Introduction
Familial hypertrophic cardiomyopathy (FHC) is an autosomal dominant disease characterized by left ventricular wall thickening, myofilament disarray and abnormal echocardiography findings. Molecular genetic studies have defined FHC as a disease of the sarcomere caused by mutations in all major sarcomeric proteins, such as |3-myosin heavy chain (44%), myosin binding protein C (35%), regulatory light chain (2%), essential light chain (1.6%), a-tropomyosin (2.5%), troponin T (7%), troponin I (5%), troponin C (~1%), a-actin (1%), and titin (<1%) (Alcalai et al., 2008).
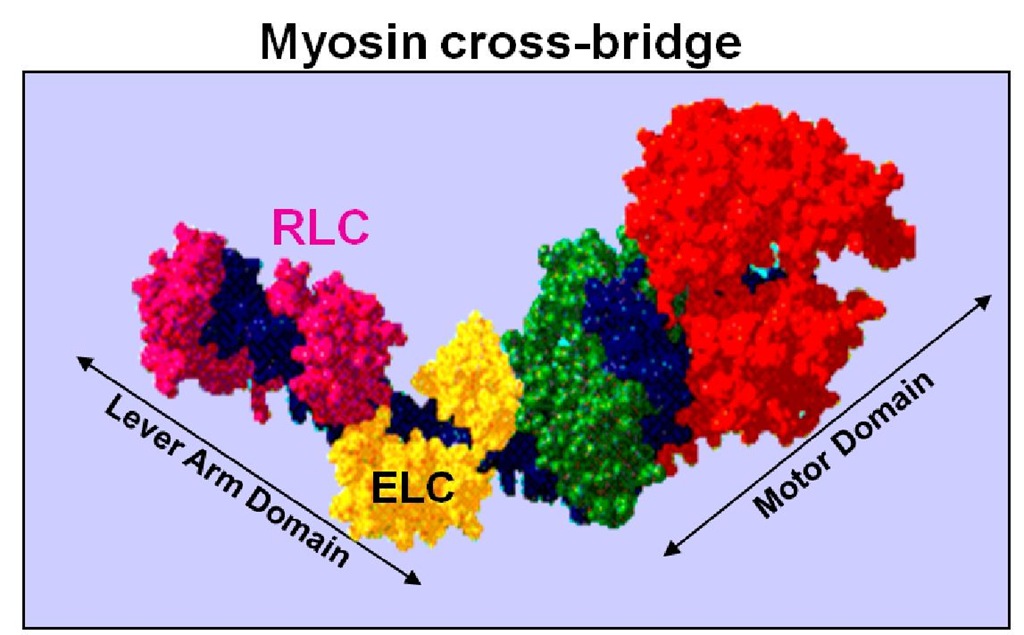
Although mutations in the regulatory light chain (RLC) of myosin are rare, they are of great significance given the importance of RLC for muscle contraction and heart function. The RLC plays an essential structural and functional role by supporting the architecture of the myosin neck region and fine-tuning the kinetics of the actin-myosin interaction (Morano, 1999; Szczesna, 2003). As shown in Fig. 1, the RLC wraps around the a-helical neck region of the myosin head by binding to a 35 amino acid IQ motif in the myosin heavy chain (MHC) (Rayment et al., 1993). This domain of MHC is anticipated to act as a lever arm, amplifying small conformational changes that originate at the catalytic site into large movements thus allowing myosin to generate motion and force (Geeves & Holmes, 2005; Lowey et al., 1993). Furthermore, this neck region has been proposed to serve as the compliant element of the myosin cross-bridge with the RLC contributing to the stiffness of the lever arm (Howard & Spudich, 1996; Pant et al., 2009). Two functionally important domains of the RLC molecule include its Ca2+-Mg2+ binding site, comprised of the N-terminal helix-loop-helix EF-hand Ca2+ binding motif, and a highly conserved N-terminal phosphorylatable serine constituting a myosin light chain kinase (MLCK)-dependent phosphorylation site.
