Introduction
Bloodstains are often found at various types of crime scene, such as homicide, hit-and-run, assault, child abuse, rape, robbery and burglary. In addition, forensic scientists are often called upon to examine clothing, weapons, vehicles and other items of physical evidence to determine whether a victim’s or a suspect’s blood has been transferred to those items. In examining blood evidence, the questions which the forensic scientist must answer are: Is it blood? If it is blood, is it human? If it is human, what information towards individualization is possible?
Since the discovery of the ABO system by Land-steiner in 1900, knowledge in human blood identification has expanded tremendously. With the advent of DNA typing technologies in the 1980s an individual bloodstain can now be identified through genetic variation at a molecular level. Further testing procedures allow for greater individualization by DNA typing than was ever possible by classical serological techniques of even just a few years ago. DNA methodologies also allow for the analysis ofsamples which are highly degraded or present in extremely small quantities. Due to the sensitivity of the polymerase chain reaction (PCR) DNA typing procedures, a minute quantity of DNA recovered from blood, as well as trace amounts of semen, tissue cells, and a variety of body fluids, can yield conclusive typing results. Therefore, it is essential to establish positively the nature of a biological stain and that a stain is, in fact, blood, before conducting further analyses or rendering an opinion concerning the genetic testing result. In addition, certain types of forensic investigations may also require the determination of the species of origin of a bloodstain. In some cases it may be necessary to confirm the presence of human blood in a questioned stain before obtaining a known sample from a suspect or a victim. Thus, it is important to determine if a blood sample is human before proceeding with further genetic testing.
Many techniques have been developed to address these questions. The present state of bloodstain evidence examination is summarized in Fig. 1 . This article is concerned only with the identification of bloodstains. Discussions of genetic marker typing of bloodstains and the identification of other body fluids can be found elsewhere.
Composition of Human Blood
Blood is a complex fluid tissue composed of a liquid portion, plasma, and cellular components. Plasma is a mixture of dissolved proteins, salts and other chemicals. The blood cells are of three main types: red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes). Table 1 identifies the major components of blood which can be used for identification purposes.
Erythrocytes are manufactured in the bone marrow. In mammals, the cells lose their nuclei before being released into the circulatory system, and are found as biconcave disks in circulating blood. The average normal red blood cell count is 4.5-5.4 million cells ml — 1. Each human red cell is about 7.5 umin diameter and 2 um thick. The membranes of human red cells contain a variety of antigens called aggluti-nogens. Red blood cells function to transport oxygen and carbon dioxide and are packed with the complex compound hemoglobin. The pigmented portion of hemoglobin, the heme molecule, gives the red color to the erythrocytes. It is this heme molecule in a stain which is used to identify the presence of blood in a sample of unknown origin.
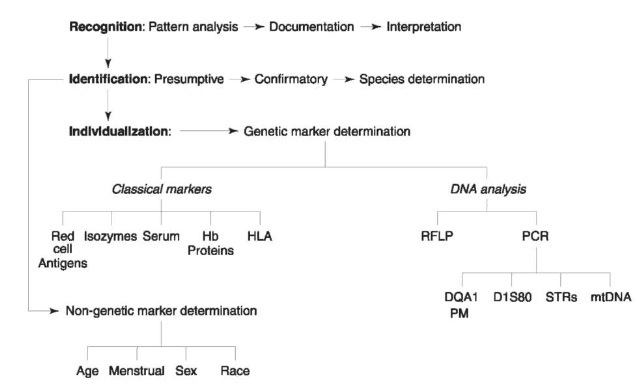
Figure 1 Present approaches to bloodstain analysis. Hb, hemoglobin; HLA, human leukocyte antigen; RFLP, restriction fragment length polymorphism; PCR, polymerase chain reaction; STRs, short tandem repeats; PM, polymarker.
