Computed tomography and magnetic resonance imaging
Cardiac computed tomography and magnetic resonance imaging yield superior anatomic data than echocardiography since they allow better definition of the anterolateral wall and tip of the left ventricle and the right ventricle. However, these procedures are more costly and the former exposes the patient to radiation (M. Maron et al., 2009). (Fig. 16 ) Nevertheless, they are very useful when thoracic deformities prevent satisfactory cardiac visualization by transthoracic echocardiography. Cardiovascular magnetic resonance has also demonstrated that mitral leaflet elongation is present in hypertrophic cardiomyopathy independently of other phenotypic variants indicating that the mitral abnormalities are primary, thus, their important role in the pathophysiology of the left ventricular outflow obstruction (M. Maron, et al.; 2011). On the other hand, gadolinium magnetic resonance imaging late enhancement allows detection of the amount of myocardial fibrosis and is a predictor of systolic dysfunction (M. Maron et al., 2008). A more recent study reports that is also effective for prognostication of adverse outcomes and which patients might require a cardioverter-defibrillator (Fig. 17) (Bruder et al., 2010).
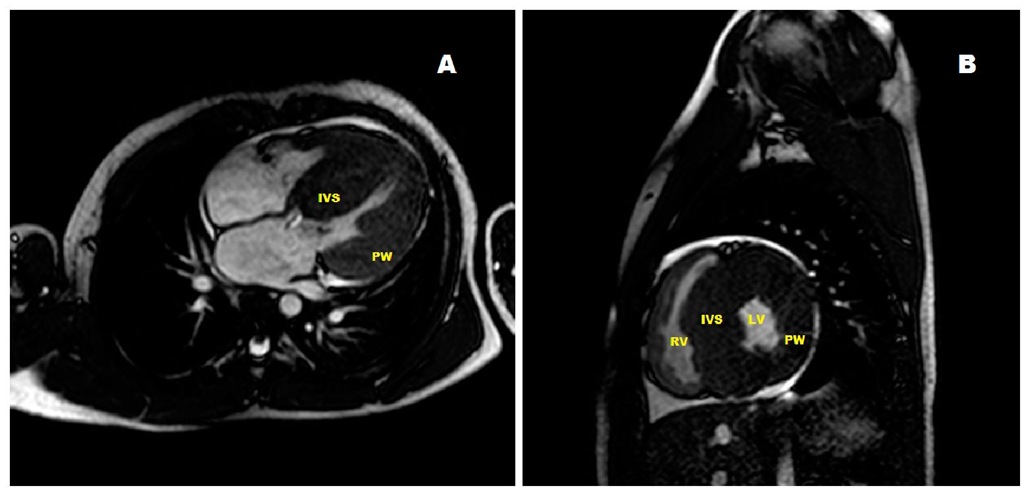
Fig. 16. A. Long axis view of a cardiac magnetic resonance image of a 5 year-old asymptomatic boy with severe hypertrophic cardiomyopathy. B: Magnetic resonance imaging of a short axis projection of the heart of a 3 year-old boy with severe heart failure. (IVS: interventricular septum, LV: left ventricle, PW: posterior wall, RV: right ventricle).
