Adherens junctions
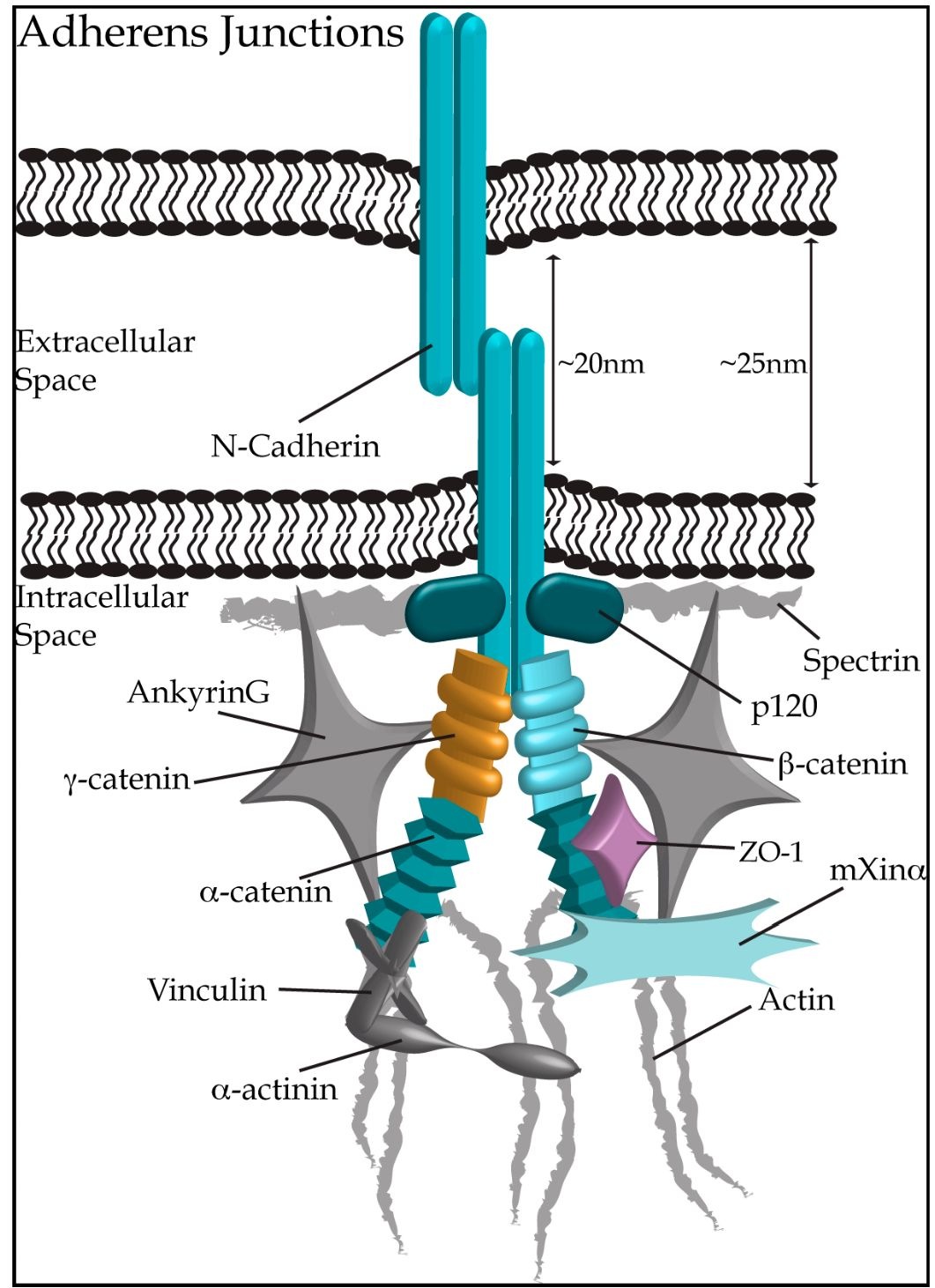
Adherens junctions are specialized structures necessary for cell-cell adhesion that provide uniform mechanical strength to the heart. Unlike gap junctions where membranes remain relatively close, opposing membranes at adherens junctions are separated by ~20 nm (Niessen, 2007). Adherens junctions frequently alternate with gap junctions along the sarcolemma at the ID, and are typically oriented perpendicular to the long axis of cardiomyocytes, optimizing the transmission of mechanical force (Hoyt et al., 1989). In addition, they can be found with desmosomes at the area composita. Functional adherens junctions require two main anchor points, one within the extracellular space where cadherins from adjacent cells tightly interact in a homophillic manner, and the other within the cytoplasmic region linking the adherens junction complex to the actin cytoskeleton through direct interactions with members of the catenin family (Fig. 3; reviewed in Niessen, 2007; Noorman et al., 2009).
