Concept
In virtually every chemistry classroom on the planet, there is a chart known as the periodic table of elements. At first glance, it looks like a mere series of boxes, with letters and numbers in them, arranged according to some kind of code not immediately clear to the observer. The boxes would form a rectangle, 18 across and 7 deep, but there are gaps in the rectangle, particularly along the top. To further complicate matters, two rows of boxes are shown along the bottom, separated from one another and from the rest of the table. Even when one begins to appreciate all the information contained in these boxes, the periodic table might appear to be a mere chart, rather than what it really is: one of the most sophisticated and usable means ever designed for representing complex interactions between the building blocks of matter.
How it works
Introduction to the Periodic Table
As a testament to its durability, the periodic table—created in 1869—is still in use today. Along the way, it has incorporated modifications involving subatomic properties unknown to the man who designed it, Russian chemist Dmitri Ivanovitch Mendeleev (1834-1907). Yet Mendeleev’s original model, which we will discuss shortly, was essentially sound, inasmuch as it was based on the knowledge available to chemists at the time.
In 1869, the electromagnetic force fundamental to chemical interactions had only recently been identified; the modern idea of the atom was less than 70 years old; and another three decades were to elapse before scientists began uncovering the substructure of atoms that causes them to behave as they do. Despite these limitations in the knowledge available to Mendeleev, his original table was sound enough that it has never had to be discarded, but merely clarified and modified, in the years since he developed it.
The rows of the periodic table of elements are called periods, and the columns are known as groups. Each box in the table represents an element by its chemical symbol, along with its atomic number and its average atomic mass in atomic mass units. Already a great deal has been said, and a number of terms need to be explained. These explanations will require the length of this essay, beginning with a little historical background, because chemists’ understanding of the periodic table—and of the elements and atoms it represents—has evolved considerably since 1869.
Elements and Atoms
An element is a substance that cannot be broken down chemically into another substance. An atom is the smallest particle of an element that retains all the chemical and physical properties of the element, and elements contain only one kind of atom. The scientific concepts of both elements and atoms came to us from the ancient Greeks, who had a rather erroneous notion of the element and—for their time, at least—a highly advanced idea of the atom.
Unfortunately, atomic theory died away in later centuries, while the mistaken notion of four “elements” (earth, air, fire, and water) survived virtually until the seventeenth century, an era that witnessed the birth of modern science. Yet the ancients did know of substances later classified as elements, even if they did not understand them as such. Among these were gold, tin, copper, silver, lead, and mercury. These, in fact, are such an old part of human history that their discoverers are unknown. The first individual credited with discovering an element was German chemist Hennig Brand (c. 1630-c. 1692), who discovered phosphorus in 1674.
Maturing concepts of atoms, elements, and molecules
The work of English physicist and chemist Robert Boyle (1627-1691) greatly advanced scientific understanding of the elements. Boyle maintained that no substance was an element if it could be broken down into other substances: thus air, for instance, was not an element. Boyle’s studies led to the identification of numerous elements in the years that followed, and his work influenced French chemists Antoine Lavoisier (1743-1794) and Joseph-Louis Proust (1754-1826), both of whom helped define an element in the modern sense. These men in turn influenced English chemist John Dalton (1766-1844), who reintroduced atomic theory to the language of science.
In A New System of Chemical Philosophy (1808), Dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted the Greek word atomos to describe these basic units. Drawing on Proust’s law of constant composition, Dalton recognized that the structure of atoms in a particular element or compound is uniform, but maintained that compounds are made up of compound “atoms.” In fact, these compound atoms are really molecules, or groups of two or more atoms bonded to one another, a distinction clarified by Italian physicist Amedeo Avogadro (1776-1856).
Dalton’s and Avogadro’s contemporary, Swedish chemist Jons Berzelius (1779-1848), developed a system of comparing the mass of various atoms in relation to the lightest one, hydrogen. Berzelius also introduced the system of chemical symbols—H for hydrogen, O for oxygen, and so on—in use today. Thus, by the middle of the nineteenth century, scientists understood vastly more about elements and atoms than they had just a few decades before, and the need for a system of organizing elements became increasingly clear. By mid-century, a number of chemists had attempted to create just such an organizational system, and though Mendeleev’s was not the first, it proved the most useful.
Mendeleev Constructs His Table
By the time Mendeleev constructed his periodic table in 1869, there were 63 known elements. At that point, he was working as a chemistry professor at the University of St. Petersburg, where he had become acutely aware of the need for a way of classifying the elements to make their relationships more understandable to his students. He therefore assembled a set of 63 cards, one for each element, on which he wrote a number of identifying characteristics for each.