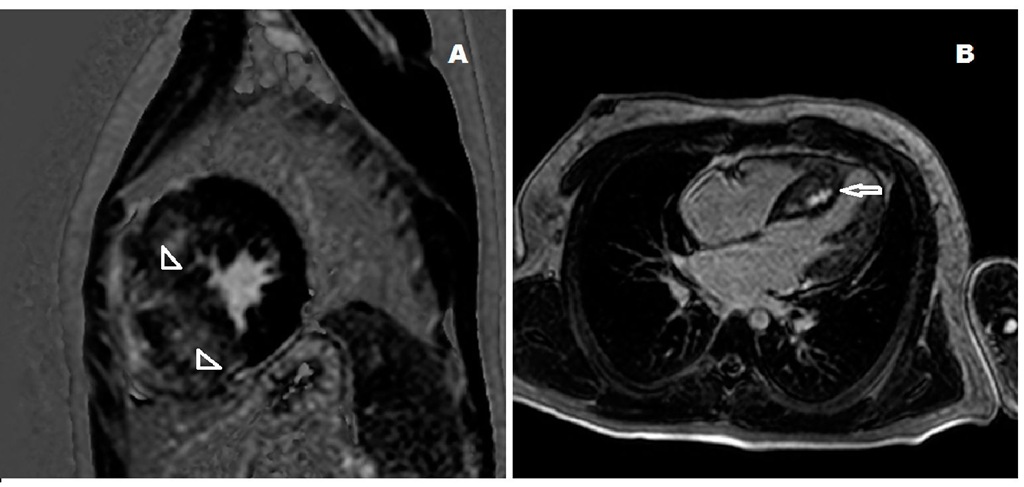
Fig. 17. A.Cardiac magnetic resonance image of the heart of a patient with positive delayed gadolinium enhancement indicating a diffuse pattern of fibrosis (arrowheads). B: Long axis view of a cardiac magnetic resonance image of the heart of a 9 year-old boy with a localized delayed gadolinium enhancement image in the interventricular septum (arrow).
Cardiac catheterization and cineangiocardiography
We owe to cardiac catheterization and cineangiocardiography the initial understanding of the physiopathology of hypertrophic cardiomyopathy shortly after its rediscovery about half a century ago (Braunwald et al., 1964; Wigle, Auger & Marquis, 1967). These techniques were then considered the gold standard for the diagnosis of hypertrophic obstructive cardiomyopathy. (Fig. 18) With the advent of the just discussed noninvasive imaging techniques like Doppler-echocardiography, computed tomography, and magnetic resonance imaging, this invasive procedure is no longer necessary for diagnostic purposes. Nowadays, this method is only used before planned surgical treatment or percutaneous interventions for septal reduction.
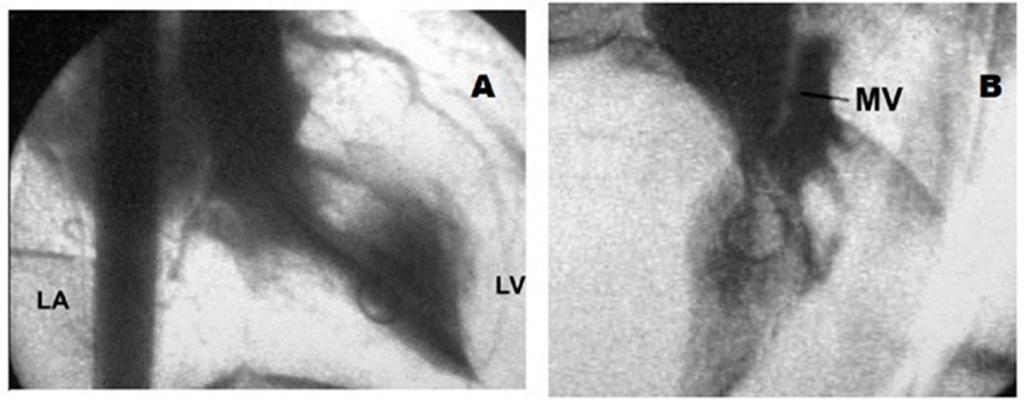
Fig. 18. A. Left ventricular cineangiocardiogram in the right anterior oblique projection showing a hypertrophied chamber with subaortic obstruction and moderate mitral insufficiency with left atrial enlargement. B. In the left anterior oblique view the anterior mitral valve can be seen contacting the septum. LV: left ventricle; LA: left atrium; MV: mitral valve.
Complications
Sudden death and congestive heart failure
The main complications in children with hypertrophic cardiomyopathy are syncope and sudden unexpected death. The latter may be the first and only manifestation of the disease and is considered to be secondary to ventricular tachycardia and fibrillation caused by myocardial fibrosis and ischemia (Basso et al., 2000; B.J. Maron et al., 2000b). Sudden death occurs more often in older children with hypertrophic cardiomyopathy either during strenuous sport activities or at rest but is infrequent in infants (B.J. Maron et al., 2003a) who are more prone to present and die with congestive heart failure specially in secondary forms (Bruno et al., 2002).
