CONCEPT
Our world is made up of atoms, yet the atomic model of the universe is nonetheless considered a “theory.” When scientists know beyond all reasonable doubt that a particular principle is the case, then it is dubbed a law. Laws address the fact that certain things happen, as well as how they happen. A theory, on the other hand, attempts to explain why things happen. By definition, an idea that is dubbed a theory has yet to be fully proven, and such is the case with the atomic theory of matter. After all, the atom cannot be seen, even with electron microscopes—yet its behavior can be studied in terms of its effects. Atomic theory explains a great deal about the universe, including the relationship between chemical elements, and therefore (as with Darwin’s theory concerning biological evolution), it is generally accepted as fact. The particulars of this theory, including the means by which it evolved over the centuries, are as dramatic as any detective story. Nonetheless, much still remains to be explained about the atom—particularly with regard to the smallest items it contains.
HOW IT WORKS
Why Study Atoms?
Many accounts of the atom begin with a history of the growth in scientists’ understanding of its structure, but here we will take the opposite approach, first discussing the atom in terms of what physicists and chemists today understand. Only then will we examine the many challenges scientists faced in developing the current atomic model: false starts, wrong theories, right roads not taken, incomplete models. In addition, we will explore the many insights added along the way as, piece by piece, the evidence concerning atomic behavior began to accumulate.
People who are not scientifically trained tend to associate studies of the atom with physics, not chemistry. While it is true that physicists study atomic structure, and that much of what scientists know today about atoms comes from the work of physicists, atomic studies are even more integral to chemistry than to physics. At heart, chemistry is about the interaction of different atomic and molecular structures: their properties, their reactions, and the ways in which they bond.
What the atom means to chemistry
Just as a writer in English works with the 26 letters of the alphabet, a chemist works with the 100-plus known elements, the fundamental and indivisible substances of all matter. And what differentiates the elements, ultimately, from one another is not their color or texture, or even the phase of matter—solid, gas, or liquid—in which they are normally found. Rather, the defining characteristic of an element is the atom that forms its basic structure.
The number of protons in an atom is the critical factor in differentiating between elements, while the number of neutrons alongside the protons in the nucleus serves to distinguish one isotope from another. However, as important as elements and even isotopes are to the work of a chemist, the components of the atom’s nucleus have little direct bearing on the atomic activity that brings about chemical reactions and chemical bonding. All the chemical “work” of an atom is done by particles vastly smaller in mass than either the protons or neutrons—fast-moving little bundles of energy called electrons.
Moving rapidly through the space between the nucleus and the edge of the atom, electrons sometimes become dislodged, causing the atom to become a positively charged ion. Conversely, sometimes an atom takes on one or more electrons, thus acquiring a negative charge. Ions are critical to the formation of some kinds of chemical bonds, but the chemical role of the electron is not limited to ionic bonds.
In fact, what defines an atom’s ability to bond with another atom, and therefore to form a molecule, is the specific configuration of its electrons. Furthermore, chemical reactions are the result of changes in the arrangement of electrons, not of any activity involving protons or neutrons. So important are electrons to the interactions studied in chemistry that a separate essay has been devoted to them.
What An Atom Is
Basic atomic structure
The definitions of atoms and elements seems, at first glance, almost circular: an element is a substance made up of only one kind of atom, and an atom is the smallest particle of an element that retains all the chemical and physical properties of the element. In fact, these two definitions do not form a closed loop, as they would if it were stated that an element is something made up of atoms. Every item of matter that exists, except for the subatomic particles discussed in this essay, is made up of atoms. An element, on the other hand, is—as stated in its definition—made up of only one kind of atom. “Kind of atom” in this context refers to the number of protons in its nucleus.
Protons are one of three basic subatomic particles, the other two being electrons and neutrons. As we shall see, there appear to be particles even smaller than these, but before approaching these “sub-subatomic” particles, it is necessary to address the three most significant components of an atom. These are distinguished from one another in terms of electric charge: protons are positively charged, electrons are negative in charge, and neutrons have no electrical charge. As with the north and south poles of magnets, positive and negative charges attract one another, whereas like charges repel. Atoms have no net charge, meaning that the protons and electrons cancel out one another.
Evolving models of the atom
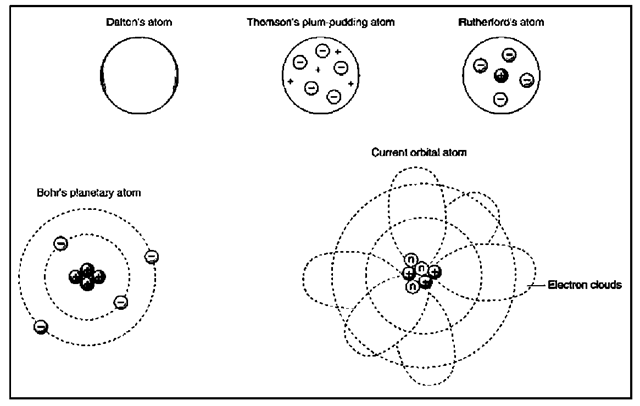
Scientists originally thought of an atom as a sort of closed sphere with a relatively hard shell, rather like a ball bearing. Nor did they initially understand that atoms themselves are divisible, consisting of the parts named above. Even as awareness of these three parts emerged in the last years of the nineteenth century and the first part of the twentieth, it was not at all clear how they fit together.