Fig. 3. Adherens Junctions connect neighbouring cardiomyocytes through homophilic dimers of N-cadherin. Connections within the intracellular space link N-cadherin to the actin cytoskeleton (depicted in grey), via additional adherens junction proteins (shown in hues of teal), ie. p120 catenin, P-catenin, a-catenin and mXina. Proteins not traditionally considered as components of adherens junctions, but localizing to the ID are shown in grey. Proteins of other ID structures, ZO-1 (gap junctions) and y-catenin (desmosomes), are depicted in light purple and gold, respectively.
N-cadherin
N-Cadherin: Cadherins are a super-family of transmembrane glycoproteins that mediate Ca2+-dependent adhesion between neighbouring cells. In the early 1980s, N-cadherin was identified as a major component of the myocardium, that localizes to the ID (Volk and Geiger, 1984). Five extracellular domains are present in N-cadherin, with the first three possessing Ca2+ binding sites; each domain is composed of up to ~110 amino acids (Fig. 2B). The extreme NH2-terminus of N-cadherin contains a highly conserved ligand recognition site composed of repeating motifs of His-Ala-Val residues, necessary for homophilic dimer formation (Nose et al., 1990). Two cadherin monomers, one from each adjacent myocyte, form a Ca2+-dependent, zipper-like homodimer (Takeichi, 1994). Although Ca2+ is necessary for maintaining cadherin homodimers, it does not mediate their initial interaction (Nagaraj et al., 1996). Following the extensive extracellular domain, N-cadherin contains a single-pass transmembrane region and a short cytoplasmic segment that associates with the actin cytoskeleton via proteins of the catenin family (Ozawa et al., 1990).
The importance of N-cadherin in the stability of IDs is evidenced in various animal models. A murine systemic knock-out model of N-cadherin resulted in embryonic lethality due to improper development of the heart tube among other abnormalities, despite the development of primitive myocardial tissue (Radice et al., 1997). Interestingly, isolated murine myocytes from the same model were able to weakly contract and aggregate in culture (Radice et al., 1997). Taken together, these results suggested that N-cadherin is critical for embryonic development of the heart and other tissues, however it is not required for electrical coupling and cell adhesion at this stage. Moreover, in a murine conditional cardiac-specific knock-out model where N-cadherin was deleted in 6-10 week old mice, sudden death occurred after ~2 months (Li et al., 2005). A significant decrease in the expression levels of gap junction proteins was also observed in this model, which was accompanied by the development of dilated cardiomyopathy and impaired left ventricular function (Li et al., 2005). In addition, the amounts of other proteins at ID were reduced, including plakoglobin and a-, P-, and p120 catenins, resulting in dissolution of the ID structure (Kostetskii et al., 2005; Li et al., 2005). Similarly, mice overexpressing N-cadherin developed early onset dilated cardiomyopathy (Ferreira-Cornwell et al., 2002), and N-cadherin/connexin-43 compound heterozygous mice were prone to cardiac arrhythmias (Li et al., 2008). Collectively, these studies suggested that N-cadherin is necessary for the maintenance and stabilization of the ID, while its absence may lead to the development of heart failure and ultimately death.
