Concept
The term “family” is used to describe elements that share certain characteristics—not only in terms of observable behavior, but also with regard to atomic structure. All noble gases, for instance, tend to be highly nonreactive: only a few of them combine with other elements, and then only with fluorine, the most reactive of all substances. Fluorine is a member of another family, the halogens, which have so many shared characteristics that they are grouped together, despite the fact that two are gases, two are solids, and one—bromine—is one of only two elements that appears at room temperature as a solid. Despite these apparent differences, common electron configurations identify the halogens as a family. Families on the periodic table include, in addition to noble gases and halogens, the alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides. The nonmetals form a loosely defined cross-family grouping, as do the metalloids.
How it works
The Basics of the Periodic Table
Created in 1869, and modified several times since then, the periodic table of the elements developed by Russian chemist Dmitri Ivanovitch Mendeleev (1834-1907) provides a highly useful means of organizing the elements. Certainly other organizational systems exist, but Mendeleev’s table is the most widely used—and with good reason. For one thing, it makes it possible to see at a glance families of elements, many of which either belong to the same group (column) or the same period (row) on the table.
The periodic table is examined in depth within the essay devoted to that subject, and among the specifics discussed in that essay are the differing systems used for periodic-table charts in North America and the rest of the world. In particular, the North American system numbers only eight groups, leaving 10 columns unnumbered, whereas the other system— approved by the International Union of Pure and Applied Chemistry (IUPAC)—numbers all 18 columns. Both versions of the periodic table show seven periods.
The groups numbered in the North American system are the two “tall” columns on the left side of the “dip” in the chart, as well as the six “tall” columns to the right of it. Group 1 in this system consists of hydrogen and the alkali metals; Group 2, the alkaline earth metals; groups 3 through 6, an assortment of metals, nonmetals, and metalloids; Group 7, halogens; and Group 8, noble gases. The “dip,” which spans 10 columns in periods 4 through 7, is the region in which the transition metals are listed. The North American system assigns no group numbers to these, or to the two rows set aside at the bottom, representing the lanthanide and actinide series of transition metals.
The IUPAC system, on the other hand, offers the obvious convenience of providing a number for each column. (Note that, like its North American counterpart, the IUPAC chart provides no column numbers for the lanthanides or actinides.) Furthermore, the IUPAC has behind it the authority of an international body, founded in 1919, which oversees a number of matters
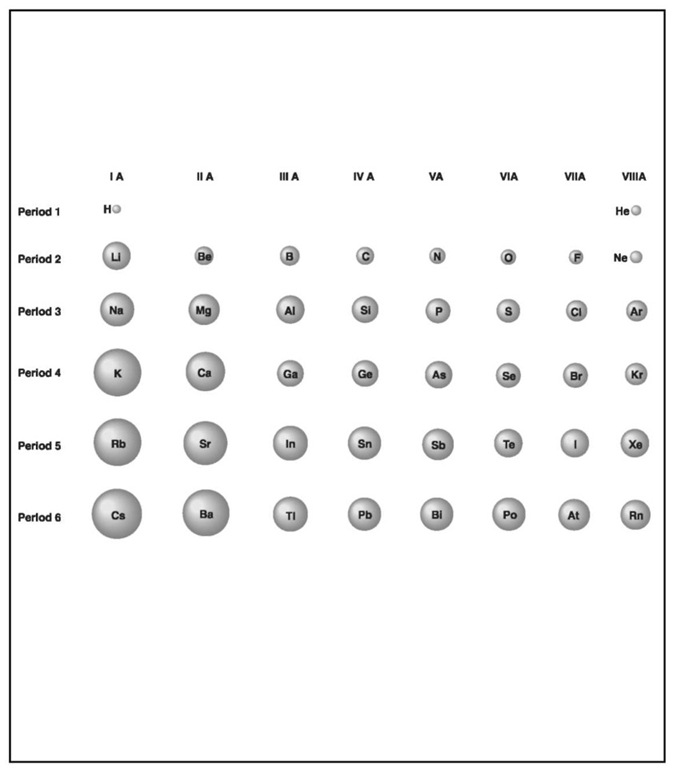
Size representation of the atomic radii of the main-group elements.
relating to the periodic table: the naming of elements, the assignment of chemical symbols to new elements, and the certification of a particular individual or research team as the discoverers of that element. For these reasons, the IUPAC system is coming into favor among North American chemists as well.