The N-terminal divalent cation-binding site of the RLC is thought to be occupied by Mg2+ when muscles are in the relaxed state and may become partially saturated with Ca2+, depending on the length of the [Ca2+] transient (Robertson et al., 1981). The MLCK phosphorylation site of RLC is also of great structural and functional importance. Phosphorylation of this site in smooth muscle activates contraction (Hartshorne & Mrwa, 1982; Small & Sobieszek, 1977; Sobieszek, 1977). In skeletal and cardiac muscle, Ca2+-calmodulin (CaM) activated MLCK phosphorylation of RLC modulates contraction by increasing the Ca2+ sensitivity and the level of force and also by modulating the kinetics of force generating myosin cross-bridges (for review see (Kamm & Stull, 2011)). Of particular importance, the phosphorylation of RLC has been shown to regulate the function of myosin in the heart (Morano, 1999; Szczesna, 2003). Attenuation of RLC phosphorylation was demonstrated to lead to ventricular myocyte hypertrophy with histological evidence of necrosis and fibrosis (Ding et al., 2010). Recent results from Szczesna-Cordary’s lab shown that RLC phosphorylation plays an essential role not only in the physiological performance of the heart, but also helps to maintain normal cardiac function in the diseased myocardium (Muthu et al., 2011). At the level of protein, the phosphorylation of RLC at Ser-15 was shown to alter the secondary structure (a-helical content) and Ca2+ binding affinity of the human ventricular RLC protein (Szczesna et al., 2001). At the level of myofilaments, RLC phosphorylation was demonstrated to result in a significantly decreased distance between the myosin heads and actin filaments bringing them closer to each other (Colson et al., 2010)
Fig. 1. Schematic representation of the myosin head (cross-bridge) containing regulatory (RLC, labeled in magenta) and essential (ELC, labeled in yellow) light chains. Indicated are 1) motor domain, and 2) lever arm (Rayment et al., 1993).
FHC-linked mutations in the regulatory light chain (RLC)
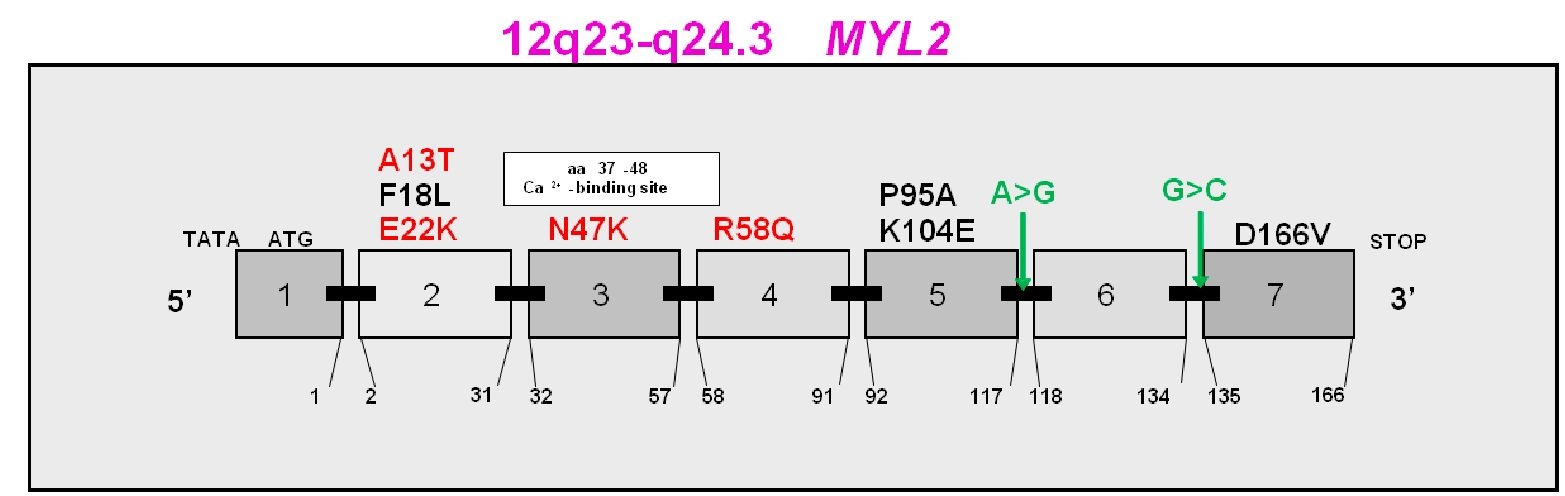
To date, eight single point mutations and two intron alternative splicing mutations in the MYL2 gene encoding for the human ventricular RLC (Swiss-Prot: P10916) have been identified to cause FHC (Fig. 2). They are A13T (alanine to threonine), F18L (phenylalanine to leucine), E22K (glutamic acid to lysine), N47K (asparagine to lysine), R58Q (arginine to glutamine), P95A (proline to alanine), K104E (lysine to glutamic acid), D166V (aspartic acid to valine), IVS5-2 (a A>G transversion in intron 5 that leads to a premature termination codon), and IVS6-1 (a G>C transversion in the acceptor splice site of intron 6). The A13T mutation arises from a replacement of alanine, an uncharged and nonpolar amino acid by threonine, an uncharged but polar amino acid. The mutation was first discovered in an American patient (Poetter et al., 1996) and was later found in a Danish proband diagnosed with hypertrophic cardiomyopathy (HCM). Two of his family members were found to be heterozygous for the mutation (Andersen et al., 2001). The proband, 42 years old, suffered from