There are normally 4000-10 000 white blood cells ml-1 of blood in the healthy, adult human. White blood cells function as part of the body’s defense system and can be of several types. The gran-ulocytes or polymorphonuclear leukocytes are the most numerous. Most granulocytes contain neutrophilic granules (neutrophils); a few contain granules that stain with acid dye (eosinophils), and some have granules that stain with basic dyes (basophils). The other white blood cell types found normally in blood are lymphocytes, cells with large round nuclei and little cytoplasm, and monocytes, cells with abundant cytoplasm and kidney-shaped nuclei. Genetic marker analysis of blood is possible because DNA can be extracted from these nucleated leukocytes.
Platelets are small, granulated bodies, 2-4 umin diameter. There are about 300 000 platelets ml-1 in circulating blood. These cells are involved in the blood clotting mechanism.
Methods for the Identification of Blood
Bloodstain identification is commonly achieved by one of five methods of analysis: (1) microscopic examination; (2) chemical methods; (3) spectrophoto-metric analysis; (4) immunological methods; and (5) DNA analysis. When using one of these methods, blood is detected by the identification of characteristic components of human blood. These techniques have different levels of sensitivity and specificity, but the test which is employed often depends on whether it is being used as a field test or as a laboratory confirmatory test.
Table 1 Components of human blood
| Component | Description | Function |
| Plasma | Fluid portion of blood: 90% water; 10% solutes, including | Transport of soluble nutrients and waste, |
| proteins (primarily albumins, globulins, fibrinogen, and | coagulation | |
| enzymes), glucose, amino acids, lipids, metabolic | ||
| compounds, respiratory gases, hormones | ||
| Cellular components | ||
| Erythrocytes | Biconcave, enucleated cells containing hemoglobin | Transport 02 and C02 |
| (red blood cells) | ||
| Leukocytes | ||
| (white blood cells) | ||
| Granular | Neutrophils, eosinophils, basophils | Phagocytosis and immune response |
| Nongranular | Lymphocytes, monocytes | Cellular antibody formation |
| Platelets | Irregular, fragment-like appearance | Initiate blood clotting |
Microscopic identification of bloodstains
As liquid blood dries and forms a bloodstain, red blood cells, like other cells, dehydrate. If placed in an environment with a higher solute concentrate, water leaves the cell by osmosis and the cells shrink and change shape. If stains are relatively fresh, it is possible to reconstitute the stain and proceed with microscopical identification of cellular components. A number of techniques have been reported for microscopic examination of erythrocytes and leukocytes in bloodstains. The results obtained with these methods are much affected by the conditions of the bloodstains. Aging, environmental factors, or heating can considerably alter blood cells and make it difficult to produce interpretable and reliable results. In addition to aiding in the identification of a sample as blood, the microscopic appearance of cells found in a stain extract may also reveal other information. For example, sickle-shaped erythrocytes may indicate that the blood sample originated from a person having sickle cell disease.
Reconstituting the blood cells with a solution to restore their original shape can be attempted with the following techniques.
1. Put a fragment of fresh blood crust on the center of a clean slide.
2. Add a drop of a solution of albumin:glycerol: 0.85% saline (20:20:60 by vol.) on the crust and mix gently until the crust is dissolved.
3. Place the slide in a moisture chamber for two hours at room temperature.
4. Prepare a thin film smear of the mixture and air dry.
5. Stain with Wright’s stain or other polychromatic stain suitable for blood samples.
6. Examine the slide under the microscope for red blood cells and white blood cells.
Chemical identification of blood
When blood dries to form a bloodstain, the cells are destroyed and their contents released into the surrounding environment. More than 250 proteins, enzymes, and other compounds have been found in the red blood cell, mostly in the soluble portion of the erythrocytes. The predominant erythrocyte protein is hemoglobin (Hb). More than 100 variants of hemoglobin have been described. Identification of blood in stains by means of chemical methods is based on the detection of heme or its derivatives in the stain sample. Such tests can be classified under one of two categories: catalytic tests and crystal tests.
Catalytic tests (screening or presumptive tests) All catalytic blood tests depend on an oxidation reaction in which an oxidant, for example, hydrogen peroxide, oxidizes a colorless material, such as phenol-phthalin or tetramethylbenzidine, to a colored one. Alternately, 3-amino-phthalhydrazide (luminol), a colorless material, can be oxidized to a product which luminesces. The general presumptive test reaction is:
H202 + reduced reagent (color 1) <— H20 + oxidized reagent (color 2).
