Introduction
The determination of alcohol (ethanol) in the blood of human subjects is one of the basic analytical procedures of an analytical toxicology laboratory. Alcohol is a commonly abused substance in most societies, taken deliberately for its mood altering effects.
When those effects result in legally harmful consequences to the imbiber, or others affected by the imbiber’s actions, then a precise determination of the concentration of alcohol in the body is required. This specific determination allows for a proper interpretation of the degree of mood alteration. More importantly, it allows a means of assessing the degree of impairment in the ability to operate complex machines, e.g. motor vehicles.
The measurement of alcohol alteration should, in theory, be in the brain but for obvious reasons this cannot be done in live subjects nor is it necessary. The alcohol concentration in the brain has a direct correlation with the arterial blood alcohol concentration. Interestingly, the arterial blood alcohol concentration can be indirectly measured via breath. Breath measurement has become the method of choice in law enforcement of drinking-driving laws, but measurement of blood is still widely used.
Because of the high risk associated with extracting arterial blood from live subjects, venous blood samples are used for alcohol determinations. Capillary blood is another alternative and offers the advantage over venous blood of being more closely correlated to the arterial blood alcohol concentration.
Collection
Venepuncture of a cubital vein in an arm is the standard technique used clinically for extracting venous blood. This procedure offers the most convenient yet relatively safest way to draw blood from a live subject. The alcohol content of these samples will represent the systemic blood concentration of alcohol, and the measured value can be readily correlated with the behavioral effects of alcohol.
An alternative to venous blood is capillary blood obtained by finger-tip puncture. This blood, obtained from the fleshy tissue of the finger-tips, represents a transition from arterial to venous blood. As such, the alcohol concentration more closely represents the concentration in arterial blood. The major disadvantage of finger-tip blood is the very limited quantity that can be extracted. The amounts extracted are usually just sufficient to perform an initial analysis with no chance of repeat if subsequently required. Thus, venous blood has become the predominant choice for forensic purposes, since the extraction is relatively more comfortable for the subject, allows more than sufficient quantity of blood for repeated analyses, and easy correlation with behavioral effects.
In deceased subjects, a wider selection of sites for blood collection becomes available. However, the wider selection is counterbalanced by the complications in interpretation arising from the differences in alcohol concentration among the sites. As for living subjects, venous blood extracted from a vein in the arm or leg provides the optimum sample for interpretive purposes. The least desirable site is an open chest cavity that results from an autopsy examination.
Blood of uncertain origin (heart, vein or artery) may gather in the chest and mix with the interstitial fluid that bathes the organs of the chest. This mixed fluid is often scooped up and submitted for alcohol analysis as a ‘blood’sample. The alcohol analysis produces a result which presents difficulties in interpretation since the sample does not truly reflect a genuine blood origin.
In forensic work, the collection of a blood sample must observe certain precautions related to the integrity and security of the sample. This is particularly so when the result will be used in the prosecution of an offense charged against an individual, such as the operation of a motor vehicle while under the influence of alcohol. Precautions are most crucial when the offense is that of exceeding a specified statutory blood alcohol concentration (BAC) in the operation of motor vehicles.
Swabbing the injection site with an alcoholic antiseptic solution is a common practice in clinical settings. Alcohol swabbing compromises the integrity of a blood sample taken for alcohol analysis and is discouraged for forensic purposes. Unfortunately, microorganisms residing on the skin, potentially on the surfaces of apparatus used for taking the blood, or suspended in the ambient air, can contaminate the blood sample. Such microorganisms utilizing blood sugars can produce alcohol through a fermentation process. Conversely, microorganisms could also use alcohol present in the blood from drinking as an energy source. Either way, the true BAC of the person is compromised, leading to difficult interpretations.