exercise-induced dyspnoea and had pronounced septal hypertrophy, diastolic filling abnormities but no significantly increased left ventricular outflow tract. One of the family members, the mother of the proband, was diagnosed with HCM late in life and died at the age of 72; while the other, 10 years old, showed no sign of HCM. In 2005, another proband carrying the A13T mutation was also identified in a Danish population (Hougs et al., 2005). Overall, this mutation is associated with a rare HCM phenotype characterized by mid left ventricular obstruction, enlarged papillary muscles and profound septal hypertrophy.
Fig. 2. Exon organization of the MYL2 gene (chromosome 12q23-q24.3) and FHC-linked mutations in human ventricular RLC (Swiss-Prot: P10916). Labeled in red, FHC mutations described in this review; in black, other identified RLC mutations; and in green, intronic splice site mutations:![]() transversion in intron 5 and
transversion in intron 5 and![]() (cytosine) transversion in intron 6.
(cytosine) transversion in intron 6.
The F18L mutation arises from a replacement of a bulky nonpolar and hydrophobic phenylalanine by the small uncharged and nonpolar leucine residue. It was found in three unrelated French families and is associated with a classic phenotype of left ventricular wall thickening and electrocardiographic (ECG) abnormities (Richard et al., 2003). The E22K mutation results from a substitution of a negatively charged glutamate with a positively charged lysine leading to potential alterations in the net charge and polarity of the mutation-bearing domain of RLC. The mutation was first identified in three persons (two brothers and one non-related individual) from two unrelated families screened together with 399 unrelated HCM patients (Poetter et al., 1996). Subsequent studies by Kabaeva, et al. identified seven individuals in a German family carrying the mutation. However, only four patients suffered from HCM while the phenotype of the remaining individuals was defined as "uncertain" (Kabaeva et al., 2002). Based on the latest clinical reports on this mutation, it is known to be associated with moderate septal hypertrophy, late onset of clinical manifestations, and benign to severe disease outcomes.
The N47K mutation results from the replacement of a polar uncharged asparagine residue by the positively charged lysine. It was first discovered in an individual of Danish descent (Andersen et al., 2001). This mutation is associated with a late onset of the disease and a rapidly progressing phenotype. The proband was diagnosed with HCM at the age of 60, and quickly progressed to a severe hypertrophic phenotype and diastolic dysfunction. It was interesting that septal hypertrophy of this patient increased rapidly (from 31 mm to 45 mm) in the two years after diagnosis. In 2005, a Danish patient carrying both N47K and a P-cardiac myosin heavy chain mutation was identified. The proband bearing both of these mutations displayed a more severe phenotype than patients with either mutation alone (Hougs et al., 2005). However, no incidence of sudden cardiac death (SCD) associated with this mutation has yet been reported.