The heme group of hemoglobin exhibits a peroxidase-like activity which may catalyze the breakdown of hydrogen peroxide. The majority of tests which have been devised for the forensic identification of blood are based on the peroxide-mediated oxidation of leukomalachite green, phenolphthalin, o-tolidine, luminol, tetramethylbenzidine, fluorescein, and other less commonly used compounds. At one time, benzi-dine and its derivatives were widely used as the color reagent in screening tests for blood. However, due to the carcinogenic nature of these compounds and the health risks involved in their use, laboratories no longer use these types of chemical reagents. The tests most commonly employed in modern crime scene procedures are phenolphthalin, leukomalachite green, luminol and tetramethylbenzidine. Reaction schemes for some of these common chemical reagents are shown in Fig. 2. All of these chemicals are highly sensitive to minute traces of hemoglobin and its derivatives, but all suffer from the occurrence of false positive reactions with some of the following materials: catalases, peroxidases, cytochromes, strong oxidizing agents and metallic salts.
Testing procedures Prior to testing, the nature, color and appearance of the stain should be noted. These are important data which will assist the scientist in interpreting any positive reactions noted with the test reagents. All efforts should be made to limit alteration of the stain or pattern while performing the screening test for blood. The screening test for blood should be performed by scraping a small sample from the stain or removing a small portion of stained material.
1. Color reagent is added to the stain material.
2. If no color develops within 30 s, a drop of 3% hydrogen peroxide is added. A resulting color change indicates that blood may be present.
3. Alternatively, the stain area may be lightly rubbed with a clean cotton swab or filter paper moistened with distilled water. Reagent and hydrogen peroxide are then added to the swab sample. This method is preferred for crime scene work and when determining which stains warrant additional testing on items of physical evidence. 4. Small samples of suspected blood mixed with other materials, such as soil, may be dissolved and the resulting supernatant tested accordingly.
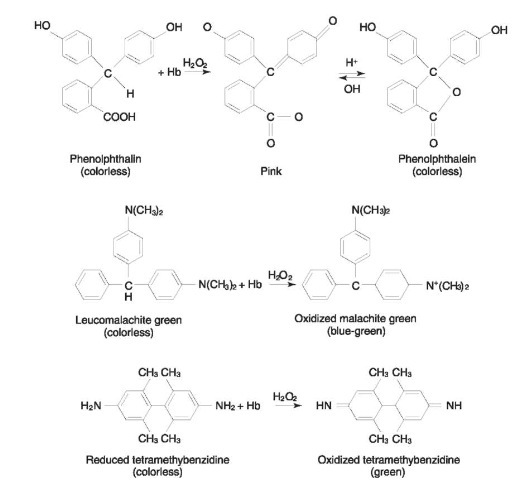
Figure 2 Reaction schemes for some presumptive test reagents.
Interpretation of results Color catalytic tests are very sensitive, but not specific. A positive color test alone should not be interpreted as positive evidence of blood. However, a negative result is proof of the absence of detectable quantities of heme or its derivatives.
When a positive result is obtained, it is necessary to consider carefully whether the test could have resulted from something other than blood. The specificity of various catalytic reagents has been studied extensively. A false positive reaction may be defined as any positive reaction given by any substance other than bloodstains. These substances may be conveniently divided into three groups.
1. Chemical oxidants and catalysts: copper and nickel salts are the chemicals which most frequently show false positive reactions. Others are rust, potassium permanganate, potassium dichro-mate, some bleaches and hypochlorites, iodine, and lead oxides. Among the common test reagents, phenolphthalin gives positive results with oxidizing compounds such as copper, potassium ferricyanide, nickel and cobalt nitrates, and some sulfocyanates; luminol reacts with some compounds of copper, cobalt and iron, potassium permanganate and hydrated sodium hypochlorite.