At one point, scientists believed that electrons floated in a cloud of positive charges. This was before the discovery of the nucleus, where the protons and neutrons reside at the heart of the atom. It then became clear that electrons were moving around the nucleus, but how? For a time, a planetary model seemed appropriate: in other words, electrons revolved around the nucleus much as planets orbit the Sun. Eventually, however—as is often the case with scientific discovery—this model became unworkable, and had to be replaced by another.
The model of electron behavior accepted today depicts the electrons as forming a cloud around the nucleus—almost exactly the opposite of what physicists believed a century ago. The use of the term “cloud” may perhaps be a bit misleading, implying as it does something that simply hovers. In fact, the electron, under normal circumstances, is constantly moving. The paths of its movement around the nucleus are nothing like that of a planet’s orbit, except inasmuch as both models describe a relatively small object moving around a relatively large one.
The furthest edges of the electron’s movement define the outer perimeters of the atom. Rather than being a hard-shelled little nugget of matter, an atom—to restate the metaphor mentioned above—is a cloud of electrons surrounding a nucleus. Its perimeters are thus not sharply delineated, just as there is no distinct barrier between Earth’s atmosphere and space itself. Just as the air gets thinner the higher one goes, so it is with an atom: the further a point is from the nucleus, the less the likelihood that an electron will pass that point on a given orbital path.
Nucleons
Mass number and atomic number
The term nucleon is used generically to describe the relatively heavy particles that make up an atomic nucleus. Just as “sport” can refer to football, basketball, or baseball, or any other item in a similar class, such as soccer or tennis, “nucleon” refers to protons and neutrons. The sum of protons and neutrons is sometimes called the nucleon number, although a more commonly used term is mass number.
Though the electron is the agent of chemical reactions and bonding, it is the number of protons in the nucleus that defines an atom as to its element. Atoms of the same element always have the same number of protons, and since this figure is unique for a given element, each element is assigned an atomic number equal to the number of protons in its nucleus. The atoms are listed in order of atomic number on the periodic table of elements.
Atomic mass and isotopes
A proton has a mass of 1.673 • 10-24 g, which is very close to the established figure for measuring atomic mass, the atomic mass unit. At one time, the basic unit of atomic mass was equal to the mass of one hydrogen atom, but hydrogen is so reactive—that is, it tends to combine readily with other atoms to form a molecule, and hence a compound—that it is difficult to isolate. Instead, the atomic mass unit is today defined as 1/12 of the mass of a carbon-12 atom. That figure is exactly 1.66053873 • 10-24 grams.
The mention of carbon-12, a substance found in all living things, brings up the subject of isotopes. The “12″ in carbon-12 refers to its mass number, or the sum of protons and neutrons. Two atoms may be of the same element, and thus have the same number of protons, yet differ in their number of neutrons—which means a difference both in mass number and atomic mass. Such differing atoms of the same element are called isotopes. Isotopes are often designated by symbols showing mass number to the upper left of the chemical symbol or element symbol—for instance, 12C for carbon-12.
Electric charge
Protons have a positive electric charge of 1, designated either as 1+ or +1. Neutrons, on the other hand, have no electric charge. It appears that the 1+ charge of a proton and the 0 charge of a neutron are the products of electric charges on the part of even smaller particles called quarks. A quark may either have a positive electric charge of less than 1+, in which case it is called an “up quark”; or a negative charge of less than 1-, in which case it is called a “down quark.”
Research indicates that a proton contains two up quarks, each with a charge of 2/3+, and
The evolution of atomic theory.
one down quark with a charge of 1/3-. This results in a net charge of 1+. On the other hand, a neutron is believed to hold one up quark with a charge of 2/3+, and two down quarks with charges of 1/3- each. Thus, in the neutron, the up and down quarks cancel out one another, and the net charge is zero.
A neutron has about the same mass as a proton, but other than its role in forming isotopes, the neutron’s function is not exactly clear. Perhaps, it has been speculated, it binds protons— which, due to their positive charges, tend to repel one another—together at the nucleus.
Electrons
An electron is much smaller than a proton or neutron, and has much less mass; in fact, its mass is equal to 1/1836 that of a proton, and 1/1839 that of a neutron. Yet the area occupied by electrons—the region through which they move— constitutes most of the atom’s volume. If the nucleus of an atom were the size of a BB (which, in fact, is billions of times larger than a nucleus), the furthest edge of the atom would be equivalent to the highest ring of seats around an indoor sports arena. Imagine the electrons as incredibly fast-moving insects buzzing constantly through the arena, passing by the BB but then flitting to the edges or points in between, and you have something approaching an image of the atom’s interior.
