CONCEPT
The term osmosis describes the movement of a solvent through a semipermeable membrane from a less concentrated solution to a more concentrated one. Water is sometimes called “the perfect solvent,” and living tissue (for example, a human being’s cell walls) is the best example of a semipermeable membrane. Osmosis has a number of life-preserving functions: it assists plants in receiving water, it helps in the preservation of fruit and meat, and is even used in kidney dialysis. In addition, osmosis can be reversed to remove salt and other impurities from water.
HOW IT WORKS
If you were to insert a hollow tube of a certain diameter into a beaker of water, the water would rise inside the tube and reach the same level as the water outside it. But suppose you sealed the bottom end of the tube with a semipermeable membrane, then half-filled the tube with salt water and again inserted it into the beaker. Over a period of time, the relative levels of the salt water in the tube and the regular water in the beaker would change, with the fresh water gradually rising into the beaker.
This is osmosis at work; however, before investigating the process, it is necessary to understand at least three terms. A solvent is a liquid capable of dissolving or dispersing one or more other substances. A solute is the substance that is dissolved, and a solution is the resulting mixture of solvent and solute. Hence, when you mix a packet of sugar into a cup of hot coffee, the coffee—which is mostly water—acts as a solvent for the sugar, a solute, and the resulting sweetened coffee is a solution. (Indeed, people who need a cup of coffee in the morning might say that it is a “solution” in more ways than one!) The relative amount of solute in the solution determines whether it can be described as more or less concentrated.
Water and Oil: Molecular Differences
In the illustrations involving the beaker and the hollow tube, water plays one of its leading roles, as a solvent. It is possible to use a number of other solvents for osmosis, but most of the ones that will be discussed here are water-based substances. In fact, virtually everything people drink is either made with water as its central component (soft drinks, coffee, tea, beer and spirits), or comes from a water-based plant or animal life form (fruit juices, wine, milk.) Then of course there is water itself, still the world’s most popular drink.
By contrast, people are likely to drink an oily product only in extreme circumstances: for instance, to relieve constipation, holistic-health practitioners often recommend a mixture of olive oil and other compounds for this purpose. Oil, unlike water, has a tendency to pass straight through a person’s system, without large amounts of it being absorbed through osmosis. In fact, oil and water differ significantly at the molecular level.
Water is the best example of a polar molecule, sometimes called a dipole. As everyone knows, water is a name for the chemical H2O, in which two relatively small hydrogen atoms bond with a large atom of oxygen. You can visualize a water molecule by imagining oxygen as a basketball with hydrogen as two baseballs fused to the
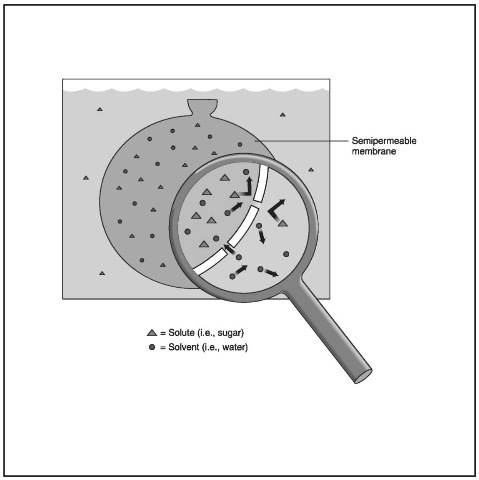
A semipermeable membrane permits only certain components of solutions, usually the solvent, to pass through.
basketball’s surface. Bonded together as they are, the oxygen tends to pull electrons from the hydrogen atoms, giving it a slight negative charge and the hydrogen a slight positive charge.
As a result, one end of a water molecule has a positive electrical charge, and the other end a negative charge. This in turn causes the positive end of one molecule to attract the negative side of its neighbor, and vice versa. Though the electromagnetic force is weak, even in relative terms, it is enough to bond water molecules tightly to one another.
By contrast, oily substances—whether the oil is animal-, vegetable-, or petroleum-based— are typically nonpolar, meaning that the positive and negative charges are distributed evenly across the surface of the molecule. Hence, the bond between oil molecules is much less tight than for water molecules. Clean motor oil in a car’s crankshaft behaves as though it were made of millions of tiny ball-bearings, each rolling through the engine without sticking. Water, on the other hand, has a tendency to stick to surfaces, since its molecules are so tightly bonded to one another.