2. Plant sources: vegetable peroxidases are the most important class of substances which show false positive reactions with chemical color tests. The following plant tissues may react with the o-tolidine or phenolphthalin reagents and be mistaken for blood: apple, apricot, bean, blackberry, Jerusalem artichoke, horseradish, potato, turnip, cabbage, onion and dandelion root. Plant material, such as horseradish, garlic, cabbage, tomato and cucumber, reacts positively with tetramethylbenzidine.
3. Animal origin. the following substances of animal origin may give positive reactions with screening reagents: pus, bone marrow, brain tissues, spinal fluid, intestine, lung, saliva and mucus. These reactions may be attributed in many cases to minute quantities of blood in the tissue or body fluid samples being tested.
1. Chemical oxidants and catalysts. the behavior of chemical oxidants is quite different from that of blood. Chemical oxidants will give a color change before the addition of the hydrogen peroxide. Therefore, a false positive reaction can be distinguished by use of a two-step test procedure in which the color reagent is added to the sample first. If no color change occurs within 30 s, the peroxide is added. A color change after peroxide addition indicates the presence of blood or other peroxidases.
2. Plant peroxidases: heme is stable at high temperatures, whereas plant peroxidases are rapidly deactivated. Therefore, heating the sample stain or extract to 100°C for 5min will differentiate the plant peroxidases from blood sources. Also, it has been found that peroxidases can be separated from hemoglobin by electrophoretic methods; however, in most instances in a laboratory or field setting this procedure is impractical. Use of some other method of blood confirmation, such as utilization of antihuman hemoglobin is most often employed.
3. Other substances of animal origin: microscopic examination of the specimen will distinguish tissue, pus, and other substances of animal origin from blood.
Crystal tests There are several crystal tests that are considered by most forensic scientists as a confirmatory test of bloodstains. Crystal tests are based on the formation of heme derivative crystals such as hema-tin, hemin and hemochromogen. In 1853, Teichmann reported that by gently heating blood with glacial acetic acid in the presence of salts, crystals were formed. The positive result is due to a combination of a halogen with ferriprotoporphyrin. The crystals are rhombic or prismatic in shape, dark brown in color, and about 10 um in size. The age of the bloodstain does not affect the formation of hematin crystals. Twelve-year-old stains have given a positive Teichmann. Bloodstains over 20 years old were also reported positive with this test. Takayama suggested a method of producing a heme complex called hemo-chromagen in 1912.
The sensitivity and specificity of the Takayama and the Teichman tests are similar: the test is positive with as little as 0.001 ml of blood or 0.1 mg of hemoglobin. While Teichman crystals may not form with stains from materials such as leather, older stains have been reported to give false negative results when tested with Takayama reagent.
Spectrophotometry methods of blood identification
Spectrophotometric procedures are seldom used at the present time in forensic analyses. This type of examination is based on the identification of hemoglobin and its derivatives through their specific absorption spectra. Absorption spectroscopy of hemoglobin was first described by Hoppe in 1862 as a means for blood identification. During the early days of the development of procedures, this method was considered one of the most conclusive tests for the identification of bloodstains. The determination of near ultraviolet and visible absorption spectra allows sufficient reliability and sensitivity for the identification of hemoglobin and derivatives such as methemoglobin, oxyhemoglobin, carboxyhemo-globin, and sulfhemoglobin.
In the near ultraviolet and visible regions of the spectrum, a complex system of absorption bands is present due to the heme portion of the hemoglobin molecule. The visible region of the spectrum of the heme derivatives differs substantially from derivative to derivative, but all have in common a strong absorption band at 400-425 nm (the Soret band). Porphyrin compounds and their derivatives from other animal or vegetable sources may share spectral characteristics with hemoglobin, hematin, or hemochromogen. Therefore, the identity of bloodstains should never be inferred solely from a single absorption spectrum.
Electrophoretic methods
Two electrophoretic approaches have been recommended for identifying bloodstains: (1) separation and identification of hemoglobin by electrophoresis and (2) separation and identification of serum proteins by immunoelectrophoresis.