Arrhythmias
Supraventricular and nonsustained ventricular tachycardias, were found in almost a third of pediatric and adolescent patients with hypertrophic cardiomyopathy studied by ambulatory electrocardiography (McKenna, et al., 1988). However, their outcome after a mean follow-up of 3 years was rather benign. In a study from our group, a quarter of the patients had symptomatic atrial or ventricular tachycardia. Three out of 7 died suddenly during follow-up (Bruno et al., 2002). Rarely, 3rd degree atrioventricular block is found in children with hypertrophic cardiomyopathy. They could present with near syncope or syncopal attacks as the 1st manifestation of the disease (Rosen et al., 1997).
Evolving phenotype
Children with hypertrophic cardiomyopathy may evolve to dilated or restrictive cardiomyopathy phenotypes in 5% of the cases. In both circumstances they have a dimmer prognosis and become candidates for heart transplantation (Biagini et al., 2005; Denfield et al., 1997; Shirani et al., 1993).
Infectious endocarditis
Bacterial endocarditis is an uncommon complication in hypertrophic cardiomyopathy though can occur affecting the left ventricular aspect of the anterior mitral valve leaflet, especially in the presence of obstruction (Aoun et al., 1994; Morgan-Hughes & Motwani, 2002). Antibiotic prophylaxis for infectious endocarditis is then recommended.
Stroke
Ischemic stroke is somewhat frequent and a cause of death in adult hypertrophic cardiomyopathy but has not been mentioned in children (B.J. Maron et al., 2000a).
Differential diagnosis
The most important differential diagnosis is with the athlete’s heart and is sometimes somewhat difficult to make. Highly trained competitive athletes may have electrocardiographic abnormalities that resemble those of hypertrophic cardiomyopathy. As in this situation, the left ventricle is hypertrophied but the wall thickness as determined by echocardiography does not exceed 15 mm in diameter and the hypertrophy is symmetrical. The left ventricular cavity is dilated and the ejection fraction is normal while hypertrophic cardiomyopathy is frequently accompanied by unusual distribution of hypertrophy, the left ventricle is smaller in size (<4.5 cm) and the ejection fraction is higher than normal. Furthermore, left ventricular diastolic function is normal in young athletes but is always impaired in hypertrophic cardiomyopathy (B.J. Maron, Spirito & Pelliccia, 1995b). Tissue Doppler studies has also been very useful to distinguish those patients in the "grey zone" (Cardim et al., 2003). When still in doubt regarding the diagnosis, cessation of physical activities usually results in regression of the left ventricular mass in a few weeks’ time (B.J. Maron et al.., 1993). Genetic screening might be useful when they are positive for a mutation which occurs in up to 70% of patients with hypertrophic cardiomyopathy. On the contrary, a negative test does not exclude the possibility of a mutation still not discovered.
Phenocopies and associations
A recent large epidemiologic study of the pediatric population with hypertrophic cardiomyopathy established that almost a quarter of patients with unexplained left ventricular hypertrophy do not have a sarcomeric etiology (Colan et al., 2007). These variants are called phenocopies and are listed in Table 5. They are classified as malformation syndromes, inborn errors of metabolism, and neuromuscular disorders and each group numbers about one third of the total.
