteristic based on a single |
| observation | |
| Verification | A confirmation process where a second qualified hair examiner microscopically reviews the results |
| from a comparison, employing the same methodology as the first examiner, and is free to agree or | |
| disagree with the conclusions of the first |
One way to increase reliability and limit the number of coincidental matches is to insure the hair examiner is: properly and thoroughly trained, experienced, allowed time to reach the decision, allowed negative and inconclusive determinations when evidence available does not warrant more, allowed access to the background and investigative information, and allowed to consult with a colleague to verify the analysis. A better quality assurance technique is verification of conclusions by another qualified examiner. Peer review is less valuable than the redundant analysis required by verification. Proficiency tests, which are difficult to design, are useful for training, practice and gaining a better understanding of the comparison process, but they cannot replace peer review and verification.
Where possible, laboratories should employ a confirmation or verification process whereby the hair examiner, having determined that questioned hairs from items of evidence and known hair standards exhibit the same microscopic characteristics, then takes these hairs to another qualified examiner. The second hair examiner reviews the hairs microscopically, employing the same methodology as the first hair examiner. The second examiner is free to agree or disagree with the first examiner’s results, but only when there is agreement are the hairs associated.
Table 6 Fundamental concepts of microscopic hair comparisons
| Concept | Definition |
| 1 | All observable characteristics of both the known and unknown hair specimens must be considered |
| 2 | A comparison involves the search for basic dissimilarities which cannot be accounted for by a logical, common sense |
| explanation | |
| 3 | A single significant difference between the two is a strong indication of two sources |
| 4 | Several repeated fundamental dissimilarities establish without a doubt that two specimens are not from a single |
| individual | |
| 5 | Sometimes inconspicuous traits distinguish the hair of two samples that appear very much alike otherwise |
| 6 | No two specimens of hair from one individual are identical in every detail |
| 7 | Known and questioned hairs need not be identical in the sense that the two sets of hair can be matched bit by bit |
| 8 | Differences between the questioned and known hairs cannot exceed the variation found in the known sample |
| 9 | As with all types of associative evidence, quality exemplars are mandatory and no conclusions are possible without |
| 10 | them Under no circumstances can an association be established by one, two, or even several unusual characteristics alone |
| 11 | A combination of a sufficient number of points of agreement with a coexistent lack of basic divergencies between the |
| questioned and known hair is required for an association | |
| 12 | If the questioned hairs were mixed with the exemplar, and the hairs could not be distinguished or retrieved, the |
| association is justified | |
| 13 | The degree of certainty given an association depends on the individuality of the hair and the amount of questioned hair |
| available for comparison | |
| 14 | The conclusions drawn from a forensic hair comparison are based on statistical considerations, related experience, |
| 15 | scientific taste, and forensic judgment |
| The conclusions must be reasonable because their impact is determined by their convincingness | |
| 16 | All associations are accepted provisionally. The provisional associations are tested by examiningalibi and elimination |
| samples. When these alibi samples are scientifically eliminated, the association gains probative value |
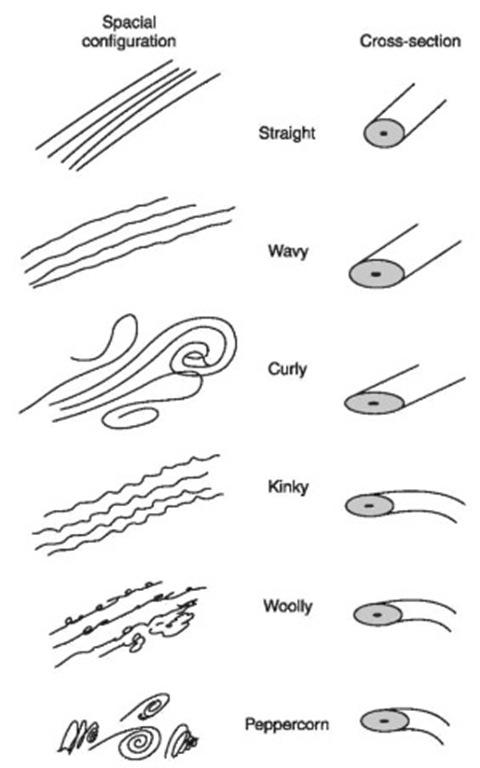
Figure 3 Human hair forms. The cross-sectional shape largely determines the spatial configuration. Hair forms for different human populations vary from straight with a round cross-section to peppercorn with a flat twisting cross-section.