Proteins of the catenin family
Within adherens junctions, N-cadherin associates with the actin cytoskeletal network through direct interactions mediated by members of the catenin/armadillo family; these include P-, a-, p120 and y-catenin (also called plakoglobin). P- and p120 catenin bind directly to the cytoplsmic domain of N-cadherin, whereas a-catenin links the actin cytoskeleton to N-cadherin, via its direct interactions with both components (reviewed in Aho et al., 1999; Butz and Larue, 1995; Niessen, 2007).
fP-Catenin: P-Catenin, like other members of the catenin/armadillo family, is characterized by a series of central domains, referred to as armadillo (arm) repeats, each composed of 42 amino acids, that form an elongated superhelix when repeated in tandem (Huber et al.,1997). P-Catenin contains twelve arm repeats (Fig. 2C; Peifer et al., 1994); deletion mutagenesis has mapped the binding site for N-cadherin to the central repeat region of P-catenin (Hulsken et al., 1994). Flanking the arm repeats are small, ~100 amino acids long, NH2- and COOH- termini that mediate the regulatory functions of P-catenin.
p120 Catenin: p120 Catenin shares a similar organization with P-catenin, and a ~22% identity within the arm repeats region (Peifer et al., 1994; Reynolds et al., 1992). Alternative splicing gives rise to four similar p120 catenin isoforms (Keirsebilck et al.,1998). Each isoform is composed of ten arm repeats that are responsible for their direct interaction with the COOH-terminus of cadherins (Fig. 2C; Daniel and Reynolds, 1995; Finnemann et al., 1997; Reynolds et al., 1992; Shibamoto et al., 1995; Staddon et al., 1995; Thoreson et al., 2000). p120 catenin does not interact with a-catenin or the actin cytoskeleton (Daniel and Reynolds, 1995), suggesting a novel, yet unidentified, function within adherens junctions.
a-Catenin: a-Catenin is a subfamily of proteins that differs significantly in both primary sequence and structural organization from the other members of the traditional catenin/armadillo family (reviewed in Kobielak and Fuchs, 2004). Instead of arm repeats, a-catenin contains three vinculin homology (VH) domains, therefore sharing considerable homology with vinculin (Fig. 2C; Rudiger, 1998). Of the main a-catenin isoforms, aT-catenin is the most prominent in the mammalian heart and localizes to the ID (Janssens et al., 2001). Through its most NH2-terminal VH domain, a-catenin dimerizes and interacts directly with P- and y-catenin (Koslov et al., 1997; Pokutta and Weis, 2000), while through its middle VH domain supports binding to vinculin and a-actinin, both of which are present within the transitional junction of the ICD (McGregor et al., 1994; Weiss et al., 1998). Similar to vinculin, a-catenin associates with filamentous actin through its last VH domain and its COOH-terminus (Rimm et al., 1995). In addition, its COOH-terminus interacts with ZO-1, which is also complexed with connexin-43 at gap junctions (Imamura et al., 1999; Talhouk et al., 2008). Taken together, these observations indicate that a-catenin functions as an intracellular adhesion protein.
It is well established that P- and p120 catenins play essential roles in diverse signaling pathways, including modulation of cell-cell adhesion; (reviewed in Anastasiadis and Reynolds, 2000; Niessen, 2007). Recently, a-catenin was also implicated in the regulation of cell adhesion and proliferation (reviewed in Kobielak and Fuchs, 2004). Although many of their suggested signaling roles originate from studies in non-cardiac cells, it is presumed that catenins may have similar regulatory activities at the ID of cardiomyocytes. In support of this, transgenic mice lacking either P- or a-catenin result in detrimental effects on the longevity of the animals, with phenotypes ranging from embryonic lethality to the development of early onset DCM (Haegel et al., 1995; Piven et al., 2011; Sheikh et al., 2006). Future studies are necessary to continue addressing this question.