How fast does an electron move? Speeds vary depending on a number of factors, but it can move nearly as fast as light: 186,000 mi (299,339 km) per second. On the other hand, for an item of matter near absolute zero in temperature, the velocity of the electron is much, much less. In any case, given the fact that an electron has enough negative charge to cancel out that of the proton, it must be highly energized. After all, this would be like an electric generator weighing 1 lb having as much power as a generator that weighed 1 ton.
According to what modern scientists know or hypothesize concerning the inner structure of the atom, electrons are not made up of quarks; rather, they are part of a class of particles called leptons. It appears that leptons, along with quarks and what are called exchange particles, constitute the elementary particles of atoms— particles on a much more fundamental level than that of the proton and neutron.
Electrons are perhaps the most intriguing parts of an atom. Their mass is tiny, even in atomic terms, yet they possess enough charge to counteract a “huge” proton. They are capable, in certain situations, of moving from one atom to another, thus creating ions, and depending on their highly complex configuration and ability to rearrange their configuration, they facilitate or prevent chemical reactions.
REAL-LIFE APPLICATIONS
Ancient Greek Theories of Matter
The first of the Greek philosophers, and the first individual in Western history who deserves to be called a scientist, was Thales (c. 625-c. 547 B.C.) of Miletus. (Miletus is in Greek Asia Minor, now part of Turkey.) Among his many achievements were the correct prediction of a solar eclipse, and one of the first-ever observations of electricity, when he noted the electrification of amber by friction.
But perhaps the greatest of Thales’s legacies was his statement that “Everything is water.” This represented the first attempt to characterize the nature of all physical reality. It set off a debate concerning the fundamental nature of matter that consumed Greek philosophers for two centuries. Later, philosophers attempted to characterize matter in terms of fire or air. In time, however, there emerged a school of thought concerned not with identifying matter as one particular thing or another, but with recognizing a structural consistency in all of matter. Among these were the philosophers Leucippus (c. 480-c. 420 B.C.) and his student Democritus (c. 460-370 B.C.)
Democritus’s “atoms”
Leucippus and Democritus proposed a new and highly advanced model for the tiniest point of physical space. Democritus, who actually articulated these ideas (far less is known about Leucip-pus) began with a “thought experiment,” imagining what would happen if an item of matter were subdivided down to its smallest piece. This tiniest fragment, representing an item of matter that could not be cut into smaller pieces, he called by a Greek term meaning “no cut”: atomos.
Democritus was not necessarily describing matter in a concrete, scientific way: his “atoms” were idealized philosophical constructs rather than purely physical units. Yet, he came amazingly close, and indeed much closer than any thinker for the next 22 centuries, to identifying the fundamental structure of physical reality. Why did it take so long for scientists to come back around to the atomic model? The principal culprit, who advanced an erroneous theory of matter, also happened to be one of the greatest thinkers of all time: Aristotle (384-322 B.C.. )
Aristotle’s “elements”
Aristotle made numerous contributions to science, including his studies in botany and zoology, as well as his explanation of the four causes, a significant attempt to explain events by means other than myth or superstition. In the area of the physical sciences, however, Aristotle’s impact was less than beneficial. Most notably, in explaining why objects fall when dropped, he claimed that the ground was their “natural” destination— a fallacy later overturned with the gravitational model developed by Galileo Galileio (1564-1642) and Sir Isaac Newton (1642-1727).
The ideas Aristotle put forward concerning what he called “natural motion” were a product of his equally faulty theories with regard to what today’s scientists refer to as chemistry. In ancient times, chemistry, as such, did not exist. Long before Aristotle’s time, Egyptian embalmers and metallurgists used chemical processes, but they did so in a practical, applied manner, exerting little effort toward what could be described as scientific theory. Philosophers such as Aristotle, who were some of the first scientists, made little distinction between physical and chemical processes. Thus, whereas physics is understood today as an important background for chemistry, Aristotle’s “physics” was actually an outgrowth of his “chemistry.”
Rejecting Democritus’s atomic model, Aristotle put forward his own view of matter. Like Democritus, he believed that matter was composed of very small components, but these he identified not as atoms, but as “elements”: earth, air, fire, and water. He maintained that all objects consisted, in varying degrees, of one or more of these, and based his explanation of gravity on the relative weights of each element. Water sits on top of the earth, he explained, because it is lighter, yet air floats above the water because it is lighter still—and fire, lightest of all, rises highest. Furthermore, he claimed that the planets beyond Earth were made up of a “fifth element,” or quintessence, of which little could be known.
In fairness to Aristotle, it should be pointed out that it was not his fault that science all but died out in the Western world during the period from about a.d. 200 to about 1200. Furthermore, he did offer an accurate definition of an element, in a general sense, as “one of those simple bodies into which other bodies can be decomposed, and which itself is not capable of being divided into others.” As we shall see, the definition used today is not very different from Aristotle’s. However, to define an element scientifically, as modern chemists do, it is necessary to refer to something Aristotle rejected: the atom. So great was his opposition to Democritus’s atomic theory, and so enormous was Aristotle’s influence on learning for more than 1,500 years following his death, that scientists only began to reconsider atomic theory in the late eighteenth century.