Along with the element symbol, discussed below, he included the atomic mass for the atoms of each. In Mendeleev’s time, atomic mass was understood simply to be the collective mass of a unit of atoms—a unit developed by Avogadro, known as the mole—divided by Avogadro’s number, the number of atoms or molecules in a mole. With the later discovery of subatomic particles, which in turn made possible the discovery of isotopes, figures for atomic mass were clarified, as will also be discussed.
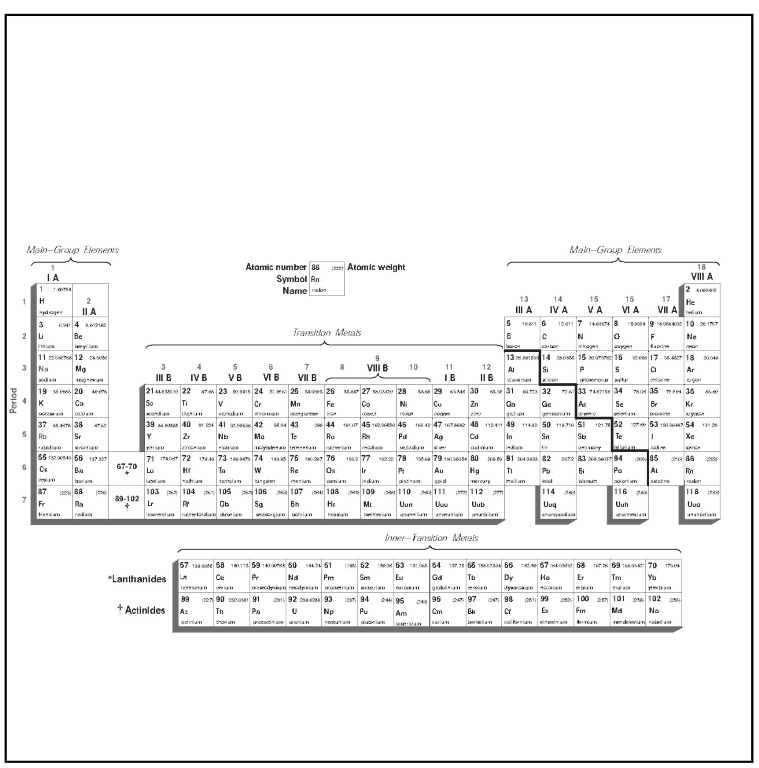
The periodic table
In addition, Mendeleev also included figures for specific gravity—the ratio between the density of an element and the density of water—as well as other known chemical characteristics of an element. Today, these items are typically no longer included on the periodic table, partly for considerations of space, but partly because chemists’ much greater understanding of the properties of atoms makes it unnecessary to clutter the table with so much detail.
Again, however, in Mendeleev’s time there was no way of knowing about these factors. As far as chemists knew in 1869, an atom was an indivisible little pellet of matter that could not be characterized by terms any more detailed than its mass and the ways it interacted with atoms of other elements. Mendeleev therefore arranged his cards in order of atomic mass, then grouped elements that showed similar chemical properties.
Confident Predictions
As Mendeleev observed, every eighth element on the chart exhibits similar characteristics, and thus, he established columns whereby element number x was placed above element number x + 8—for instance, helium (2) above neon (10). The patterns he observed were so regular that for any “hole” in his table, he predicted that an element to fill that space would be discovered.
Indeed, Mendeleev was so confident in the basic soundness of his organizational system that in some instances, he changed the figures for the atomic mass of certain elements because he was convinced they belonged elsewhere on the table.
Later discoveries of isotopes, which in some cases affected the average atomic mass considerably, confirmed his suppositions. Likewise the undiscovered elements he named “eka-aluminum,” “eka-boron,” and “eka-silicon” were later identified as gallium, scandium, and germanium, respectively.
Real-Life Applications
Subatomic Structures Clarify the Periodic Table
Over a period of 35 years, between the discovery of the electron in 1897 and the discovery of the neutron in 1932, chemists’ and physicists’ understanding of atomic structure changed completely. The man who identified the electron was English physicist J. J. Thomson (1856-1940). The electron is a negatively charged particle that contributes little to an atom’s mass; however, it has a great deal to do with the energy an atom possesses. Thomson’s discovery made it apparent that something else had to account for atomic mass, as well as the positive electric charge offsetting the negative charge of the electron.
Thomson’s student Ernest Rutherford (1871-1937)—for whom, incidentally, ruther-fordium (104 on the periodic table) is named— identified that “something else.” In a series of experiments, he discovered that the atom has a nucleus, a center around which electrons move, and that the nucleus contains positively charged particles called protons. Protons have a mass 1,836 times as great as that of an electron, and thus, this seemed to account for the total atomic mass.