Desmosomes
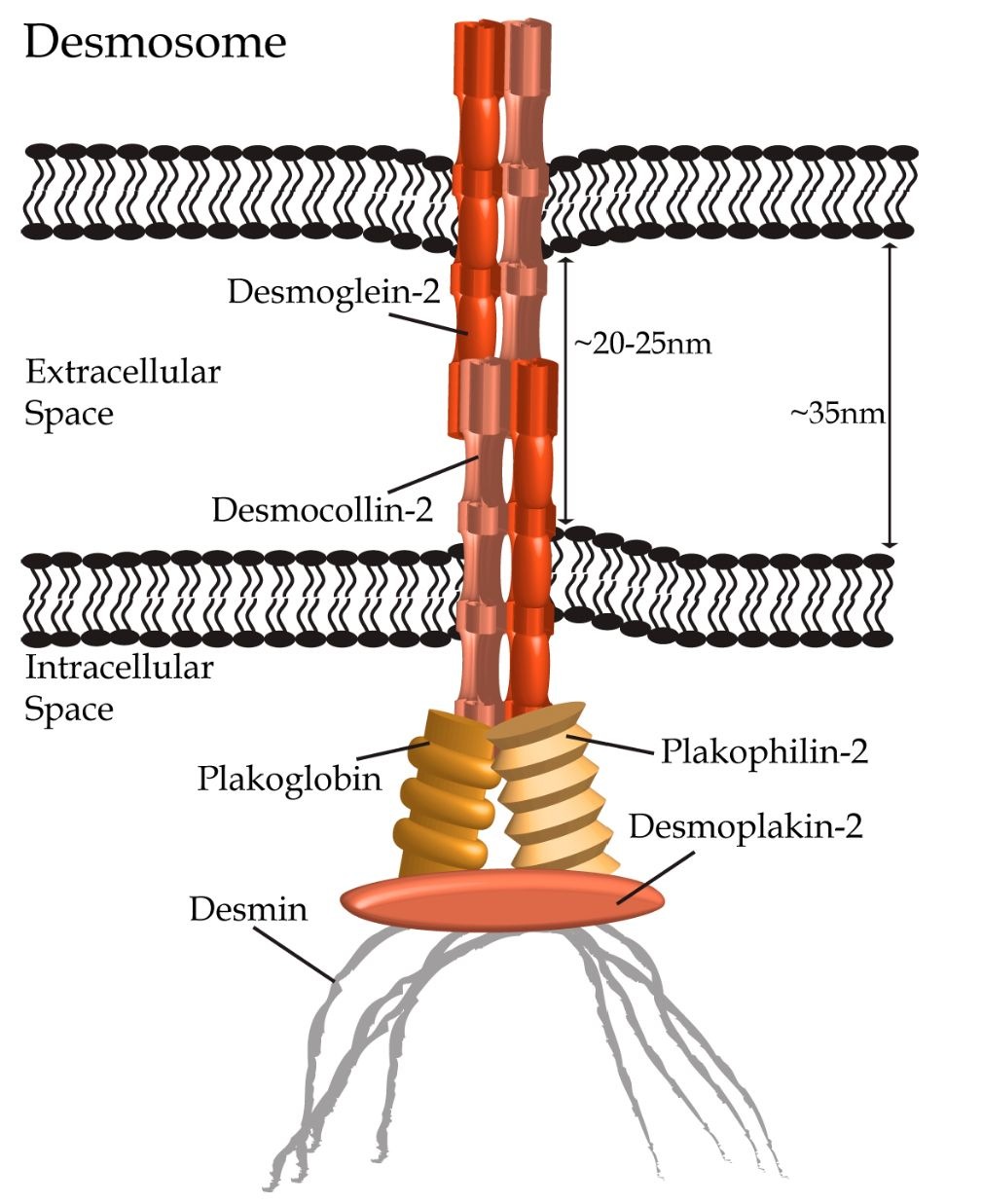
Similar to adherens junctions, desmosomes are also symmetrical protein complexes with intercellular elements connecting adjacent cells, and intracellular components associating with intermediate filaments. First identified as adhesive structures of epithelial cells by Giulio Bizzozero in the late 19th century.In the middle of the 20th century, desmosomes were identified as a major component of the cardiac ID (Fawcett and McNutt, 1969; Grimley and Edwards, 1960; Muir, 1957; Sjostrand and Andersson, 1954), where its main function is to provide structural support to neighboring cardiomyocytes (reviewed in Delmar and McKenna, 2010; Delva et al., 2009; Thomason et al., 2010). Desmosomes bring apposing cells within 20-35 nm of each other (Noorman et al., 2009), and are typically found in close proximity to gap junctions, although recent studies indicate that they are also present next to adherens junctions within the area composita. They consist of proteins from three families: the desmosomal cadherins, the catenin/armadillo family and the plakins (Fig. 4).
Fig. 4. Desmosomes connect neighbouring cardiomyocytes through heterophilic dimers of desmocollin-2 and desmoglein-2 (shown in hues of orange) forming within the extracellular space. Interactions with plakophilin-2, plakoglobin (a.k.a. y-catenin) and desmoplakin-1 (depicted in hues of gold and orange) link the desmosomal complex to the intermediate filament protein desmin (shown in gray) in cardiomyocytes.