A Maturing Concept of Elements
Boyle’s Idea of Elements
One of the first steps toward an understanding of the chemical elements came with the work of English physicist and chemist Robert Boyle (1627-1691). Building on the usable definition of an element provided by Aristotle, Boyle maintained no substance was an element if it could be broken down into other substances. Thus, air could be eliminated from the list of “elements,” because, clearly, it could be separated into more than one elemental substance. (In fact, none of the four “elements” identified by Aristotle even remotely qualifies as an element in modern chemistry.)
Boyle, nonetheless, still clung to aspects of alchemy, a pseudo-science based on the transformation of “base metals,” for example, the metamorphosis of iron into gold. Though true chemistry grew out of alchemy, the fundamental proposition of alchemy was faulty: if one metal can be turned into another, then that means that metals are not elements, which, in fact, they are. Nonetheless, Boyle’s studies led to the identification of numerous elements—that is, items that really are elements—in the years that followed.
Lavoisier and Proust:Constant Composition
A few years after Boyle came two French chemists who extended scientific understanding of the elements. Antoine Lavoisier (1743-1794) affirmed the definition of an element as a simple substance that could not be broken down into a simpler substance, and noted that elements always react with one another in the same proportions.
Joseph-Louis Proust (1754-1826) put forward the law of constant composition, which holds that a given compound always contains the same proportions of mass between elements. Another chemist of the era had claimed that the composition of a compound varies in accordance with the reactants used to produce it. Proust’s law of constant composition made it clear that any particular compound will always have the same composition.
Early Modern Understanding of the Atom
Dalton And Avogadro:Atoms And Molecules
The work of Lavoisier and Proust influenced a critical figure in the development of the atomic model: English chemist John Dalton (1766-1844). In A New System of Chemical Philosophy (1808), Dalton put forward the idea that nature is composed of tiny particles, and in so doing he adopted Democritus’s word “atom” to describe these basic units. This idea, which Dalton had formulated five years earlier, marked the starting-point of modern atomic theory.
Dalton recognized that the structure of atoms in a particular element or compound is uniform, but maintained that compounds are made up of compound atoms: in other words, water, for instance, is a compound of “water atoms.” However, water is not an element, and thus, it was necessary to think of its atomic composition in a different way—in terms of molecules rather than atoms. Dalton’s contemporary Amedeo Avogadro (1776-1856), an Italian physicist, became the first scientist to clarify the distinction between atoms and molecules.
The later development of the mole, which provided a means whereby equal numbers of molecules could be compared, paid tribute to Avogadro by designating the number of molecules in a mole as “Avogadro’s number.” Another contemporary, Swedish chemist Jons Berzelius (1779-1848), maintained that equal volumes of gases at the same temperature and pressure contained equal numbers of atoms. Using this idea, he compared the mass of various reacting gases, and developed a system of comparing the mass of various atoms in relation to the lightest one, hydrogen. Berzelius also introduced the system of chemical symbols—H for hydrogen, O for oxygen, and so on—in use today.
Brownian motion and kinetic theory
Yet another figure whose dates overlapped with those of Dalton, Avogadro, and Berzelius was Scottish botanist Robert Brown (1773-1858). In 1827, Brown noted a phenomenon that later had an enormous impact on the understanding of the atom. While studying pollen grains under a microscope, Brown noticed that the grains underwent a curious zigzagging motion in the water. The pollen assumed the shape of a colloid, a pattern that occurs when particles of one substance are dispersed—but not dissolved—in another substance. At first, Brown assumed that the motion had a biological explanation—that is, it resulted from life processes within the pollen—but later, he discovered that even pollen from long-dead plants behaved in the same way.
Brown never understood what he was witnessing. Nor did a number of other scientists, who began noticing other examples of what came to be known as Brownian motion: the constant but irregular zigzagging of colloidal particles, which can be seen clearly through a microscope. Later, however, Scottish physicist James Clerk Maxwell (1831-1879) and others were able to explain this phenomenon by what came to be known as the kinetic theory of matter.
Kinetic theory is based on the idea that molecules are constantly in motion: hence, the water molecules were moving the pollen grains Brown observed. Pollen grains are many thousands of times as large as water molecules, but since there are so many molecules in even a drop of water, and their motion is so constant but apparently random, they are bound to move a pollen grain once every few thousand collisions.
Mendeleev and the Periodic Table
In 1869, Russian chemist Dmitri Mendeleev (1834-1907) introduced a highly useful system for organizing the elements, the periodic table. Mendeleev’s table is far more than just a handy chart listing elements: at once simple and highly complex, it shows elements in order of increasing atomic mass, and groups together those exhibiting similar forms of chemical behavior and structure.