Isotopes and atomic mass
Later, working with English chemist Frederick Soddy (1877-1956), Rutherford discovered that when an atom emitted certain types of particles, its atomic mass changed. Rutherford and Soddy named these atoms of differing mass isotopes, though at that point—because the neutron had yet to be discovered—they did not know exactly what change had caused the change in mass. Certain types of isotopes, Soddy and Rutherford went on to conclude, had a tendency to decay by emitting particles or gamma rays, moving (sometimes over a great period of time) toward stabilization. In the process, these radioactive isotopes changed into other isotopes of the same element—and sometimes even to isotopes of other elements.
Soddy concluded that atomic mass, as measured by Berzelius, was actually an average of the mass figures for all isotopes within that element. This explained a problem with Mendeleev’s periodic table, in which there seemed to be irregularities in the increase of atomic mass from element to element. The answer to these variations in mass, it turned out, related to the number of isotopes associated with a given element: the greater the number of isotopes, the more these affected the overall measure of the element’s mass.
A clearer definition of atomic number
Just a few years after Rutherford and Soddy discovered isotopes, Welsh physicist Henry Moseley (1887-1915) uncovered a mathematical relationship between the amount of energy a given element emitted and its atomic number. Up to this point, the periodic table had assigned atomic number in order of mass, beginning with the lightest element, hydrogen. Using atomic mass and other characteristics as his guides, Mendeleev had been able to predict the discovery of new elements, but such predictions had remained problematic. Thanks to Moseley’s work, it became possible to predict the existence of undiscovered elements with much greater accuracy.
As Moseley discovered, the atomic number corresponds to the number of positive charges in the nucleus. Thus carbon, for instance, has an atomic number of 6 not because there are five lighter elements—though this is also true—but because it has six protons in its nucleus. The ordering by atomic number happens to correspond to the ordering by atomic mass, but atomic number provides a much more precise means of distinguishing elements. For one thing, atomic number is always a whole integer—1 for hydrogen, for instance, or 17 for chlorine, or 92 for uranium. Figures for mass, on the other hand, are almost always rendered with whole numbers and decimal fractions (for example, 1.008 for hydrogen).
If atoms have no electric charge, meaning that they have the same number of protons as electrons, then why do chemists not say that atomic number represents the number of protons or electrons? The reason is that electrons can easily be lost or gained by atoms to form ions,which have an electric charge. However, protons are very hard to remove.
Neutrons and atomic mass
By 1932, scientists had come a long way toward understanding the structure of the atom. Not only had the electron, nucleus, and proton been discovered, but the complex model of electron configuration (described later in this essay) had begun to evolve. Yet, one nagging question remained: the mass of the protons in the nucleus simply could not account for the entire mass of the atom. Neither did the electrons make a significant contribution to mass.
Suppose a proton was “worth” $1,836, while an electron had a value of only $1. In the “bank account” for deuterium, an isotope of hydrogen, there is $3,676, which poses a serious discrepancy in accounting. Because deuterium is a form of hydrogen, it has one proton as well as one electron, but that only accounts for $1,837. Where does deuterium get the other $1,839? These numbers are not chosen at random, as we shall see.
The answer to the problem of atomic mass came when English physicist James Chadwick (1891-1974) identified the neutron, a particle with no electric charge, residing in the nucleus alongside the protons. Whereas the proton has a mass 1,836 times as large as that of the electron, the neutron’s mass is slightly larger—1,839 times that of an electron. This made it possible to clarify the values of atomic mass, which up to that time had been problematic, because a mole of atoms representing one element is likely to contain numerous isotopes.
Average Atomic Mass
Today, the periodic table lists, along with chemical symbol and atomic number, the average atomic mass of each element. As its name suggests, the average atomic mass provides the average value of mass—in atomic mass units (amu)—for a large sample of atoms. According to Berzelius’s system for measuring atomic mass, 1 amu should be equal to the mass of a hydrogen atom, even though that mass had yet to be measured, since hydrogen almost never appears alone in nature. Today, in accordance with a 1960 agreement among members of the international scientific community, measurements of atomic mass take carbon-12, an isotope found in all living things, as their reference point.
It is inconvenient, to say the least, to measure the mass of a single carbon-12 atom, or indeed of any other atom. Instead, chemists use a large number of atoms, a value known as Avogadro’s number, which in general is the number of atoms in a mole (abbreviated mol). Avogadro’s number is defined as 6.02214199 • 1023, with an uncertainty of 4.7 • 1016. In other words, the number of particles in a mole could vary by as much as 47,000,000,000,000,000 on either side of the value for Avogadro’s number. This might seem like a lot, but in fact it is equal to only about 80 parts per billion.