Malformation syndromes
Noonan’s and LEOPARD syndromes
The most common malformation syndromes associated with hypertrophic cardiomyopathy included in the European Society of Cardiology classification of familial hypertrophic cardiomyopathy, are Noonan’s syndrome and its allelic variant LEOPARD syndrome, acronym for lentiginosis, electrocardiographic anomalies, ocular hypertelorism, pulmonic stenosis, abnormal male genitalia, retardation of growth, and deafness. The prevalence of Noonan,s syndrome, initially described as the Turner phenotype with normal karyotype (Noonan, 1968), is estimated in 1:2,000 births (Nora et al., 1974). (Fig. 19) It is inherited as an autosomic dominant form and nearly 80% have congenital heart disease, most frequently pulmonic valve and arterial stenoses, and atrial and ventricular septal defects. The introduction of echocardiography as a diagnostic tool, allowed the recognition of an association with hypertrophic cardiomyopathy in almost a quarter of patients (Nora et al., (1975). In the Australian epidemiologic study of childhood cardiomyopathies, 28% of 80 patients with hypertrophic cardiomyopathy had Noonan’s syndrome (Nugent et al., 2005). These patients presented earlier and had more frequent biventricular involvement. However, they did not find greater adverse events rate than in sarcomeric hypertrophic cardiomyopathy. In about 40-50% of patients a mutation of protein tyrosine phosphatase nonreceptor type 11 (PTPN11) is found (Type 1 Noonan’s syndrome), but this is present in 90% of LEOPARD patients (Sznajer et al., 2007; Tartaglia et al., 2001). This gene controls a series of developmental processes, among them the genesis of semilunar valves. The mutation is then mainly present in patients with heart defects but not in those with hypertrophic cardiomyopathy.
Related conditions
Some related genetic conditions to Noonan’s syndrome like the Costello syndrome, the cardiofaciocutaneous syndrome, and palmo-plantar hyperkeratosis with woolly hair may also be associated with hypertrophic cardiomyopathy (Peirone et al., 2005, Roberts et al., 2006).
Inborn errors of metabolism
Pompe’s disease
Pompe’s disease or type II glycogen storage disease is an autosomal recessive inherited disorder caused by absence of the acid alpha-glucosidase enzyme (GAA) preventing the normal degradation of glycogen in the cardiac myocyte (Hers, 1963). There is an infantile type with massive cardiac enlargement as seen in hypertrophic cardiomyopathy with obstruction leading to congestive heart failure and early death (Ehlers et al., 1962). A typical electrocardiogram is shown in Fig. 20. Late onset Pompe’s disease with a better outcome has also been described (Winkle et al., 2005). Enzyme replacement therapy is now available for treatment of GAA deficiency.
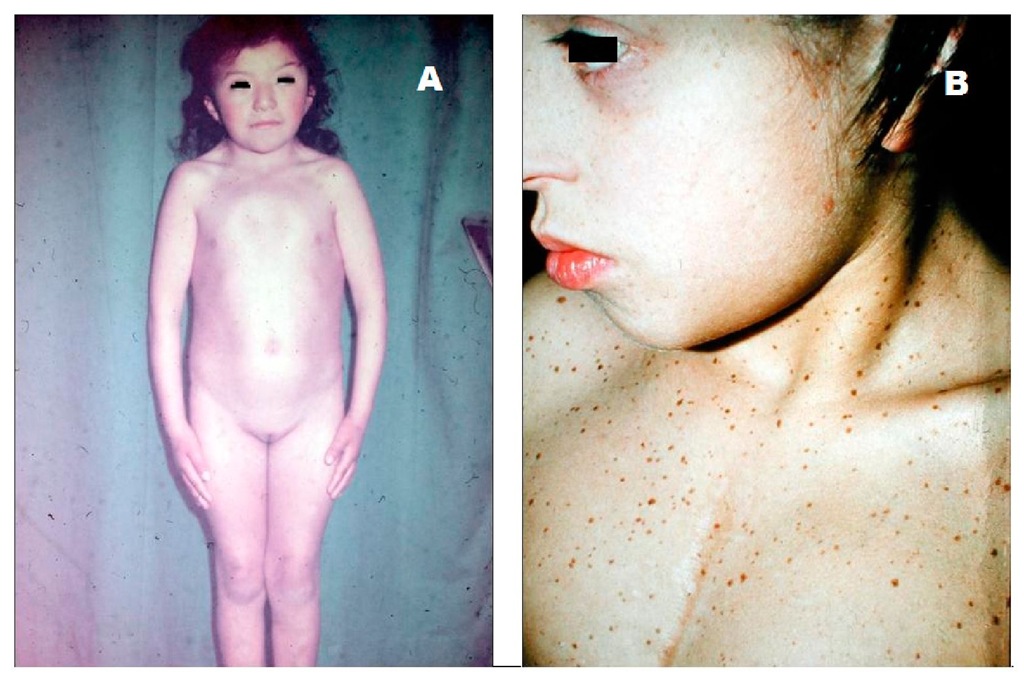
Fig. 19. A. Phenotype of a 10-year-old girl with Noonan’s syndrome and hypertrophic cardiomyopathy. There is short stature, peculiar face, eyelid ptosis with downward slant, ocular and mammillar hypertelorism, low set ears, pterigium colli, and a prominent chest. B. Ten-year-old girl with LEOPARD syndrome. The multiple lentigines and hypertrophic obstructive cardiomyopathy became apparent long after she had been operated on for severe pulmonary valve stenosis when she was 6-month old.