Reading from right to left and top to bottom, the periodic table, as it is configured today, lists atoms in order of atomic number, generally reflected by a corresponding increase in average atomic mass. As Mendeleev observed, every eighth element on the chart exhibits similar characteristics, and thus the chart is organized in columns representing specific groups of elements.
The patterns Mendeleev observed were so regular that for any “hole” in his table, he predicted that an element would be discovered that would fill that space. For instance, at one point there was a gap between atomic numbers 71 and 73 (lutetium and tantalum, respectively). Mendeleev indicated that an atom would be found for the space, and 15 years after this prediction, the element germanium was isolated.
However, much of what defines an element’s place on the chart today relates to subatomic particles—protons, which determine atomic number, and electrons, whose configurations explain certain chemical similarities. Mendeleev was unaware of these particles: from the time he created his table, it was another three decades before the discovery of the first of these particles, the electron. Instead, he listed the elements in an order reflecting outward characteristics now understood to be the result of the quantity and distribution of protons and electrons.
Electromagnetism and Radiation
The contribution of Mendeleev’s contemporary, Maxwell, to the understanding of the atom was not limited to his kinetic theory. Building on the work of British physicist and chemist Michael Faraday (1791-1867) and others, in 1865 he published a paper outlining a theory of a fundamental interaction between electricity and magnetism. The electromagnetic interaction, as it later turned out, explained something that gravitation, the only other form of fundamental interaction known at the time, could not: the force that held together particles in an atom.
The idea of subatomic particles was still a long time in coming, but the model of electro-magnetism helped make it possible. In the long run, electromagnetism was understood to encompass a whole spectrum of energy radiation, including radio waves; infrared, visible, and ultraviolet light; x rays; and gamma rays. But this,too, was the product of work on the part of numerous individuals, among whom was English physicist William Crookes (1832-1919).
In the 1870s, Crookes developed an apparatus later termed a Crookes tube, with which he sought to analyze the “rays”—that is, radiation— emitted by metals. The tube consisted of a glass bulb, from which most of the air had been removed, encased between two metal plates or electrodes, referred to as a cathode and an anode. A wire led outside the bulb to an electric source, and when electricity was applied to the electrodes, the cathodes emitted rays. Crookes concluded that the cathode rays were particles with a negative electric charge that came from the metal in the cathode plate.
Radiation
In 1895, German physicist Wilhelm Rontgen (1845-1923) noticed that photographic plates held near a Crookes tube became fogged, and dubbed the rays that had caused the fogging “x rays” A year after Rontgen’s discovery, French physicist Henri Becquerel (1852-1908) left some photographic plates in a drawer with a sample of uranium. Uranium had been discovered more than a century before; however, there were few uses for it until Becquerel discovered that the uranium likewise caused a fogging of the photographic plates.
Thus radioactivity, a type of radiation brought about by atoms that experience radioactive decay was discovered. The term was coined by Polish-French physicist and chemist Marie Curie (1867-1934), who with her husband Pierre (1859-1906), a French physicist, was responsible for the discovery of several radioactive elements.
The Rise and Fall of the Plum Pudding Model
Working with a Crookes tube, English physicist J. J. Thomson (1856-1940) hypothesized that the negatively charged particles Crookes had observed were being emitted by atoms, and in 1897, he gave a name to these particles: electrons. The discovery of the electron raised a new question: if Thomson’s particles exerted a negative charge, from whence did the counterbalancing positive charge come?
An answer, of sorts, came from William Thomson, not related to the other Thomson and, in any case, better known by his title as Lord Kelvin (1824-1907). Kelvin compared the structure of an atom to an English plum pudding: the electrons were like raisins, floating in a positively charged “pudding”—that is, an undifferentiated cloud of positive charges.
Kelvin’s temperature scale contributed greatly to the understanding of molecular motion as encompassed in the kinetic theory of matter. However, his model for the distribution of charges in an atom—charming as it may have been—was incorrect. Nonetheless, for several decades, the “plum pudding model,” as it came to be known, remained the most widely accepted depiction of the way that electric charges were distributed in an atom. The overturning of the plum pudding model was the work of English physicist Ernest Rutherford (1871-1937), a student of J. J. Thomson.
Rutherford identifies the nucleus
Rutherford did not set out to disprove the plum pudding model; rather, he was conducting tests to find materials that would block radiation from reaching a photographic plate. The two materials he identified, which were, respectively, positive and negative in electric charge, he dubbed alpha and beta particles. (An alpha particle is a helium nucleus stripped of its electrons, such that it has a positive charge of 2; beta particles are either electrons or positively charged subatomic particles called positrons. The beta particle Rutherford studied was an electron emitted during radioactive decay.)
Using a piece of thin gold foil with photographic plates encircling it, Rutherford bombarded the foil with alpha particles. Most of the alpha particles went straight through the foil—as they should, according to the plum pudding model. However, a few particles were deflected from their course, and some even bounced back. Rutherford later said it was as though he had fired a gun at a piece of tissue paper, only to see the tissue deflect the bullets. Analyzing these results, Rutherford concluded that there was no “pudding” of positive charges: instead, the atom had a positively charged nucleus at its center.