Blood alcohol kits, such as pictured in Fig. 1, have been developed expressly for forensic purposes. These kits are self-contained, requiring no additional apparatus whose cleanliness and alcohol-free status may be open to question. The kits contain tubes that are sterile and contain a preservative, such as sodium fluoride with an anticoagulant. Tubes used in Canada contain sodium fluoride to produce a final concentration of 1% w/v and potassium oxalate as anticoagulant to produce a final concentration of 0.2% w/v. The preservative stabilizes the blood sample for an indefinite period of up to several months, if required. The anticoagulant prevents clotting, an undesirable feature when analyzing the blood. Although not essential, the anticoagulant nevertheless simplifies the analysis by eliminating the step of homogenizing clotted blood. This step would otherwise require the use of homogenizing apparatus, a messy and somewhat cumbersome procedure when dealing with whole blood. Refrigeration of the blood samples at approximately 4°C is recommended for prolonged storage. Experimental data have shown that blood concentrations decrease slightly over time when stored at room temperatures. The exact cause of the decrease is not certain but is thought to be either evaporation of the alcohol around the rubber stopper, or oxidation to acetaldehyde using oxygen from oxy-hemoglobin (the red pigment in the blood). Refrigeration stabilizes the blood alcohol for periods of up to six months. The preservative, sodium fluoride, and the commonly used anticoagulants, do not interfere with the analytical procedures in current forensic use.

Figure 1 Blood collection kit with two vacuumblood tubes (a, b), sterile needle (c), needle holder (d), nonalcoholic swab (e), seals (f) and packaging (g, h).
It should be noted that analysis for forensic purposes requires whole blood. Hospital and clinical labs routinely separate the fluid portion, plasma, from the blood. The plasma can be conveniently analyzed for a number of chemicals, including alcohol, using automated equipment specifically designed for that purpose. Forensic or toxicology laboratories, however, are more likely to analyze whole blood for alcohol since statutory limits for legal operation of motor vehicles are expressed in terms of whole blood, e.g. 80 mg of alcohol in 100 ml of blood. Alcohol concentrations expressed in terms of plasma have to be converted into the equivalent concentration in whole blood. Since this conversion is directly related to the water content of the blood of the individual, a degree of uncertainty is introduced at this point. The water content, or hematocrit value, varies not only among individuals but within the same individual. Hence, the equivalent whole blood concentration cannot be predicted precisely, but only within a range of values with acknowledgment to possible values outside that range.
Analysis Chemical
Analysis of blood for alcohol has been performed for the better part of the twentieth century. Wet chemical methods using an oxidation-reduction reaction were developed to analyze alcohol condensate distilled from the blood. One of the more widely used methods was developed by E.M.P. Widmark, a Swedish scientist considered the classic pioneer worker in systematic studies of alcohol in the human body. This method of incubating blood samples suspended above a dichromate-acid solution at raised temperatures in enclosed flasks proved a reliable method. The procedure was modified in subsequent years, most notably in the early 1950s. This procedure, labeled the modified Widmark procedure, is still used currently for verification of alcohol content in blood standards, or as a crosscheck on other procedures. The wet chemical methods provide satisfactory accuracy and precision, but the nature of the procedures, including corrosive chemicals, make them less than desirable to use. In addition, these procedures lack the degree of specificity for ethanol considered necessary for forensic purposes. These procedures cannot distinguish ethanol from other common alcohols such as methanol or isopropanol. Methanol is widely used as an industrial solvent, labeled as methyl hydrate, and in such products as windshield washer fluid, or gasoline line antifreeze. It is a toxic substance sometimes found in illicitly distilled alcoholic beverages. In some instances it has been added to beverages as a cheap alternative to ethanol. Isopropanol (2-propanol) is also an industrial solvent but can also be used as a rubbing alcohol in pharmaceutical preparations. Again this toxic substance is sometimes ingested resulting in poisoning in humans. Hence, the usefulness of oxidation-reduction methods in a broad forensic context is limited to samples taken from motor vehicle drivers who have consumed alcoholic beverages only. Alternative methods are required for subjects who may have ingested the other volatile substances, either deliberately or unintentionally. In post-mortem examinations, the possibility of micro-bial-induced deterioration of the body, or putrefaction, becomes a distinct possibility. Microorganisms not only produce ethanol but can also produce other volatile substances which can interfere with the alcohol analysis. These other volatile substances are indicative of putrefaction and can be used as confirmation for the putrefaction process. Chemical methods, such as the modified Widmark procedure, are unable to distinguish these volatile substances from ethanol and therefore are unsuitable for identifying possible putrefaction in exhibit material.