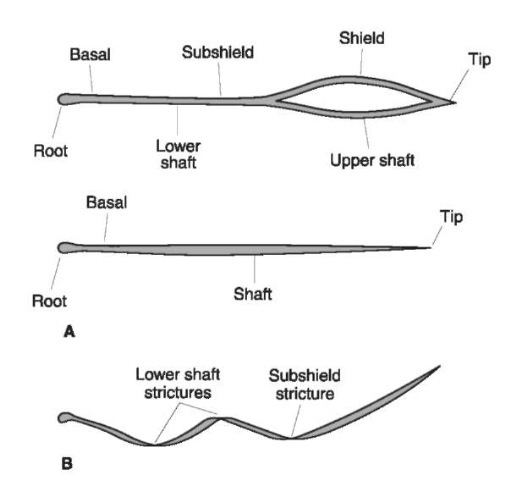
Figure 4 Regions of shielded and non shielded hair. The parts (A) and locations of strictures (B) of a common animal hair shaft are noted from tip to root including the blade.
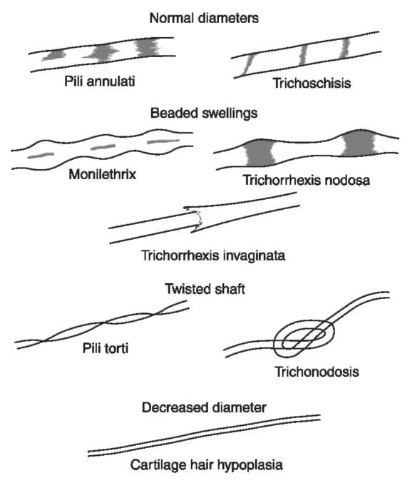
Figure 5 Hair shaft abnormalities. Sometimes human hair grows in abnormal ways due to disease. These hairs, when viewed microscopically, sometimes have normal diameters with crossway markings, beaded swellings, twisted shafts or decreased diameters.

Figure 6 Scale terminology and patterns. The scale patterns on the hair of wild animals vary greatly between different families of animals and sometimes along the length of a single hair. These patterns can be visualized when the surface scales are reproduced with a cast or viewed with a scanning electron microscope.
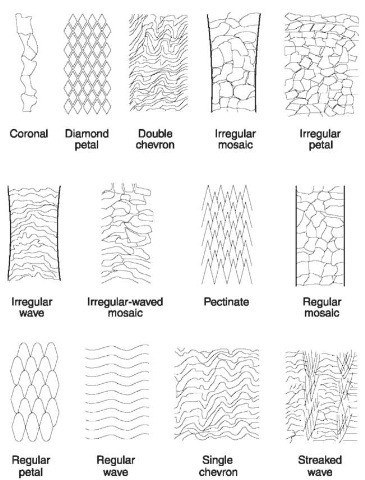
Figure 7 Scale patterns. Details of patterns seen on hairs of different families of animals.
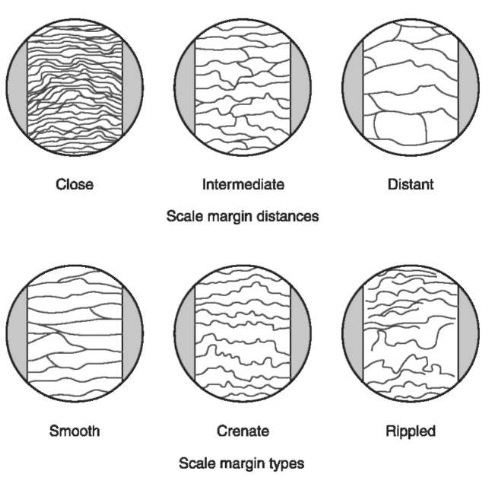
Figure 8 Scale margins. At higher magnifications, three common patterns on the leading edge (exposed margins) of animal hair scales are found. The distances between the scale margins can be described as close, intermediate or distant or simply measured with a microscope.