Despite the international acceptance of the IUPAC system, as well as its merits in terms of convenience, the North American system is generally the one used in this topic. The reason, in part, is that most American schools still use this system; furthermore, there is a reasoning behind the assignment of numbers to only eight groups, as will be discussed. Where necessary or appropriate, however, group numbers in the IUPAC system will be provided as well.
Principal Energy Levels
Group numbers in the North American system indicate the number of valence electrons, or the electrons that are involved in chemical bonding.
Valence electrons also occupy the highest energy level in the atom—which might be thought of as the orbit farthest from the nucleus, though in fact the term “orbit” is misleading when applied to the ways an electron moves.
Electrons do not move around the nucleus of an atom in regular orbits, like planets around the Sun; rather, their paths can only be loosely defined in terms of orbitals, a pattern of probabilities regarding the areas through which an electron is likely to move. The pattern of orbitals is determined by the principal energy level of the atom, which indicates the distance an electron may move away from the nucleus.
Principal energy level is designated by a whole-number integer, beginning with 1 and moving upward to 7: the higher the number, the further the electron is from the nucleus, and hence the greater the energy in the atom. The relationship between principal energy level and period is relatively easy to demonstrate. The number n of a period on the periodic table is the same as the number of the highest principal energy level for the atoms on that row—that is, the principal energy level occupied by its valence electrons. Thus, elements on period 1 have a highest principal energy level of 1, and so on.
Valence Electron Configurations
When discussing families of elements, however, the periods or rows on the periodic table are not as important as the groups or columns. These are defined by the valence electron configurations, a subject more complicated than principal energy levels—though the latter requires a bit more explanation in order to explain electron configurations.
Each principal energy level is divided into sublevels corresponding to the number n of the principal energy level: thus, principal energy level 1 has one sublevel, principal energy level 2 has two, and so on. As one might expect, with an increase in principal energy levels and sublevels, there are increases in the complexity of the orbitals.
Orbital Patterns
The four basic types of orbital patterns are designated as s, p,d, and f. The s shape might be described as spherical, though when talking about electrons, nothing is quite so neat: orbital patterns, remember, only identify regions of probability for the electron. In other words, in an s orbital, the total electron cloud will probably end up being more or less like a sphere.
The p shape is like a figure eight around the nucleus, and the d like two figure eights meeting at the nucleus. Again, these and other orbital patterns do not indicate that the electron will necessary follow that path. What it means is that, if you could take millions of photographs of the electron during a period of a few seconds, the resulting blur of images in a p orbital would somewhat describe the shape of a figure eight.
The orbital pattern is so complex that most basic chemistry text topics do not even attempt to explain it, and beyond f are other, even more complicated, patterns designated in alphabetical order: g, h, and so on. In the discussion that follows, we will not be concerned with these, since even for the lanthanides and the actinides, an atom at the ground state does not fill orbital patterns beyond an f.
Sublevels and orbital filling
Principal energy level 1 has only an s sublevel; 2 has an s and a p, the latter with three possible orientations in space; 3 has an s, p, and d (five possible spatial orientations); and 4 has an s, p, d, and f(seven possible spatial orientations.)
According to the Pauli exclusion principle, only two electrons can occupy a single orbital pattern—that is, the s sublevel or any one of the spatial orientations in p, d, and f—and those two electrons must be spinning in opposite directions. Thus, two electrons can move in an s orbital pattern or shell, six in a p, 10 in a d, and 14 in an forbital pattern or shell. Valence shell configurations are therefore presented with superscript figures indicating the number of electrons in that orbital pattern—for instance, s1 for one electron in the s orbital, or d10, indicating a d orbital that has been completely filled.
Real-Life Applications
Representative Elements
Hydrogen (atomic number 1), with the simplest of all atomic structures, has just one electron on principal energy level 1, so, in effect, its valence electron is also a core electron. The valence configuration for hydrogen is thus written as 1 s1.It should be noted, as described in the Electrons essay, that if a hydrogen atom (or any other atom) is in an excited state, it may reach energy levels beyond its normal, or ground, state.
Moving straight down the periodic table to francium (atomic number 87), which is in the same column as hydrogen, one finds that it has a valence electron configuration of 7s1.Thus, although francium is vastly more complex and energy-filled than hydrogen, the two elements have the same valence shell configuration; only the number of the principal energy level is different. All the elements listed below hydrogen in Group 1 are therefore classified together as alkali metals. Obviously, hydrogen—a gas—is not part of the alkali metal family, nor does it clearly belong to any other family: it is the “lone wolf” of the periodic table.