Biochemical
Enzyme oxidation is another methodology first developed in the 1950s for measuring alcohol. A number of variants have been subsequently developed, centred around the basic enzymatic reaction:
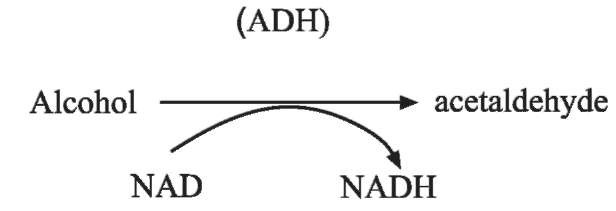
where ADH is alcohol dehydrogenase and NAD+ is the coenzyme nicotinamide adenine dinucleotide. NAD+ is reduced to NADH in direct proportion to the concentration of alcohol present in the sample being analyzed. The NADH can be measured by its absorption of ultraviolet (UV) radiation at 340 nm.
The ADH analysis was developed into a kit form which has found widespread use in hospital or clinical laboratories. The kit form is best suited to laboratories that only perform alcohol analysis on an infrequent basis. In these situations the use of more expensive and elaborate methods would not be feasible. In more recent years, automated analyses using batch analyzers were developed which allowed the processing of more samples.
In addition the ADH procedures were designed for measuring ethanol only, a limitation in forensic work. In the forensic field, toxicology laboratories must be capable of detecting, confirming and, if necessary, quantifying any volatile substance present that is foreign to the human body.
Instrumental
The advent of gas chromatography (GC) proved a welcome advance in forensic analysis for alcohol. Although the equipment has a high capital cost and requires high purity compressed gases, the principle of operation is relatively simple, fast, accurate and precise with a high degree of specificity.
The basic principle of GC is shown in Fig. 2.A sample is vaporized in an injection block and then carried through a long thin column, usually stainless steel, glass, or fibrous material, by an inert carrier gas such as nitrogen or helium. The column can either be of the packed variety, i.e. packed with an adsorbent material, or an open tube of very small internal diameter. The latter type, known as capillary columns, have internal diameters of less than 1 mm. The inner walls of capillary columns are coated with an adsorbent film.
The vaporized sample emerges from the other end of the column where the components come in contact with a detector. The volatile components of the original sample can be visually displayed either on a computer screen or on paper.
The separation and movement of the components in the blood sample are determined by the rate of carrier gas flow, the temperature of the column and the type of adsorbent material utilized. The gas flow and oven temperature can be adjusted to achieve optimum conditions for the clear separation of volatile components from one another. The adsorbent

Figure 2 Schematic diagram of GC instrument.
material will retain the volatile components to varying degrees depending on the degree of molecular attraction of each component with the adsorbent material. Passage of the components can take 15-20 minutes or so. Capillary columns, on the other hand, not only allow very fast passage, 5 minutes or less, but also result in sharper, cleaner separation of the components. Hence, capillary columns have become the separatory columns of choice in GC analysis.
The type of detector most commonly used is the flame ionization detector (FID). Water does not register on FID hence this detector is the one of choice for water-based samples such as blood. Although the signal produced by the detector is an analog one, the electrical impulses can be digitized. This process takes place in an analog-to-digital (A-D) converter from whence the digital impulses can be quantified. By measuring the number of impulses or counts from a known concentration of alcohol, one can compare that number with the number produced by an unknown concentration of alcohol. The concentration of the unknown can be calculated from the known since the response of the detector is directly proportional to the concentration of alcohol.
Although this quantification is a simple process, it is highly subject to variances in the volume of sample injected onto the GC column. Consequently, a more reliable technique employed by laboratories is the use of an added internal standard (ISTD). A blood sample is mixed with a solution containing another volatile compound (ISTD) such as n-propanol to produce a diluted sample. This diluted sample is easier to handle than straight blood and the volume injected onto the GC column is not critical. The ISTD must be volatile and must be clearly separable from the alcohol being measured. The ratio of counts between the ISTD and the alcohol is a constant regardless of the variation in volumes mixed or the volume of diluted sample injected onto the GC column.