When 1 is divided by Avogadro’s number, the result is 1.66 • 10-24—the value, in grams, of 1 amu. However, according to the 1960 agreement, 1 amu is officially 1/12 the mass of a carbon-12 atom, whose exact value (re-tested in 1998), is 1.6653873 X 10-24 g. Carbon-12, sometimes represented as (12/6)C, contains six protons and six neutrons, so the value of 1 amu thus obtained is, in effect, an average of the mass for a proton and neutron.
Though atoms differ, subatomic particles do not. There is no such thing, for instance, as a “hydrogen proton”—otherwise, these subatomic particles, and not atoms, would constitute the basic units of an element. Given the unvarying mass of subatomic particles, combined with the fact that the neutron only weighs 0.16% more than a proton, the established value of 1 amu provides a convenient means of comparing mass. This is particularly useful in light of the large numbers of isotopes—and hence of varying figures for mass—that many elements have.
Atomic mass units and the periodic table
The periodic table as it is used today includes figures in atomic mass units for the average mass of each atom. As it turns out, Berzelius was not so far off in his use of hydrogen as a standard, since its mass is almost exactly 1 amu—but not quite. The value is actually 1.008 amu, reflecting the presence of slightly heavier deuterium isotopes in the average sample of hydrogen
Figures increase from hydrogen along the periodic table, though not by a regular pattern. Sometimes the increase from one element to the next is by just over 1 amu, and in other cases, the increase is by more than 3 amu. This only serves to prove that atomic number, rather than atomic mass, is a more straightforward means of ordering the elements.
Mass figures for many elements that tend to appear in the form of radioactive isotopes are usually shown in parentheses. This is particularly true for elements with very, very high atomic numbers (above 92), because samples of these elements do not stay around long enough to be measured. Some have a half-life—the period in which half the isotopes decay to a stable form— of just a few minutes, and for others, the half-life is a fraction of a second. Therefore, atomic mass figures represent the mass of the longest-lived isotope.
Elements
As of 2001, there were 112 known elements, of which about 90 occur naturally on Earth. Uranium, with an atomic number of 92, was the last naturally occurring element discovered: hence some sources list 92 natural elements. Other sources, however, subtract those elements with a lower atomic number than uranium that were first created in laboratories rather than discovered in nature. In any case, all elements with atomic numbers higher than 92 are synthetic, meaning that they were created in laboratories. Of these 20 elements—all of which have appeared only in the form of radioactive isotopes with short half-lives—the last three have yet to receive permanent names.
In addition, three other elements—designated by atomic numbers 114, 116, and 118, respectively—are still on the drawing board, as it were, and do not yet even have temporary names. The number of elements thus continues to grow, but these “new” elements have little to do with the daily lives of ordinary people. Indeed, this is true even for some of the naturally occurring elements: for example, few people who are not chemically trained would be able to identify yttrium, which has an atomic number of 39.
Though an element can exist theoretically as a gas, liquid, or a solid, in fact, the vast majority of elements are solids. Only 11 elements exist in the gaseous state at a normal temperature of about 77°F (25°C). These are the six noble gases; fluorine and chlorine from the halogen family; as well as hydrogen, nitrogen, and oxygen. Just two are liquids at normal temperature: mercury, a metal, and the nonmetal halogen bromine. It should be noted that the metal gallium becomes
liquid at just 85.6°F (29.76°C); below that temperature, however, it— like the elements other than those named in this paragraph—is a solid.
Chemical Names and Symbols
For the sake of space and convenience, elements are listed on the periodic table by chemical symbol or element symbol—a one-or two-letter abbreviation for the name of the element according to the system first developed by Berzelius. These symbols, which are standardized and unvarying for any particular element, greatly aid the chemist in writing out chemical formulas, which could otherwise be quite cumbersome.
Many of the chemical symbols are simple one-letter designations: H for hydrogen, O for oxygen, and F for fluorine. Others are two-letter abbreviations, such as He for helium, Ne for neon, and Si for silicon. Note that the first letter is always capitalized, and the second is always lowercase. In many cases, the two-letter symbols indicate the first and second letters of the element’s name, but this is not nearly always the case. Cadmium, for example, is abbreviated Cd, while platinum is Pt.
Many of the one-letter symbols indicate elements discovered early in history. For instance, carbon is represented by C, and later “C” elements took two-letter designations: Ce for cerium, Cr for chromium, and so on. Likewise, krypton had to take the symbol Kr because potassium had already been assigned K. The association of potassium with K brings up one of the aspects of chemical symbols most confusing to students just beginning to learn about the periodic table: why K and not P? The latter had in fact already been taken by phosphorus, but then why not Po, assigned many years later instead to polonium?