Fig. 20. Typical electrocardiogram of an infant with Pompe’s disease. There is short atrioventricular conduction and biventricular hypertrophy with large voltages and no repolarization abnormalities.
Other glycogen storage diseases
A genetic study of 75 patients with hypertrophic cardiomyopathy found that 3 out of 35 patients with negative sarcomere protein mutations had genetical defects in lysosome-associated membrane protein 2 (LAMP2) responsible for the X-linked disorder Danon’s disease/Wolff-Parkinson-White in 2 of the 3, and in AMP-activated protein kinase gamma 2 (PRKAG2) in the remaining, causing Wolff-Parkinson-White/hypertrophic cardiomyopathy (Arad et al., 2005). Both conditions produce glycogen storage and might wrongly be considered as sarcomeric hypertrophic cardiomyopathies. The presence of preexcitation should point to the correct diagnosis. Type IIIa and IV glycogen storage diseases are very rare conditions caused by deficiencies in debrancher and branching enzymes respectively. These patients have muscular hypotonia with elevated creatine kinase and heart and liver involvement. There is heterogenous severity of the disease and the hypertrophic cardiomyopathy is concentric. The inheritance is autosomal recessive (Kishnani et al., 2010). (Fig. 21)
Fabry’s disease
Fabry’s disease, an X-linked disorder characterized by intracellular accumulation of glycosphingolipids caused by deficiency of the lysosomal enzyme alpha-galactoside A (GLA) may result in late onset hypertrophic cardiomyopathy, therefore it is rare in children. The reported prevalences in adult males and females with hypertrophic cardiomyopathy are 7.5 and 12% respectively (Chimenti et al., 2004; Sachdev et al., 2002). Enzyme replacement therapy for these patients is available (Eng et al., 2001).
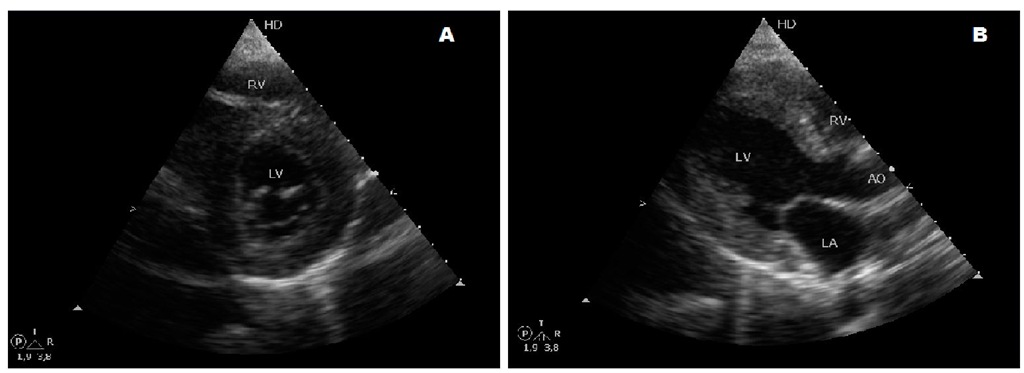
Fig. 21. Short and long axis parasternal echocardiographic views of the heart of a 19-year-old female with type III glycogen storage disease with concentric hypertrophic cardiomyopathy.