This tight bond gives water highly unusual properties compared to other substances close to its molecular weight. Among these are its high boiling point, its surprisingly low density when frozen, and the characteristics that make osmosis possible. Thanks to its intermolecular structure, water is not only an ideal solvent, but its closely

Osmosis equalizes concentration.
packed structure enables easy movement, as, for instance, from an area of low concentration to an area of high concentration.
In the beaker illustration, the “pure” water is almost pure solvent. (Actually, because of its solvent qualities, water seldom appears in a pure state unless one distills it: even water flowing through a “pure” mountain stream carries all sorts of impurities, including microscopic particles of the rocks over which it flows.) In any case, the fact that the water in the beaker is almost pure makes it easy for it to flow through the semipermeable membrane in the bottom of the tube. By contrast, the solute particles in the saltwater solution have a much harder time passing through, and are much more likely to block the openings in the membrane. As a result, the movement is all in one direction: water in the beaker moves through the membrane, and into the tube.
A few points of clarification are in order here. A semipermeable membrane is anything with a structure somewhere between that of, say, plastic on the one hand and cotton on the other. Were the tube in the beaker covered with Saran wrap, for instance, no water would pass through. On the other hand, if one used a piece of cotton in the bottom of the tube, the water would pass straight through without osmosis taking place. In contrast to cotton, Gore-tex is a fabric containing a very thin layer of plastic with billions of tiny pores which let water vapor flow out without allowing liquid water to seep in. This accounts for the popularity of Gore-tex for outdoor gear—it keeps a person dry without holding in their sweat. So Gore-tex would work well as a semipermeable membrane.
Also, it is important to consider the possibilities of what can happen during the process of osmosis. If the tube were filled with pure salt, or salt with only a little water in it, osmosis would reach a point and then stop due to osmotic pressure within the substance. Osmotic pressure results when a relatively concentrated substance takes in a solvent, thus increasing its pressure until it reaches a point at which the solution will not allow any more solvent to enter.
REAL-LIFE APPLICATIONS
Cell Behavior and Salt Water
Cells in the human body and in the bodies of all living things behave like microscopic bags of solution housed in a semipermeable membrane. The health and indeed the very survival of a person, animal, or plant depends on the ability of

In this 1966 photo, a girl is undergoing kidney dialysis, a crucial modern medical application that relies on osmosis.
the cells to maintain their concentration of solutes.
Two illustrations involving salt water demonstrate how osmosis can produce disastrous effects in living things. If you put a carrot in salty water, the salt water will “draw” the water from inside the carrot—which, like the human body and most other forms of life, is mostly made up of water. Within a few hours, the carrot will be limp, its cells shriveled.
Worse still is the process that occurs when a person drinks salt water. The body can handle a little bit, but if you were to consume nothing but salt water for a period of a few days, as in the case of being stranded on the proverbial desert island, the osmotic pressure would begin drawing water from other parts of your body. Since a human body ranges from 60% water (in an adult male) to 85% in a baby, there would be a great deal of water available—but just as clearly, water is the essential ingredient in the human body. If you continued to ingest salt water, you would eventually experience dehydration and die.
How, then, do fish and other forms of marine life survive in a salt-water environment? In most cases, a creature whose natural habitat is the ocean has a much higher solute concentration in its cells than does a land animal. Hence,
for them, salt water is an isotonic solution, or one that has the same concentration of solute—and hence the same osmotic pressure—as in their own cells.
Osmosis in Plants
Plants depend on osmosis to move water from their roots to their leaves. The further toward the edge or the top of the plant, the greater the solute concentration, which creates a difference in osmotic pressure. This is known as osmotic potential, which draws water upward. In addition, osmosis protects leaves against losing water through evaporation.
Crucial to the operation of osmosis in plants are “guard cells,” specialized cells dispersed along the surface of the leaves. Each pair of guard cells surrounds a stoma, or pore, controlling its ability to open and thus release moisture.
In some situations, external stimuli such as sunlight may cause the guard cells to draw in potassium from other cells. This leads to an increase in osmotic potential: the guard cell becomes like a person who has eaten a dry biscuit, and is now desperate for a drink of water to wash it down. As a result of its increased osmotic potential, the guard cell eventually takes on
Water through osmosis
The guard cells then swell with water, opening the stomata and increasing the rate of gas exchange through them. The outcome of this action is an increase in the rate of photosynthesis and plant growth.