Chemical symbols based in other languages
In fact, potassium’s symbol is one of the more unusual examples of a chemical symbol, taken from an ancient or non-European language. Soon after its discovery in the early nineteenth century, the element was named kalium, apparently after the Arabic qali or “alkali.” Hence, though it is known as potassium today, the old symbol still stands.
The use of Arabic in naming potassium is unusual in the sense that “strange” chemical symbols usually refer to Latin and Greek names. Latin names include aurum, or “shining dawn” for gold, symbolized as Au; or ferrum, the Latin word for iron, designated Fe. Likewise, lead (Pb) and sodium (Na) are represented by letters from their Latin names, plumbum and natrium, respectively.
Some chemical elements are named for Greek or German words describing properties of the element. Consider, for instance, the halogens, collectively named for a Greek term meaning “salt producing” Chloros, in Greek, describes a sickly yellow color, and was assigned to chlorine; the name of bromine comes from a Greek word meaning “stink”; and that of iodine is a form of a Greek term meaning “violet-colored.” Astatine, last-discovered of the halogens and the rarest of all natural elements, is so radioactive that it was given a name meaning “unstable.” Another Greek-based example outside the halogen family is phosphorus, or “I bring light”—appropriate enough, in view of its phosphorescent properties.
Names of later elements
The names of several elements with high atomic numbers—specifically, the lanthanides, the transuranium elements of the actinide series, and some of the later transition metals—have a number of interesting characteristics. Several reflect the places where they were originally discovered or created: for example, germanium, americium, and californium. Other elements are named for famous or not-so-famous scientists. Most people could recognize einsteinium as being named after Albert Einstein (1879-1955), but the origin of the name gadolinium—Finnish chemist Johan Gadolin (1760-1852)—is harder for the average person to identify. Then of course there is element 101, named mendelevium in honor of the man who created the periodic table.
Two elements are named after women: curium after French physicist and chemist Marie Curie (1867-1934), and meitnerium after Austrian physicist Lise Meitner (1878-1968). Curie, the first scientist to receive two Nobel Prizes—in both physics and chemistry—herself discovered two elements, radium and polonium. In keeping with the trend of naming transuranium elements after places, she commemorated the land of her birth, Poland, in the name of polonium. One of Curie’s students, French physicist Marguerite Perey (1909-1975), also discovered an element and named it after her own homeland: francium.
Meitnerium, the last element to receive a name, was created in 1982 at the Gesellschaft fur Schwerionenforschung, or GSI, in Darmstadt, Germany, one of the world’s three leading centers of research involving transuranium elements. The other two are the Joint Institute for Nuclear Research in Dubna, Russia, and the University of California at Berkeley, for which berkelium is named.
The iupac and the naming of elements
One of the researchers involved with creating berkelium was American nuclear chemist Glenn T. Seaborg (1912-1999), who discovered plutonium and several other transuranium elements. In light of his many contributions, the scientists who created element 106 at Dubna in 1974 proposed that it be named seaborgium, and duly submitted the name to the International Union of Pure and Applied Chemistry (IUPAC).
Founded in 1919, the IUPAC is, as its name suggests, an international body, and it oversees a number of matters relating to the periodic table: the naming of elements, the assignment of chemical symbols to new elements, and the certification of a particular research team as the discoverers of that element. For many years, the IUPAC refused to recognize the name seaborgium, maintaining that an element could not be named after a living person. The dispute over the element’s name was not resolved until the 1990s, but finally the IUPAC approved the name, and today seaborgium is included on the international body’s official list.
Elements 110 through 112 had yet to be named in 2001, and hence were still designated by the three-letter symbols Uun, Uuu, and Uub respectively. These are not names, but alphabetic representations of numbers: un for 1, nil for 0, and bium for 2. Thus, the names are rendered as ununnilium, unununium, and ununbium; the undiscovered elements 114, 116, and 118 are respectively known as ununquadium, ununhexi-um, and ununoctium.
Layout of the Periodic Table
Two systems for labeling groups
Having discussed the three items of information contained in the boxes of the periodic table—atomic number, chemical symbol/ name, and average atomic mass—it is now possible to step back from the chart and look at its overall layout. To reiterate what was stated in the introduction to the periodic table above, the table is arranged in rows called periods, and columns known as groups. The deeper meaning of the periods and groups, however—that is, the way that chemists now understand them in light of what they know about electron configurations—will require some explanation.
All current versions of the periodic table show seven rows—in other words, seven periods—as well as 18 columns. However, the means by which columns are assigned group numbers varies somewhat. According to the system used in North America, only eight groups are numbered. These are the two “tall” columns on the left side of the “dip” in the chart, as well as the six “tall” columns to the right of it. The “dip,” which spans 10 columns in periods 4 through 7, is the region in which the transition metals are listed. The North American system assigns no group numbers to these, or to the two rows set aside at the bottom, representing the lanthanide and actinide series of transition metals.