Now look at two elements in Group 2, with beryllium (atomic number 4) and radium (88) at the top and bottom respectively. Beryllium has a valence shell configuration of 2s2. This means its valence shell is at principal energy level 2, where there are two electrons on an s orbital pattern. Radium, though it is on period 7, nonetheless has the same valence shell configuration: 7s2. This defines the alkaline earth metals family in terms of valence shell configuration.
For now, let us ignore groups 3 through 6— not to mention the columns between groups 2 and 3, unnumbered in the North American system—and skip over to Group 7. All the elements in this column, known as halogens, have valence shell configurations of ns2np5. Beyond Group 7 is Group 8, the noble gases, all but one of whom have valence shell configurations of ns2np6.The exception is helium, which has an s2 valence shell. This seems to put it with the alkaline earth metals, but of course helium is not a metal. In terms of its actual behavior, it clearly belongs to the noble gases family.
The configurations of these valence shells have implications with regard to the ways in which elements bond, a subject developed at some length in the Chemical Bonding essay. Here we will consider it only in passing, to clarify the fact that electron configuration produces observable results. This is most obvious with the noble gases, which tend to resist bonding with most other elements because they already have eight electrons in their valence shell—the same number of valence electrons that most other atoms achieve only after they have bonded.
From the Representative Elements to the Transition Elements
Groups 3 through 6, along with hydrogen and the four families so far identified, constitute the 44 representative or main-group elements. In 43 of these 44, the number of valence shell electrons is the same as the group number in the North American system. (Helium, which is in Group 8 but has two valence electrons, is the lone exception.) By contrast, the 40 elements listed in the “dip” at the middle of the chart—the transition metals—follow a less easily defined pattern. This is part of the reason why the North American system does not list them by group number, and also why neither system lists the two other families within the transition elements—the lan-thanides and actinides.
Before addressing the transition metals, however, let us consider patterns of orbital filling, which also differentiate the representative elements from the transition elements. Each successive representative element fills all the orbitals of the elements that precede it (with some exceptions that will be explained), then goes on to add one more possible electron configuration. The total number of electrons—not just valence shell electrons—is the same as the atomic number. Thus fluorine, with an atomic number of 9, has a complete configuration of 1 s22s22p5.Neon, directly following it with an atomic number of 10, has a total configuration of 1s22s22p6. (Again, this is not the same as the valence shell configuration, which is contained in the last two sub-levels represented: for example, 2s22p6 for neon.)
The chart that follows shows the pattern by which orbitals are filled. Note that in several places, the pattern of filling becomes “out of order,” something that will be explained below.
Orbital Filling by Principal Energy Level
•1s (2) •2s (2) •2p (6) •3s (2) •3p (6)
•4s (2)
•3d (10)
•4p (6)
•5s (2)
•4d (10)
•5p (6)
•6s (2) •4f (14)
•5d (10)
•6p (6)
•7s (2)
•5f (14)
•6d (10)
Patterns of orbital filling
Generally, the 44 representative elements follow a regular pattern of orbital filling, and this is particularly so for the first 18 elements. Imagine a small amphitheater, shaped like a cone, with smaller rows of seats at the front. These rows are also designated by section, with the section number being the same as the number of rows in that section.
The two seats in the front row comprise a section labeled 1 or 1 s, and this is completely filled after helium (atomic number 2) enters the auditorium. Now the elements start filling section 2, which contains two rows. The first row of section 2, labeled 2s, also has two seats, and after beryllium (4), it too is filled. Row 2p has 6 seats, and it is finally filled with the entrance of neon (10). Now, all of section 2 has been filled; therefore, the eleventh element, sodium, starts filling section 3 at the first of its three rows. This row is 3 s—which, like all s rows, has only two seats. Thus, when element 13, aluminum, enters the theatre, it takes a seat in row 3p, and eventually argon (18), completes that six-seat row.
By the pattern so far established, element 19 (potassium) should begin filling row 3d by taking the first of its 10 seats. Instead, it moves on to section 4, which has four rows, and it takes the first seat in the first of those rows, 4s. Calcium (20) follows it, filling the 4s row. But when the next element, scandium (21), comes into the theatre, it goes to row 3d, where potassium “should have” gone, if it had continued filling sections in order. Scandium is followed by nine companions (the first row of transition elements) before another representative element, gallium (31), comes into the theatre. (For reasons that will not be discussed here, chromium and copper, elements 24 and 29, respectively, have valence electrons in 4s—which puts them slightly off the transition metal pattern.)