The Nucleus Emerges
Protons and Isotopes
In addition to defining the nucleus, Rutherford also gave a name to the particles that imparted its positive charge: protons. But just as the identification of the electron had raised new questions that, in being answered, led to the discovery of the proton, Rutherford’s achievement only brought up new anomalies concerning the behavior of the nucleus.
Together with English chemist Frederick Soddy (1877-1956), Rutherford discovered that when an atom emitted alpha or beta particles, its atomic mass changed. Soddy had a name for atoms that displayed this type of behavior: isotopes. Certain types of isotopes, Soddy and Rutherford went on to conclude, had a tendency to decay, moving toward stabilization, and this decay explained radioactivity.
Clarifying the Periodic Table
Soddy concluded that atomic mass, as measured by Berzelius, was actually an average of the mass figures for all isotopes within that element. This explained a problem with Mendeleev’s periodic table, in which there seemed to be irregularities in the increase of atomic mass from element to element. The answer to these variations in mass, it turned out, related to the number of isotopes associated with a given element: the greater the number of isotopes, the more these affected the overall measure of the element’s mass.
By this point, physicists and chemists had come to understand that various levels of energy in matter emitted specific electromagnetic wavelengths. Welsh physicist Henry Moseley (18871915) experimented with x rays, bombarding atoms of different elements with high levels of energy and observing the light they gave off as they cooled. In the course of these tests, he uncovered an astounding mathematical relationship: the amount of energy a given element emitted was related to its atomic number.
Furthermore, the atomic number corresponded to the number of positive charges—this was in 1913, before Rutherford had named the proton—in the nucleus. Mendeleev had been able to predict the discovery of new elements, but such predictions had remained problematic. When scientists understood the idea of atomic number, however, it became possible to predict the existence of undiscovered elements with much greater accuracy.
Neutrons
Yet again, discoveries—the nucleus, protons, and the relationship between these and atomic number—only created new questions. (This, indeed, is one of the hallmarks of an active scientific theory. Rather than settling questions, science is about raising new ones, and thus improving the quality of the questions that are asked.) Once Rutherford had identified the proton, and Moseley had established the number of protons, the mystery at the heart of the atom only grew deeper.
Scientists had found that the measured mass of atoms could not be accounted for by the number of protons they contained. Certainly, the electrons had little to do with atomic mass: by then it had been shown that the electron weighed about 0.06% as much as a proton. Yet for all elements other than protium (the first of three hydrogen isotopes), there was a discrepancy between atomic mass and atomic number. Clearly, there had to be something else inside the nucleus.
In 1932, English physicist James Chadwick (1891-1974) identified that “something else.” Working with radioactive material, he found that a certain type of subatomic particle could penetrate lead. All other known types of radiation were stopped by the lead, and therefore, Chad-wick reasoned that this particle must be neutral in charge. In 1932, he won the Nobel Prize in Physics for his discovery of the neutron.
The Nuclear Explosion
isotopes and radioactivity. Chadwick’s discovery clarified another mystery, that of the isotope, which had been raised by Rutherford and Soddy several decades earlier. Obviously, the number of protons in a nucleus did not change, but until the identification of the neutron, it had not been clear what it was that did change. At that point, it was understood that two atoms may have the same atomic number— and hence be of the same element—yet they may differ in number of neutrons, and thus be isotopes.
As the image of what an isotope was became clearer, so too did scientists’ comprehension of radioactivity. Radioactivity, it was discovered, was most intense where an isotope was the most unstable—that is, in cases where an isotope had the greatest tendency to experience decay. Uranium had a number of radioactive isotopes, such as 235U, and these found application in the burgeoning realm of nuclear power—both the destructive power of atomic bombs, and later the constructive power of nuclear energy plants.
Fission vs. Fusion
In nuclear fission, or the splitting of atoms, uranium isotopes (or other radioactive isotopes) are bombarded with neutrons, splitting the uranium nucleus in half and releasing huge amounts of energy. As the nucleus is halved, it emits several extra neutrons, which spin off and split more uranium nuclei, creating still more energy and setting off a chain reaction. This explains the destructive power in an atomic bomb, as well as the constructive power—providing energy to homes and businesses—in a nuclear power plant. Whereas the chain reaction in an atomic bomb becomes an uncontrolled explosion, in a nuclear plant the reaction is slowed and controlled.
Yet nuclear fission is not the most powerful form of atomic reaction. As soon as scientists realized that it was possible to force particles out of a nucleus, they began to wonder if particles could be forced into the nucleus. This type of reaction, known as fusion, puts even nuclear fission, with its awesome capabilities, to shame: nuclear fusion is, after all, the power of the Sun. On the surface of that great star, hydrogen atoms reach incredible temperatures, and their nuclei fuse to create helium. In other words, one element actually transforms into another, releasing enormous amounts of energy in the process.