Hemoglobins are conjugated proteins. After selection of an appropriate buffer pH, the charged hemoglobin molecules are moved by electrophoresis through a support medium toward the electrode with the opposite charge. Most of the substances which give false positives with chemical tests for blood are either uncharged or have a different charge from hemoglobin and are thus eliminated by this method. After electrophoretic separation, the hemoglobin fractions are visualized by staining with leu-komalachite green solution or any other catalytic color test reagent. Banding patterns may then be compared with known standards.
Immunoelectrophoresis involves the combination
of the techniques of immunodiffusion and electro-phoresis for the analysis of biological fluids. In this procedure, bloodstain extract is placed in wells in agar on a glass slide and then subjected to electro-phoresis by application of an electric current. A bloodstain extract contains hemoglobin as well as serum proteins. Under these conditions, the individual proteins move at different rates. After electropho-resis, antihuman serum is placed in a trough running the length of the slide and parallel to the path of migration. The separated proteins and antiserum diffuse toward one another, permitting the corresponding human serum proteins to undergo an antigen-antibody reaction with the antibodies and forming preciption lines at the points where these complexes form. The hemoglobin will remain near the point of origin and give a pinkish ring around the sample well. These white precipitin lines and the pinkish hemoglobin ring are a positive indication of blood. There are no other substances besides blood that will give this pattern combination. Another advantage of this method is that the species of origin of the bloodstain can be determined at the same time.
Immunological (antihemoglobin) tests
Anti-hemoglobin precipitin sera have been used for the identification of human bloodstains. The highly specific reaction obtained between human bloodstains and the antihuman hemoglobin serum allows a stain to be identified in a single operation as blood of human origin. This test can be carried out through either one-dimensional or two-dimensional diffusion techniques. A positive result with this test is not only absolute identification of a stain as blood, but also shows the stain is from a human source.
Determination of Species of Origin
After a stain has been identified as blood, it is necessary for the forensic scientist to determine whether that blood is of human origin. If it is not human, it may then be necessary to determine to what species the blood does belong. Most methods in common use for determining the species of origin are immunological in nature. If an animal is injected with a protein molecule from another species, it will sometimes recognize this protein as a foreign substance (antigen) and will produce an antiserum (antibody) which will react with such protein both in vivo and in vitro. The immunological precipitin test for medicolegal species determination in bloodstains was first used in 1901.
The in vitro antibody-antigen reaction is detected by the formation of an antigen-antibody (Ag-Ab) complex. The reaction requires the presence of three elements: antiserum, bloodstain extract (antigen) and buffer. The temperature, pH, incubation time, and ionic strength at which a precipitin reaction is performed have a direct influence on the precipitin band formation. For example, the most favorable temperature is usually between 25°C and 37°C and the optimal pH is between 7 and 8. However, the exact conditions which are optimal for a system must be determined for each new antigen-antibody system under investigation.
The specificity of the antiserum plays the most important role in species determination. Traces of contaminating antibodies in commercially prepared antisera could cause serious error. Therefore, the precise specificity of the antiserum in use must be known. Antihuman sera can be produced by injecting human serum or hemoglobin into various animals. The most commonly used antisera created by this method are produced by rabbits, goats or sheep. These antisera produce a stable precipitate. Monoclonal antibodies are also commercially available for species testing. To ensure the specificity of the anti-serum, it is imperative that laboratory scientists select by direct testing for crossreactivity and determine the strength of the antiserum by a titration method. During species determination, the same batch of tested antiserum must always be used. Only by such strict controls can the forensic scientist maintain the degree of certainty and reproducibility required for a reliable species determination.
Figure 3 depicts several methods for the determination of the Ag-Ab complex in species tests.
The following are the most commonly used methods for species determination in forensic laboratories.
Precipitin methods
Ouchterlony method: double diffusion in two directions This diffusion method was first described by Ouchterlony in 1949. It involves the use of agar gel plates with wells for both antibodies and antigens. The two reactants diffuse into the gel where the soluble antigens and antibodies form an insoluble complex -a precipitate. The Ouchterlony method allows both qualitative and semiquantitative evaluation of the reactants. Precipitin band formation gives the scientist considerable information regarding the identity, partial identity or nonidentity of the antigen and antibody reaction. It also yields information on the diffusion coefficients and concentrations of the reactants.