According to the “proper” order of filling seats, now that 3d (and hence all of section 3) is filled, gallium should take a seat in 4s. But those seats have already been taken by the two preceding representative elements, so gallium takes the first of six seats in 4p. After that row fills up at krypton (36), it is again “proper” for the next representative element, rubidium (37), to take a seat in 4d. Instead, just as potassium skipped 3d, rubidium skips 4d and opens up section 5 by taking the first of two seats in 5s.
Just as before, the next transition element— yttrium (39)—begins filling up section 4d, and is followed by nine more transition elements until cadmium (48) fills up that section. Then, the representative elements resume with indium (49), which, like gallium, skips ahead to section 5p. And so it goes through the remainder of the periodic table, which ends with two representative elements followed by the last 10 transition metals.
Transition Metals
Given the fact that it is actually the representative elements that skip the d sublevels, and the transition metals that go back and fill them, one might wonder if the names “representative” and “transition” (implying an interruption) should be reversed. However, remember the correlation between the number of valence shell electrons and group number for the representative elements. Furthermore, the transition metals are the only elements that fill the d orbitals.
This brings us to the reason why the lan-thanides and actinides are set apart even from the transition metals. In most versions of the periodic table, lanthanum (57) is followed by hafnium (72) in the transition metals section of the chart. Similarly, actinium (89) is followed by ruther-fordium (104). The “missing” metals—lan-thanides and actinides, respectively—are listed at the bottom of the chart. There are reasons for this, as well as for the names of these groups.
After the 6s orbital fills with the representative element barium (56), lanthanum does what a transition metal does—it begins filling the 5d orbital. But after lanthanum, something strange happens: cerium (58) quits filling 5d, and moves to fill the 4f orbital. The filling of that orbital continues throughout the entire lanthanide series, all the way to lutetium (71). Thus, lan-thanides can be defined as those metals that fill the 4f orbital; however, because lanthanum exhibits similar properties, it is usually included with the lanthanides. Sometimes the term “lan-thanide series” is used to distinguish the other 14 lanthanides from lanthanum itself.
Key Terms
Actinides: Those transition metals that fill the 5f orbital. Because actinium— which does not fill the 5f orbital—exhibits characteristics similar to those of the actinides, it is usually considered part of the actinides family.
Alkali metals: All members, except hydrogen, of Group 1 on the periodic table of elements, with valence electron configurations of ns1.
Alkaline earth metals: Group 2 on the periodic table of elements, with valence electron configurations of ns2.
Electron cloud: A term used to describe the pattern formed by orbitals.
Families of elements: Related elements, including the noble gases, halogens, alkali metals, alkaline earth metals, transition metals,lanthanides, and actinides. In addition, metals, nonmetals, and metalloids form loosely defined families. Other family designations—such as carbon family—are sometimes used.
Ground state: A term describing the state of an atom at its ordinary energy level.
Groups: Columns on the periodic table of elements. These are ordered according to the numbers of valence electrons in the outer shells of the atoms for the elements represented.
Halogens: Group 7 of the periodic table of elements, with valence electron configurations of ns2np5.
Ion: An atom or atoms that has lost or gained one or more electrons, and thus has a net electric charge.
Lanthanides: The transition metals that fill the 4forbital. Because lan-thanum—which does not fill the 4f orbital—exhibits characteristics similar to those of the lanthanides, it is usually considered part of the lanthanide family.
Main-group elements: The 44 elements in Groups 1 through 8 on the periodic table of elements, for which the number of valence electrons equals the group number. (The only exception is helium.) The main-group elements, also called representative elements, include the families of alkali metals, alkali earth metals, halogens, and noble gases, as well as other metals, nonmetals, and metalloids.
Metalloids: Elements which exhibit characteristics of both metals and non-metals. Metalloids are all solids, but are not lustrous or shiny, and they conduct heat and electricity moderately well. The six metalloids occupy a diagonal region between the metals and nonmetals on the right side of the periodic table. Sometimes astatine is included with the metalloids, but in this topic it is treated within the context of the halogens family.
Metals: A collection of 87 elements that includes numerous families—the alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides, as well as seven elements in groups 3 through 5. Metals, which occupy the left, center, and part of the right-hand side of the periodic table, are lustrous or shiny in appearance, and malleable, meaning that they can be molded into different shapes without breaking. They are excellent conductors of heat and electricity, and tend to form positive ions by losing electrons.
Noble gases: Group 8 of the periodic table of elements, all of whom (with the exception of helium) have valence electron configurations of ns2np6.