Nuclear Energy in War and Peace
The atomic bombs dropped by the United States on Japan in 1945 were fission bombs. These were the creation of a group of scientists—legendary figures such as American physicist J. Robert Oppenheimer (1904-1967), American mathematician John von Neumann (1903-1957), American physicist Edward Teller (1908-), and Italian physicist Enrico Fermi (1901-1954)—involved in the Manhattan Project at Las Alamos, New Mexico.
Some of these geniuses, particularly Oppen-heimer, were ambivalent about the moral implications of the enormous destructive power they created. However, most military historians believe that far more lives—both Japanese and American—would have been lost if America had been forced to conduct a land invasion of Japan. As it was, the Japanese surrendered shortly after the cities of Hiroshima and Nagasaki suffered the devastating effects of fission-based explosions.
By 1952, U.S. scientists had developed a “hydrogen,” or fusion bomb, thus raising the stakes greatly. This was a bomb that possessed far more destructive capability than the ones dropped over Japan. Fortunately, the Hiroshima and Nagasaki bombs were the only ones dropped in wartime, and a ban on atmospheric nuclear testing has greatly reduced the chances of human exposure to nuclear fallout of any kind. With the end of the arms race between the United States and the Soviet Union, the threat of nuclear destruction has receded somewhat—though it will perhaps always be a part of human life.
Nonetheless, fear of nuclear power, spawned as a result of the arms race, continues to cloud the future of nuclear plants that generate electricity—even though these, in fact, emit less radioactive pollution than coal- or gas-burning power plants. At the same time, scientists continue to work on developing a process of power generation by means of nuclear fusion, which, if and when it is achieved, will be one of the great miracles of science.
Particle Accelerators
One of the tools used by scientists researching nuclear fusion is the particle accelerator, which moves streams of charged particles—protons, for instance—faster and faster. These fast particles are then aimed at a thin plate composed of a light element, such as lithium. If the proton manages to be “captured” in the nucleus of a lithium atom, the resulting nucleus is unstable, and breaks into alpha particles.
This method of induced radioactivity is among the most oft- used means of studying nuclear structure and subatomic particles. In 1932, the same year that Chadwick discovered the neutron, English physicist John D. Cockcroft (1897-1967) and Irish physicist Ernest Walton (1903-1995) built the first particle accelerator. Some particle accelerators today race the particles in long straight lines or, to save space, in ringed paths several miles in diameter.
Quantum Theory and Beyond
The Contribution of Relativity
It may seem strange that in this lengthy (though, in fact, quite abbreviated!) overview of developments in understanding of the atom, no mention has been made of the figure most associated with the atom in the popular mind: German-American physicist Albert Einstein (18791955). The reasons for this are several. Einstein’s relativity theory addresses physical, rather than chemical, processes, and did not directly contribute to enhanced understanding of atomic structure or elements. The heart of relativity theory is the famous formula E = mc2, which means that every item of matter possesses energy proportional to its mass multiplied by the squared speed of light.
The value of mc2, of course, is an enormous amount of energy, and in order to be released in significant quantities, an article of matter must experience the kinetic energy associated with very, very high speeds—speeds close to that of light. Obviously, the easiest thing to accelerate to such a speed is an atom, and hence, nuclear energy is a result of Einstein’s famous equation. Nonetheless, it should be stressed that although Einstein is associated with unlocking the power of the atom, he did little to explain what atoms are.
However, in the course of developing his relativity theory in 1905, Einstein put to rest a question about atoms and molecules that still remained unsettled after more than a century. Einstein’s analysis of Brownian motion, combined with the confirmation of his results by French physicist Jean Baptiste Perrin (18701942), showed conclusively that yes, atoms and molecules do exist. It may seem amazing that as recently as 1905, this was still in doubt; however, this only serves to illustrate the arduous path scientists must tread in developing a theory that accurately explains the world.
Planck’s Quantum Theory
A figure whose name deserves to be as much a household word as Einstein’s—though it is not—is German physicist Max Planck (1858-1947). It was Planck who initiated the quantum theory that Einstein developed further, a theory that prevails today in the physical sciences.
At the atomic level, Planck showed, energy is emitted in tiny packets or “quanta.” Each of these energy packets is indivisible, and the behavior of quanta redefine the old rules of physics handed down from Newton and Maxwell. Thus, it is Planck’s quantum theory, rather than Einstein’s relativity, that truly marks the watershed, or “before and after,” between classical physics and modern physics.
Quantum theory is important not only to physics, but to chemistry as well. It helps to explain the energy levels of electrons, which are not continuous, as in a spectrum, but jump between certain discrete points. The quantum model is now also applied to the overall behavior of the electron; but before this could be fully achieved, scientists had to develop a new understanding of the way electrons move around the nucleus.
Bohr’s Planetary Model of the Atom
As was often the case in the history of the atom, a man otherwise respected as a great scientist put forward a theory of atomic structure that at first seemed convincing, but ultimately turned out to be inaccurate. In this case, it was Danish physicist Bohr (18851962), a seminal figure in the development of nuclear fission.