Crossed-over electrophoresis The crossed-over elec-trophoretic technique can be used for both quantitative and qualitative determination of a blood sample.
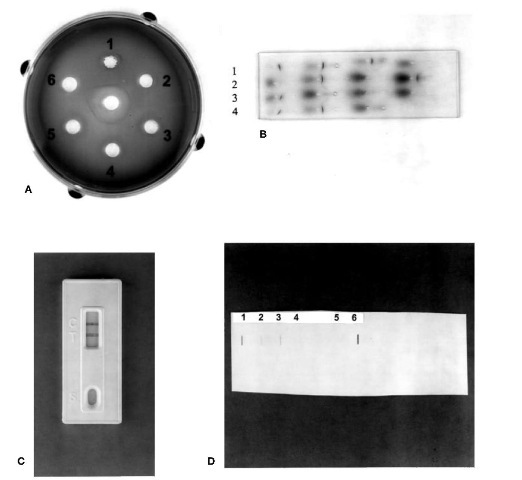
Figure 3 Common methods for species determination. (A) Ouchterlony double diffusion: samples Ithrough 5 show a positive reaction with antiserum (center well). (B) Crossover electrophoresis: samples 1, 3 and 4 are positive; sample 2 is a negative control. (C) Rapid immunoassay: T, test sample with a positive result; C, positive control. (D) Human DNA quantitation: slot blot showing various positive DNA concentration standards (1-4), negative control (5) and positive test sample (6).
Under the influence of an electric field, the antigen and the antibody migrate toward each other and a precipitate is formed at the point of their interaction. Small wells about 1.5 mm in diameter are punched in an agar gel. The stain extract is placed in the cathodic well of a neighboring pair, and the antiserum in the opposite well. The antiserum travels towards the cathode (negative electrode), while the stain extract migrates anodically (toward the positive electrode). A precipitin band will form at the site of the interaction of the antibodies and the antigens.
Rapid immunoassay
Immunoassay test strips for human blood have become commercially available in recent years. These tests offer high sensitivity and specificity as confirmatory tests for the presence of human blood in stain extracts. Such procedures involve the reaction of antigens in the extract with monoclonal antibodies within the test strip. The combined antigen-antibody complex moves up the strip to an area where it is immobilized. The complex reacts with dye particles in the area where it has been halted to create visible reactions. Some assays combine built-in positive controls on the test strips. These tests have a reported sensitivity of 0.05 ug hemoglobin ml-1 and, thus, are suitable for use with highly dilute stain extracts or older stain materials.
Rapid immunoassay procedures are highly specific, easily performed, applicable to various types of samples, and produce results in a timely manner, usually within minutes of application to the test strips. This last feature allows for confirmation of blood in super-natants of samples which will be subject to DNA analysis prior to testing, without consumption of stain portions necessary for obtaining DNA. Such rapid assays also prevent extensive delay in genetic marker testing. Rapid confirmation of human blood may also be conducted at crime scenes by trained personnel. The disadvantage of this method is that reported ‘high dosage’ effects may yield false negative results. However, this effect is readily avoided by appropriate dilution of the sample prior to application of the stain extract to the test strips.
Human DNA quantitation
A sample can be determined to be blood of human origin by reaction with a probe specific for human DNA. Probes complementary to primate specific DNA sequences, such as those found at the locus D17Z1, are readily available and used primarily to determine the amount of human DNA extracted from a sample prior to DNA typing. DNA extracted from a sample is spotted on a membrane along with known concentrations of human DNA. After reaction with the human-specific probe, results obtained from the unknown sample are compared to the signal intensity of the known standards; the amount of human DNA in the sample can be estimated in this manner. The sensitivity of the human DNA quantitation test is commonly in the 0.15-0.20 ng range when a color reagent detection method is employed. One disadvantage of this technique is that human DNA from any tissue or cells will produce a positive reaction. Thus, it is necessary to determine that the DNA obtained is from blood by using one of the heme identification techniques discussed previously.