When there is a water shortage, however, other cells transmit signals to the guard cells that cause them to release their potassium. This decreases their osmotic potential, and water passes out of the guard cells to the thirsty cells around them. At the same time, the resultant shrinkage in the guard cells closes the stomata, decreasing the rate at which water transpires through them and preventing the plant from wilting.
Osmosis and Medicine
Osmosis has several implications where medical care is concerned, particularly in the case of the storage of vitally important red blood cells. These are normally kept in a plasma solution which is isotonic to the cells when it contains specific proportions of salts and proteins. However, if red blood cells are placed in a hypotonic solution, or one with a lower solute concentration than in the cells themselves, this can be highly detrimental.
Hence water, a life-giving and life-preserving substance in most cases, is a killer in this context. If red blood cells were stored in pure water, osmosis would draw the water into the cells, causing them to swell and eventually burst. Similarly, if the cells were placed in a solution with a higher solute concentration, or hypertonic solution, osmosis would draw water out of the cells until they shriveled.
In fact, the plasma solution used by most hospitals for storing red blood cells is slightly hypertonic relative to the cells, to prevent them from drawing in water and bursting. Physicians use a similar solution when injecting a drug intravenously into a patient. The active ingredient of the drug has to be suspended in some kind of medium, but water would be detrimental for the reasons discussed above, so instead the doctor uses a saline solution that is slightly hyper-tonic to the patient’s red blood cells.
One vital process closely linked to osmosis is dialysis, which is critical to the survival of many victims of kidney diseases. Dialysis is the process by which an artificial kidney machine removes waste products from a patients’ blood—performing the role of a healthy, normally functioning kidney. The openings in the dialyzing membrane are such that not only water, but salts and other waste dissolved in the blood, pass through to a surrounding tank of distilled water. The red blood cells, on the other hand, are too large to enter the dialyzing membrane, so they return to the patient’s body.
Preserving Fruits and Meats
Osmosis is also used for preserving fruits and meats, though the process is quite different for the two. In the case of fruit, osmosis is used to dehydrate it, whereas in the preservation of meat, osmosis draws salt into it, thus preventing the intrusion of bacteria.
Most fruits are about 75% water, and this makes them highly susceptible to spoilage. To preserve fruit, it must be dehydrated, which—as in the case of the salt in the meat— presents bacteria with a less-than-hospitable environment. Over the years, people have tried a variety of methods for drying fruit, but most of these have a tendency to shrink and harden the fruit. The reason for this is that most drying methods, such as heat from the Sun, are relatively quick and drastic; osmosis, on the other hand, is slower, more moderate—and closer to the behavior of nature.
Osmotic dehydration techniques, in fact, result in fruit that can be stored longer than fruit dehydrated by other methods. This in turn makes it possible to provide consumers with a wider variety of fruit throughout the year. Also, the fruit itself tends to maintain more of its flavor and nutritional qualities while keeping out microorganisms.
Because osmosis alone can only remove about 50% of the water in most ripe fruits, however, the dehydration process involves a secondary method as well. First the fruit is blanched, or placed briefly in scalding water to stop enzymatic action. Next it is subjected to osmotic dehydration by dipping it in, or spreading it with, a specially made variety of syrup whose sugar draws out the water in the fruit. After this, air drying or vacuum drying completes the process. The resulting product is ready to eat; can be preserved on a shelf under most climatic conditions; and may even be powdered for making confectionery items.
Whereas osmotic dehydration of fruit is currently used in many parts of the world, the salt-curing of meat in brine is largely a thing of the past, due to the introduction of refrigeration. Many poorer families, even in the industrialized world, however, remained without electricity long after it spread throughout most of Europe and North America. John Steinbeck’s Grapes of Wrath (1939) offers a memorable scene in which a contemporary family, the Joads, kill and cure a pig before leaving Oklahoma for California. And a Web site for Walton Feed, an Idaho company specializing in dehydrated foods, offers reminiscences by Canadians whose families were still salt-curing meats in the middle of the twentieth century. Verla Cress of southern Alberta, for instance, offered a recipe from which the following details are drawn.
KEY TERMS
Hypertonic: Of higher osmotic pressure.
Hypotonic: Of lower osmotic pressure.
Isotonic: Of equal osmotic pressure.
Osmosis: The movement of a solvent through a semipermeable membrane from a less concentrated solution to a more concentrated one.
Osmotic potential: A difference in osmotic pressure that draws water from an area of less osmotic pressure to an area of greater osmotic pressure.
Osmotic pressure: The pressure that builds in a substance as it experiences osmosis, and eventually stops that process.