As for the columns that the North American system does number, this numbering may appear in one of four forms: either by Roman numerals; Roman numerals with the letter A (for example, IIIA); Hindu-Arabic numbers (for example, 3); or Hindu-Arabic numerals with the letter A. Throughout this topic, the North American system of assigning Hindu-Arabic numerals without the letter A has been used. However, an attempt has been made in some places to include the group designation approved by the IUPAC, which is used by scientists in Europe and most parts of the world outside of North America. (Some scientists in North America are also adopting the IUPAC system.)
The IUPAC numbers all columns on the chart, so that instead of eight groups, there are 18. The table below provides a means of comparing the North American and IUPAC systems. Columns are designated in terms of the element family or families, followed in parentheses by the atomic numbers of the elements that appear at the top and bottom of that column. The first number following the colon is the number in the North American system (as described above, a
Hindu-Arabic numerical without an “A”), and the second is the number in the IUPAC system.
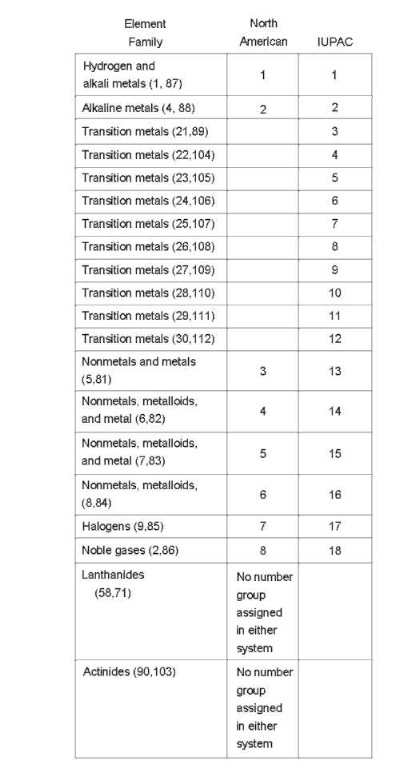
Valence Electrons, Periods, and Groups
The merits of the IUPAC system are easy enough to see: just as there are 18 columns, the IUPAC lists 18 groups. Yet the North American system is more useful than it might seem: the group number in the North American system indicates the number of valence electrons, the electrons that are involved in chemical bonding. Valence electrons also occupy the highest energy level in the atom—which might be thought of as the orbit farthest from the nucleus, though in fact the reality is more complex.
A more detailed, though certainly far from comprehensive, discussion of electrons and energy levels, as well as the history behind these discoveries, appears in the Electrons essay. In what follows, the basics of electron configuration will be presented with the specific aim of making it clear exactly why elements appear in particular columns of the periodic table.
Principal energy levels and periods
At one time, scientists thought that electrons moved around a nucleus in regular orbits, like planets around the Sun. In fact the paths of an electron are much more complicated, and can only be loosely defined in terms of orbitals, a set of probabilities regarding the positions that an electron is likely to occupy as it moves around the nucleus. The pattern of orbitals is determined by the principal energy level of the atom, which indicates a distance that an electron may move away from the nucleus.
Principal energy level is designated by a whole-number integer, beginning with 1 and moving upward: the higher the number, the further the electron is from the nucleus, and hence the greater the energy in the atom. Each principal energy level is divided into sublevels corresponding to the number n of the principal energy level: thus, principal energy level 1 has one sub-level, principal energy level 2 has two, and so on.
The relationship between principal energy level and period is relatively easy to demonstrate: the number n of a period on the periodic table is the same as the number of the highest principal energy level for the atoms on that row—that is, the principal energy level occupied by its valence electrons. Thus, elements on period 4 have a highest principal energy level of 4, whereas the valence electrons of elements on period 7 are at principal energy level 7. Note the conclusion that this allows us to draw: the further down the periodic table an element is positioned, the greater the energy in a single atom of that element. Not surprisingly, most of the elements used in nuclear power come from period 7, which includes the actinides.
Valence electron configurations and groups
Now to a more involved subject, whereby group number is related to valence electron configuration. As mentioned earlier, the principal energy levels are divided into sublevels, which are equal in number to the principal energy level number: principal energy level 1 has one sublevel, level 2 has two sublevels, and so on. As one might expect, with an increase in principal energy levels and sub-levels, there are increases in the complexity of the orbitals.