Using the observation, derived from quantum theory, that electrons only occupied specific energy levels, Bohr hypothesized that electrons orbited around a nucleus in the same way that planets orbit the Sun. There is no reason to believe that Bohr formed this hypothesis for any sentimental reasons—though, of course, scientists are just as capable of prejudice as anyone. His work was based on his studies; nonetheless, it is easy to see how this model seemed appealing, showing as it did an order at the subatomic level reflecting an order in the heavens.
Electron Clouds
Many people today who are not scientifically trained continue to think that an atom is structured much like the Solar System. This image is reinforced by symbolism, inherited from the 1950s, that represents “nuclear power” by showing a dot (the nucleus) surrounded by ovals at angles to one another, representing the orbital paths of electrons. However, by the 1950s, this model of the atom had already been overturned.
In 1923, French physicist Louis de Broglie (1892-1987) introduced the particle-wave hypothesis, which indicated that electrons could sometimes have the properties of waves—an eventuality not encompassed in the Bohr model. It became clear that though Bohr was correct in maintaining that electrons occupy specific energy levels, his planetary model was inadequate for explaining the behavior of electrons.
Two years later, in 1925, German physicist Werner Heisenberg (1901-1976) introduced what came to be known as the Heisenberg Uncertainty Principle, showing that the precise position and speed of an electron cannot be known at the same time. Austrian physicist Erwin Schrodinger (1887-1961) developed an equation for calculating how an electron with a certain energy moves, identifying regions in an atom where an electron possessing a certain energy level is likely to be. Schrodinger’s equation cannot, however, identify the location exactly.
KEY TERMS
Atom: The smallest particle of an element that retains all the chemical and physical properties of the element. An atom can exist either alone or in combination with other atoms in a molecule. Atoms are made up of protons, neutrons, and electrons.
Atomic mass unit: An SI unit (abbreviated amu), equal to 1.66 • 10-24 g, for measuring the mass of atoms.
Atomic number: The number of protons in the nucleus of an atom. Since this number is different for each element, elements are listed on the periodic table of elements in order of atomic number.
Average atomic mass: A figure used by chemists to specify the mass—in atomic mass units—of the average atom in a large sample.
Chemical symbol: A one- or two-letter abbreviation for the name of an element.
Compound: A substance made up of atoms of more than one element. These atoms are usually joined in molecules.
Electron: Negatively charged particles in an atom. Electrons, which spin around the protons and neutrons that make up the atom’s nucleus, constitute a very small portion of the atom’s mass. The number of electrons and protons is the same, thus canceling out one another; on the other hand, if an atom loses or gains electrons, it becomes an ion.
Element symbol: Another term for chemical symbol.
Ion: An atom or atoms that has lost or gained one or more electrons, and thus has a net electric charge.
Isotopes: Atoms that have an equal number of protons, and hence are of the same element, but differ in their number of neutrons.
Mass number: The sum of protons and neutrons in an atom’s nucleus.
Molecule: A group of atoms, usually (but not always) representing more than one element, joined in a structure. Compounds are typically made up of molecules.
Neutron: A subatomic particle that has no electric charge. Neutrons are found at the nucleus of an atom, alongside protons.
Nucleon: A generic term for the heavy particles—protons and neutrons— that make up the nucleus of an atom.
Nucleon number: Another term for mass number.
Nucleus: The center of an atom, a region where protons and neutrons are located, and around which electrons spin.
Periodic table of elements: A chart that shows the elements arranged in order of atomic number, along with chemical symbol and the average atomic mass (in atomic mass units) for that particular element. Vertical columns within the periodic table indicate groups or “families” of elements with similar chemical characteristics.
Proton: A positively charged particle in an atom. Protons and neutrons, which together form the nucleus around which electrons spin, have approximately the same mass—a mass that is many times greater than that of an electron.
Quark: A particle believed to be a component of protons and neutrons. A quark may either have a positive electric charge of less than 1+, in which case it is called an “up quark”; or a negative charge of less than 1-, in which case it is called a “down quark.”
Radiation: In a general sense, radiation can refer to anything that travels in a stream, whether that stream be composed of subatomic particles or electromagnetic waves. In a more specific sense, the term relates to the radiation from radioactive materials, which can be harmful to human beings.
Radioactivity: A term describing a phenomenon whereby certain isotopes are subject to a form of decay brought about by the emission of high-energy particles or radiation, such as alpha particles, beta particles, or gamma rays.
Rather than being called orbits, which suggest the orderly pattern of Bohr’s model, Schrodinger’s regions of probability are called orbitals. Moving within these orbitals, electrons describe the shape of a cloud, as discussed much earlier in this essay; as a result, the “electron cloud” theory prevails today. This theory incorporates aspects of Bohr’s model, inasmuch as electrons move from one orbital to another by absorbing or emitting a quantum of energy.