Solvent: A liquid capable of dissolving or dispersing one or more other substances.
Solute: A substance capable of being dissolved in a solvent.
Solution: A mixture of solvent and solute.
First a barrel is filled with a solution containing 2 gal (7.57 l) of hot water and 8 oz (.2268 kg) of salt, or 32 parts hot water to one part salt, as well as a small quantity of vinegar. The pig or cow, which would have just been slaughtered, should then be cut up into what Cress called “ham-sized pieces (about 10-15 lb [5-7 kg]) each.” The pieces are then soaked in the brine barrel for six days, after which the meat is removed, dried, “and put… in flour or gunny sacks to keep the flies away. Then hang it up in a cool dry place to dry. It will keep like this for perhaps six weeks if stored in a cool place during the Summer. Of course, it will keep much longer in the Winter. If it goes bad, you’ll know it!”
Cress offered another method, one still used on ham today. Instead of salt, sugar is used in a mixture of 32 oz (.94 l) to 3 gal (11.36 l) of water. After being removed, the meat is smoked—that is, exposed to smoke from a typically aromatic wood such as hickory, in an enclosed barn—for three days. Smoking the meat tends to make it last much longer: four months in the summer, according to Cress.
The Walton Feeds Web page included another brine-curing recipe, this one used by the women of the Stirling, Alberta, Church of the Latter-Day Saints in 1973. Also included were reminiscences by Glenn Adamson (born 1915): “…When we butchered a pig, Dad filled a wooden 45-gal (170.34 l) barrel with salt brine. We cut up the pig into maybe eight pieces and put it in the brine barrel. The pork soaked in the barrel for several days, then the meat was taken out, and the water was thrown away…. In the hot summer days after they [the pieces of meat] had dried, they were put in the root cellar to keep them cool. The meat was good for eating two or three months this way.”
For thousands of years, people used salt to cure and preserve meat: for instance, the sailing ships that first came to the New World carried on board barrels full of cured meat, which fed sailors on the voyage over. Meat was not the only type of food preserved through the use of salt or brine, which is hypertonic—and thus lethal—to bacteria cells. Among other items packed in brine were fish, olives, and vegetables.
Even today, some types of canned fish come to the consumer still packed in brine, as do olives. Another method that survives is the use of sugar—which can be just as effective as salt for keeping out bacteria—to preserve fruit in jam.
Reverse Osmosis
Given the many ways osmosis is used for preserving food, not to mention its many interactions with water, it should not be surprising to discover that osmosis can also be used for desalination, or turning salt water into drinking water. Actually, it is not osmosis, strictly speaking, but rather reverse osmosis that turns salt water from the ocean—97% of Earth’s water supply—into water that can be used for bathing, agriculture, and in some cases even drinking.
When you mix a teaspoon of sugar into a cup of coffee, as mentioned in an earlier illustration, this is a non-reversible process. Short of some highly complicated undertaking—for instance, using ultrasonic sound waves— it would be impossible to separate solute and solvent.
Osmosis, on the other hand, can be reversed. This is done by using a controlled external pressure of approximately 60 atmospheres, an atmosphere being equal to the air pressure at sea level—14.7 pounds-per-square-inch (1.013 X 105 Pa.) In reverse osmosis, this pressure is applied to the area of higher solute concentration—in this case, the seawater. As a result, the pressure in the seawater pushes water molecules into a reservoir of pure water.
If performed by someone with a few rudimentary tools and a knowledge of how to provide just the right amount of pressure, it is possible that reverse osmosis could save the life of a shipwreck victim stranded in a location without a fresh water supply. On the other hand, a person in such a situation may be able to absorb sufficient water from fruits and plant life, as Tom Hanks’s character did in the 2001 film Cast Away.
Companies such as Reverse Osmosis Systems in Atlanta, Georgia, offer a small unit for home or business use, which actually performs the reverse-osmosis process on a small scale. The unit makes use of a process called cross flow, which continually cleans the semipermeable membrane of impurities that have been removed from the water. A small pump provides the pressure necessary to push the water through the membrane. In addition to an under-the-sink model, a reverse osmosis water cooler is also available.
Not only is reverse osmosis used for making water safe, it is also applied to metals in a variety of capacities, not least of which is its use in treating wastewater from electroplating. But there are other metallurgical methods of reverse osmosis that have little to do with water treatment: metal finishing, as well as recycling of metals and chemicals. These processes are highly complicated, but they involve the same principle of removing impurities that governs reverse osmosis.