The four types of orbital patterns are designated as s, p,d, and f. Two electrons can move in an s orbital pattern or shell, six in a p, 10 in a d,and 14 in an f orbital pattern or shell. This says nothing about the number of electrons that are actually in a particular atom; rather, the higher the principal energy level and the larger the number of sublevels, the greater the number of ways that the electrons can move. It does happen to be the case, however, that with higher atomic numbers—which means more electrons to offset the protons—the higher the energy level, the larger the number of orbitals for those electrons.
Let us now consider a few examples of valence shell configurations. Hydrogen, with the simplest of all atomic structures, has just one electron on principal energy level 1, so in effect its valence electron is also a core electron. The valence configuration for hydrogen is thus written as 1 s1. Moving straight down the periodic table to francium (atomic number 87), which is in the same column as hydrogen, one finds that it has a valence electron configuration of 7s1. Thus, although francium is vastly more complex and energy-filled than hydrogen, the two elements have the same valence-shell configuration; only the number of the principal energy level is different.
Now look at two elements in Group 3 (Group 13 in the IUPAC system): boron and thallium, which respectively occupy the top and bottom of the column, with atomic numbers of 5 and 81. Boron has a valence-shell configuration of 2s22p1. This means its valence shell is at principal energy level 2, where there are two electrons in an s orbital pattern, and 2 in a p orbital pattern. Thallium, though it is on period 6, nonetheless has the same valence-shell configuration: 6s26p1.
Notice something about the total of the superscript figures for any element in Group 3 of the North American system: it is three. The same is true in the other columns numbered on North American charts, in which the total number of electrons equals the group number. Thus in Group 7, the valence shell configuration is ns2np5,where n is the principal energy level. There is only one exception to this: helium, in Group 8 (the noble gases), has a valence shell configuration of 1s2. Were it not for the fact that it clearly fits with the noble gases due to shared properties, helium would be placed next to hydrogen at the top of Group 2, where all the atoms have a valence-shell configuration of ns2.
Obviously the group numbers in the IUPAC system do not correspond to the number of valence electrons, because the IUPAC chart includes numbers for the columns of transition metals, which are not numbered in the North American system. In any case, in both systems the columns contain elements that all have the same number of electrons in their valence shells. Thus the term “group” can finally be defined in accordance with modern chemists’ understanding, which incorporates electron configurations of which Mendeleev was unaware. All the members of a group have the same number of valence electrons in the same orbital patterns, though at different energy levels. (Once again, helium is the lone exception.)
Some Challenges of the Periodic Table
Irregular Patterns
The groups that are numbered in the North American system are referred to as “representative” elements, because they follow a clearly established pattern of adding valence shell electrons. By contrast, the 40 elements listed in the “dip” at the middle of the chart—the transition elements— do not follow such a pattern. This is why the North American system does not list them by group number, and also why neither system lists two “branches” of the transition-metal family, the lanthanides and actinides.
Even within the representative elements, there are some challenges as far as electron configuration. For the first 18 elements—1 (hydrogen) to 18 (argon)—there is a regular pattern of orbital filling. Beginning with helium (2) onward, all of principal level 1 is filled; then, beginning with beryllium (4), sublevel 2s begins to fill. Sublevel 2p—and hence principal level 2 as a whole—becomes filled at neon (10).
After argon, as one moves to the element occupying the nineteenth position on the periodic table—potassium—the rules change. Argon, in Group 8 of the North American system, has a valence shell of 3s23p6, and by the pattern established with the first 18 elements, potassium should begin filling principal level 3d. Instead, it “skips” 3d and moves on to 4s. The element following argon, calcium, adds a second electron to the 4s sublevel.
After calcium, as the transition metals begin with scandium (21), the pattern again changes:indeed, the transition elements are defined by the fact that they fill the d orbitals rather than the p orbitals, as was the pattern up to that point. After the first period of transition metals ends with zinc (30), the next representative element—gallium (31)—resumes the filling of the p orbital rather than the d. And so it goes, all along the four periods in which transition metals break up the steady order of electron configurations.
As for the lanthanide and actinide series of transitions metals, they follow an even more unusual pattern, which is why they are set apart even from the transition metals. These are the only groups of elements that involve the highly complex fsublevels. In the lanthanide series, the seven 4forbital shells are filled, while the actinide series reflects the filling of the seven 5forbital shells.
Why these irregularities? One reason is that as the principal energy level increases, the energy levels themselves become closer—i.e., there is less difference between the energy levels. The atom is thus like a bus that fills up: when there are just a few people on board, those few people (analogous to electrons) have plenty of room, but as more people get on, the bus becomes increasingly more crowded, and passengers jostle against one another. In the atom, due to differences in energy levels, the 4s orbital actually has a lower energy than the 3d, and therefore begins to fill first. This is also true for the 6s and 4forbitals.