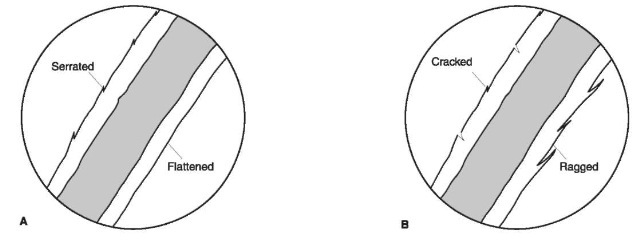
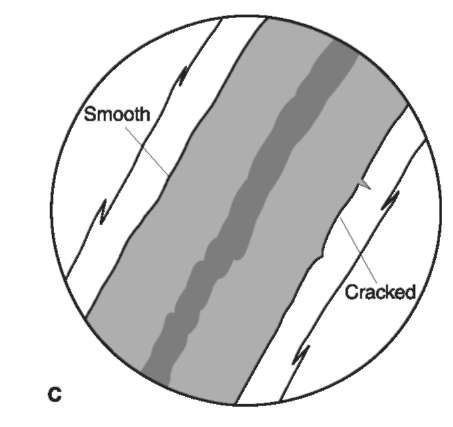
Figure 9 Outer and inner cuticular margins. When viewed with transmitted light, in a medium with a refractive index near that of hair, the cuticle of human hairs appears as a transparent thin band on either side of the hair shaft. The outer cuticular margins (outside surface of the hair shaft) (A, B) can be flattened, serrated, cracked or ragged whereas the inner margin (interface between cuticle and cortex) (C), if distinct, can be smooth or cracked.
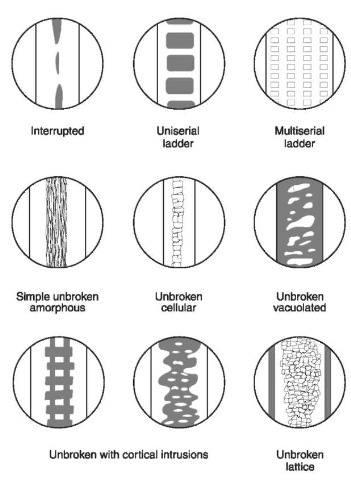
Figure 10 Medulla types. The medulla in animals varies greatly. In humans the medulla is always less than half the width of the hair shaft; in animals it usually fills more than half of the shaft diameter. The various patterns for medullae found in domesticated and wild animals are shown.
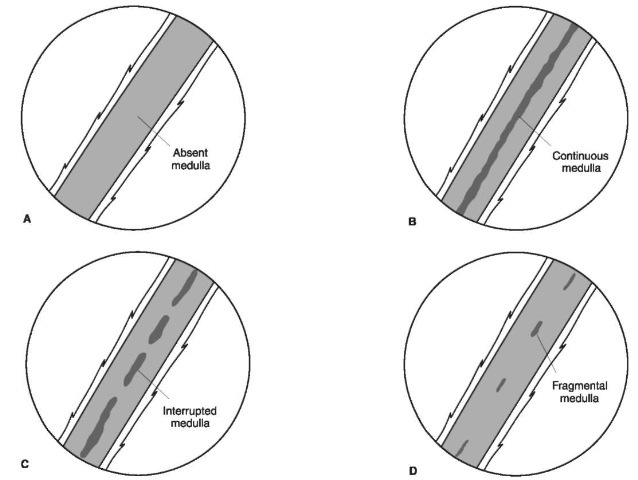
Figure 11 Medullation. In humans the medulla may be absent (A), continuous (B) or discontinuous (C, D). In an interrupted, medulla (C) the length of the medullary band is greater than the length of the space between. In a fragmental medulla (D) the medullary band length is shorter than the distance between.
Conclusions
In order to conclude that a questioned hair is associated with a known source, it must first be determined that the characteristics exhibited by the questioned hair fit within the range of characteristics present in the known sample. In addition, an association can be strengthened if there is also a one to one correspondence of all characteristics between the questioned hair and one or two known hairs with no significant differences over the entire length of the hair.