Nonmetals: Elements that have a dull appearance; are not malleable; are poor conductors of heat and electricity; and tend to gain electrons to form negative ions. They are thus the opposite of metals in most regards, as befits their name. Aside from hydrogen, the other 18 nonmetals occupy the upper right-hand side of the periodic table, and include the noble gases, halogens, and seven elements in groups 3 through 6.
Orbital: A pattern of probabilities regarding the position of an electron for an atom in a particular energy state. The higher the principal energy level, the more complex the pattern of orbitals. The four types of orbital patterns are designated as s, p, d, and f—each of which is more complex than the one before.
Periodic table of elements: A chart that shows the elements arranged in order of atomic number, along with chemical symbol and the average atomic mass (in atomic mass units) for that particular element.
Periods: Rows of the periodic table of elements. These represent successive energy levels in the atoms of the elements involved.
Principal energy level: A value indicating the distance that an electron may move away from the nucleus of an atom. This is designated by a whole-number integer, beginning with 1 and moving upward. The higher the principal energy level, the greater the energy in the atom, and the more complex the pattern of orbitals.
Representative elements: See main-group elements.
Transition metals: A group of 40 elements, which are not assigned a group number in the North American version of the periodic table. These are the only elements that fill the d orbitals.
Valence electrons: Electrons that occupy the highest energy levels in an atom. These are the electrons involved in chemical bonding.
A similar pattern occurs for the actinides. The 7s orbital fills with radium (88), after which actinium (89) begins filling the 6d orbital. Next comes thorium, first of the actinides, which begins the filling of the 5forbital. This is completed with element 103, lawrencium. Actinides can thus be defined as those metals that fill the 5f orbital; but again, because actinium exhibits similar properties, it is usually included with the actinides.
Metals, Nonmetals, and Metalloids
The reader will note that for the seven families so far identified, we have generally not discussed them in terms of properties that can more easily be discerned—such as color, phase of matter, bonding characteristics, and so on. Instead, they have been examined primarily from the standpoint of orbital filling, which provides a solid chemical foundation for identifying families. Macroscopic characteristics, as well as the ways that the various elements find application in daily life, are discussed within essays devoted to the various groups.
Note, also, that the families so far identified account for only 92 elements out of a total of112 listed on the periodic table: hydrogen; six alkali metals; six alkaline earth metals; five halogens; six noble gases; 40 transition metals; 14 lanthanides; and 14 actinides. What about the other 20? Some discussions of element families assign these elements, all of which are in groups 3 through 6, to families of their own, which will be mentioned briefly. However, because these “families” are not recognized by all chemists, in this topic the 20 elements of groups 3 through 6 are described generally as metals, nonmetals, and metalloids.
Metals and nonmetals.
Metals are lustrous or shiny in appearance, and malleable, meaning that they can be molded into different shapes without breaking. They are excellent conductors of heat and electricity, and tend to form positive ions by losing electrons. On the periodic table, metals fill the left, center, and part of the right-hand side of the chart. Thus it should not come as a surprise that most elements (87, in fact) are metals. This list includes alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides, as well as seven elements in groups 3 through 6—aluminum, gallium, indium, thallium, tin, lead, and bismuth.
Nonmetals have a dull appearance; are not malleable; are poor conductors of heat and electricity; and tend to gain electrons to form negative ions. They are thus the opposite of metals in most regards, as befits their name. Nonmetals, which occupy the upper right-hand side of the periodic table, include the noble gases, halogens, and seven elements in groups 3 through 5. These nonmetal “orphans” are boron, carbon, nitrogen, oxygen, phosphorus, sulfur, and selenium. To these seven orphans could be added an eighth, from Group 1: hydrogen. As with the metals, a separate essay—with a special focus on the “orphans”—is devoted to nonmetals.
Metalloids and other “family” designations
Occupying a diagonal region between the metals and non-metals are metalloids, elements which exhibit characteristics of both metals and nonmetals. They are all solids, but are not lustrous, and conduct heat and electricity moderately well. The six metalloids are silicon, germanium, arsenic, antimony, tellurium, and polonium. Astatine is sometimes identified as a seventh metalloid; however, in this topic, it is treated as a member of the halogen family.
Some sources list “families” rather than collections of”orphan” metals, metalloids, and non-metals, in groups 3 through 6. These designations are not used in this topic; however, they should be mentioned briefly. Group 3 is sometimes called the boron family; Group 4, the carbon family; Group 5, the nitrogen family; and Group 6, the oxygen family. Sometimes Group 5 is designated as the pnictogens, and Group 6 as the chalcogens.