Figure 3 shows a typical apparatus, known as an automatic dilutor, which will extract an aliquot of blood from the tubes featured in the background then dispense that aliquot automatically mixed with an aliquot of ISTD solution. The ratio of blood to ISTD solution is usually in the order of 1:10 to produce a sufficiently watery blood dilution to minimize the matrix effect that can affect the analytical results.
Although the diluted sample can be manually injected, this procedure is labor intensive and time consuming. Hence, manual injection has largely been replaced with automatic injector systems. These systems sit on top of a GC instrument and automatically extract small aliquots of the diluted samples and inject them onto the GC column.
The injection of diluted blood samples even with automated injector systems, has the disadvantage of clogging GC columns and injection apparatus with blood proteins. These proteins shorten the life of GC columns and easily clog the small bore tubes and needles of the injection system. As a result, the technique known as headspace (HS) sampling introduced during the 1960s has become the preferred method for GC analysis of blood samples. Instead of injecting an aliquot of the diluted blood sample, a portion of the vapor sitting above the aqueous sample is injected onto the GC column. GC columns do not deteriorate as rapidly, injection systems do not clog with blood proteins, and many thousands of injections can be made on the same column.

Figure 3 Automatic diluter apparatus for mixing blood samples with ISTD solution.
Reliable analysis using HS-GC requires that the diluted samples be incubated in sealed vials with large headspace volumes at controlled temperatures. This incubation period allows full equilibrium of the vapor portion above the liquid sample. Fig. 4 shows a typical HS-GC set up. The large instrument in the middle is the GC instrument itself with a control panel on the right side to set injector port, column and FID temperatures. On the left side are the controls to regulate carrier gas flow. Two sets of controls are shown since most GC instruments permit two separate columns to be housed in the same oven. To the left of the GC instrument is the headspace apparatus in which the vials are placed. This apparatus requires its own carrier gas supply which is injected into the vials to flush the headspace and carry the alcohol-ISTD vapor sample onto the GC column. The control box for the apparatus is to the left. On the right of the GC instrument is an optional integrator with printout if a computer system is not available, or cannot be justified on financial grounds.
On the extreme left of the figure is an A-D converter which relays the FID signal to a computer (not shown) which processes the signal and reports the BAC.
Ideally a GC system, especially HS-GC, will separate the common volatile substances likely to be encountered in forensic work accurately and precisely. Fig. 5 and 6 show representative separations of a mixture of four such substances together with the ISTD (n-propanol). Two different columns on different GC instruments result in different retention times (time taken for each component to move through the columns) and in a different order.

Figure 4 HS-GC instrument system with GC instrument (a), HS analyzer (b), A-D converter (c), and optional integrator with printout (d).
Analytical Quality Assurance
A laboratory that conducts forensic alcohol analysis on body fluids should have documented procedures in place. These procedures should identify such items as methods of analysis, quality control procedures, and performance criteria for accuracy, precision, and specificity.
A suggested protocol is outlined as follows:
I All exhibit samples should be analyzed for ethanol and other similar volatile substances using validated methods.
Validation of methods:
1. Compare the new method with an accredited method.
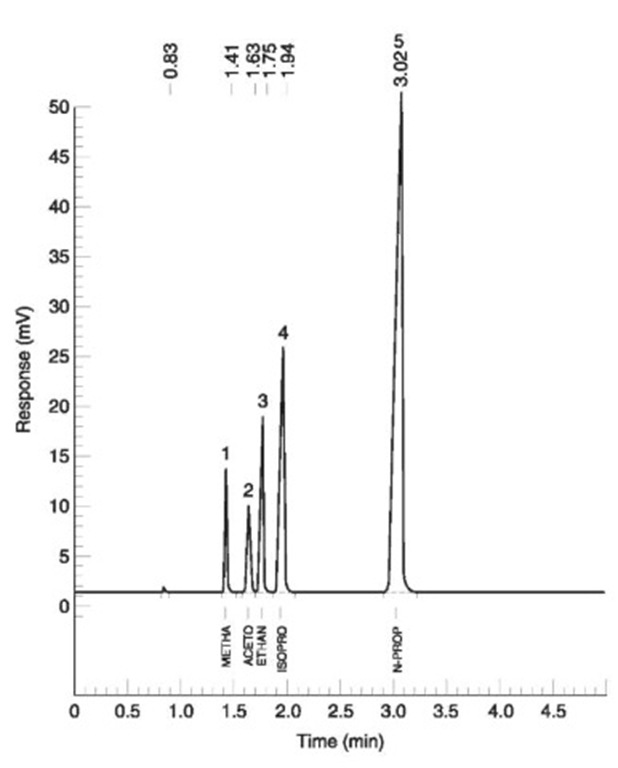
Figure 5 FID #1. Column: DB Wax x 0.32 mm x 10 m, 0.5 urn film; joined to DB-1 0.32 mm x 20 m, 5.0 umfilm. 1, Methanol; 2, acetone; 3, ethanol; 4, isopropanol; 5, n-propanol (internal standard).

Figure 6 FID #2. Column: DB-1 0.32 mm x 30 m, 5.0 umfilm.1, Methanol; 2, ethanol; 3, acetone; 4, isopropanol; 5, n-propa-nol (internal standard).
2. Perform a minimum of ten replicate analysis on five different concentrations of alcohol standards specified in the range of 0-400 mg in 100 ml of solution (mg%).
(a) Perform a statistical comparison of the data obtained to determine if there is a significant difference between the two methods; and
(b) Perform a statistical analysis to determine the limit of detection (LOD) and the limit of quantitation (LOQ) for the method.
(c) Determine the specificity of the method.
(d) If no statistical difference between the new method and an accredited method is determined, and the limits of detection and quantification and the specificity are acceptable; then approve the new method for use.
II A quality control procedure of a validated method should be conducted with exhibit analysis:
1. Quality control standards could be prepared either by weight or by volume in either water purified by distillation or deionization or whole blood. (Sheep’s blood is recommended, but human blood could be substituted).
2. For calibration checks, replicate analytical results from the quality control standards with concentrations less than 100 mg% should not exceed + 3 mg% of the target value; and replicate analytical results from quality control standards with concentrations of 100 mg% or greater should not exceed +3% of the target value. 3. When calibration check results do not meet the specified performance standards, analysts should identify and redress any nonconformance in the quality control procedure prior to conducting analysis on exhibit material.
III All exhibit material should be analyzed using at least two aliquots of the sample.
1. For volatile substance concentrations of less than 100mg%, all replicate results should not exceed + 5 mg% of the analytical mean result. For volatile substance concentrations of 100 mg% or greater, all replicate analytical results should not exceed + 5% of the analytical mean result.
2. Any reported volatile substance in samples should be confirmed by a second method.
3. The volatile substance concentration should be reported in terms of the body fluid analyzed.
4. Ethanol, acetone, methanol and isopropanol should be reported when their concentrations exceed 3 mg%. Other volatile substances, detected and verified, should be reported when the results are of pharmacological significance.
Laboratories that conduct forensic alcohol analysis should hold available for potential court testimony all documentation pertaining to the testing procedures. All test materials should be treated as evidence with appropriate security, proper documentation, retention and storage of records and items. Laboratories engaged in forensic alcohol analysis should employ the services and advice of at least one qualified forensic scientist who specializes in the analysis of body fluids for alcohol. A qualified forensic scientist is a person who, using the appropriate combination of knowledge, skill, experience and integrity, undertakes one or more of the following tasks: analysis of evidence materials, interpretation of evidence, presentation of expert testimony.
Laboratories should participate in recognized external proficiency tests for alcohol. Such tests should be conducted on at least an annual basis. Corrective action should be taken whenever deficiencies are revealed by the proficiency test process. Analysis of exhibit material should be suspended until such deficiencies are corrected.