Changes in atomic size
The subject of element families is a matter unto itself, and therefore a separate essay in this topic has been devoted to it. The reader is encouraged to consult the Families of Elements essay, which discusses aspects of electron configuration as well as the properties of various element families.
One last thing should be mentioned about the periodic table: the curious fact that the sizes of atoms decreases as one moves from left to right across a row or period, even though the sizes increase as one moves from top to bottom along a group. The increase of atomic size in a group, as a function of increasing atomic number, is easy enough to explain. The higher the atomic number, the higher the principal energy level, and the greater the distance from the nucleus to the furthest probability range for the electron.
On the other hand, the decrease in size across a period is a bit more challenging to comprehend; however, it just takes a little explaining. As one moves along a period from left to right, there is a corresponding increase in the number of protons within the nucleus. This means a stronger positive charge pulling the electrons inward. Therefore, the “cloud” of electrons is drawn ever closer toward the increasingly powerful charge at the center of the atom, and the size of the atom decreases because the electrons cannot move as far away from the nucleus.
Key Terms
Atom: The smallest particle of an element that retains all the chemical and physical properties of the element.
Atomic mass unit: An SI unit (abbreviated amu), equal to 1.66 • 10-24 g, for measuring the mass of atoms.
Atomic number: The number of protons in the nucleus of an atom. Since this number is different for each element, elements are listed on the periodic table of elements in order of atomic number.
Average atomic mass: A figure used by chemists to specify the mass—in atomic mass units—of the average atom in a large sample.
Avogadro’s number: A figure,named after Italian physicist Amedeo Avogadro (1776-1856), equal to 6.022137 x 1023. Avogadro’s number indicates the number of atoms or molecules in a mole.
Chemical symbol: A one- or two-letter abbreviation for the name of an element.
Compound: A substance made of two or more elements that have bonded chemically. These atoms are usually, but not always, joined in molecules.
Electron: A negatively charged particle in an atom. The configurations of valence electrons define specific groups on the periodic table of elements, while the principal energy levels of those valence electrons define periods on the table.
Element: A substance made up of only one kind of atom, which cannot be chemically broken into other substances.
Element symbol: Another term for chemical symbol.
Groups: Columns on the periodic table of elements. These are ordered according to the numbers of valence electrons in the outer shells of the atoms for the elements represented.
Half-life: The length of time it takes a substance to diminish to one-half its initial amount.
Ion: An atom or atoms that has lost or gained one or more electrons, thus acquiring a net electric charge.
Isotopes: Atoms that have an equal number of protons, and hence are of the same element, but differ in their number of neutrons. This results in a difference of mass. Isotopes may be either stable or unstable. The latter type, known as radioisotopes, are radioactive.
Mole: The SI fundamental unit for “amount of substance.” A mole is, generally speaking, Avogadro’s number of atoms, molecules, or other elementary particles; however, in the more precise SI definition, a mole is equal to the number of carbon atoms in 12.01 g of carbon.
Molecule: A group of atoms, usually but not always representing more than one element, joined by chemical bonds. Compounds are typically made of up molecules.
Neutron: A subatomic particle that has no electric charge. Neutrons, together with protons, account for the majority of average atomic mass. When atoms have the same number of protons—and hence are the same element—but differ in their number of neutrons, they are called isotopes.
Nucleus: The center of an atom, a region where protons and neutrons are located. The nucleus accounts for the vast majority of the average atomic mass.
Orbital: A pattern of probabilities regarding the regions that an electron can occupy within an atom in a particular energy state. The higher the principal energy level, the more complex the pattern of orbitals.
Periodic table of elements: A chart that shows the elements arranged in order of atomic number, along with chemical symbol and the average atomic mass (in atomic mass units) for that particular element.
Periods: Rows of the periodic table of elements. These represent successive principal energy levels for the valence electrons in the atoms of the elements involved.
Principal energy level: A value indicating the distance that an electron may move away from the nucleus of an atom. This is designated by a whole-number integer, beginning with 1 and moving upward. The higher the principal energy level, the greater the energy in the atom, and the more complex the pattern of orbitals.
Proton: A positively charged particle in an atom. The number of protons in the nucleus of an atom is the atomic number of an element.
Radioactivity: A term describing a phenomenon whereby certain isotopes known as radioisotopes are subject to a form of decay brought about by the emission of high-energy particles. “Decay” does not mean that the isotope “rots”; rather, it decays to form another isotope— either of the same element or another—until eventually it becomes stable. This stabilizing process may take a few seconds, or many years.
Valence electrons: Electrons that occupy the highest energy levels in an atom. These are the electrons involved in chemical bonding.