The R58Q mutation occurs when the bulky positively charged arginine is replaced by an uncharged but polar glutamine. It was first discovered by Flavigny, et al. in 1998 in three unrelated French families with HCM (Flavigny et al., 1998). The mutation was associated with a classic form of FHC characterized by left ventricular wall thickness, abnormal ECG findings and SCD (Flavigny et al., 1998). In 2002, Kabaeva, et al. detected this R58Q mutation in a German proband with a clinical phenotype of moderate septal hypertrophy, early onset of disease and premature SCD (Kabaeva et al., 2002). In 2003, the R58Q mutation was once more identified in two independent population studies (from France and Sweden), and again was associated with a malignant disease phenotype (Morner et al., 2003; Richard et al., 2003). Out of all identified FHC RLC mutations, the R58Q mutation was found to be the most prevalent, occurring independently in multiple families with different ethnic backgrounds. The mutation is associated with a phenotype of severe cardiac hypertrophy and multiple incidences of SCD.
The P95A mutation occurs when a bulky hydrophobic proline residue is substituted with a smaller alanine residue. It was discovered together with the A13T and E22K mutations in an American family and shares a rare clinical phenotype, similar to that of A13T and E22K positive patients (Poetter et al., 1996).
The K104E mutation results from a replacement of the positively charged lysine with the negatively charged glutamic acid. The mutation was first observed in a Danish family and was mistakenly reported as L103E (Andersen et al., 2001). The parents carrying this mutation were asymptomatic with a normal ECG pattern. However, their son was diagnosed with pronounced septal hypertrophy at the age of 17 and progressed to diastolic dysfunction. His sister, 42 years old, was also positive for the mutation, but no typical hypertrophic phenotype was observed in her case. This mutation is associated with pronounced septal hypertrophy and diastolic filling abnormalities.
The D166V mutation occurs when a negatively charged aspartic acid is substituted with a bulky polar valine residue. This mutation was identified in a French proband, with the potential to cause SCD at a young age. It was first mistakenly presented as D166L in Richard, et al., 2003 and later corrected to D166V (Richard et al., 2003; 2004). Similar to R58Q, this mutation is associated with a malignant disease phenotype and SCD. Intron mutations: Intron 6-1 G>C mutation (IVS 6-1) was discovered along with K104E in the Danish population and is associated with pronounced septal hypertrophy (Andersen et al., 2001). The other intronic mutation 5-2 A>G, (IVS 5-2) was first discovered in the French population and is associated with a malignant form of FHC (Richard et al., 2003). The mutation is predicted to lead to a premature codon termination.
The clinical phenotype associated with FHC-linked mutations in the regulatory light chain and current to date literature citations are illustrated in Table 1.
This review focuses on the functional phenotypes associated with five (A13T, E22K, N47K, R58Q and D166V) RLC mutations extensively studied in vitro using RLC-mutant reconstituted muscle systems and in vivo, using cardiac muscle preparations from transgenic mice expressing FHC-RLC mutations.
|
Mutation in RLC |
Clinical phenotype |
Population |
Major findings |
|
A13T |
Massive hypertrophy of cardiac papillary muscles, mid-cavity left ventricular obstruction, pronounced septal and ventricular hypertrophy and diastolic filling abnormalities |
Danish (Andersen et al., 2001; Hougs et al., 2005); American (Poetter et al., 1996) |
Mutation-induced changes in a-helical content and in Ca2+ binding properties of RLC (Szczesna et al., 2001). No change in Ca2+sensitivity of myofibrillar ATPase activity (Szczesna et al., 2001). Increased force production in skinned papillary muscle fibers from Tg-A13T mice (unpublished data). Histopathological changes in left ventricles and inter-ventricular septa of Tg-A13T mice (unpublished data) |
|
F18L |
Classic form of HCM -increased left ventricular wall thickness, abnormal ECG findings, no mid left ventricular obstruction |
French (Flavigny et al., 1998; Richard et al., 2003) |
Decrease in Ca2+ binding affinity to RLC (Szczesna et al., 2001). Increase in a-helical content of RLC (Szczesna et al., 2001). Decrease in Ca2+ sensivity of myofibrillar ATPase activity (Szczesna-Cordary et al., 2004a). Compromised maximal tension, cooperativity and Ca2+ sensitivity of force (Roopnarine, 2003). |
|
E22K |
Moderate septal hypertrophy, late onset of clinical manifestation or no symptoms of FHC (Kabaeva). Also associated with massive hypertrophy of cardiac papillary muscles and adjacent venstricular tissue causing midcavity obstruction (Poetter) |
German (Kabaeva et al., 2002), American (Poetter et al., 1996) |
Protein non-phosphorylatable (Szczesna et al., 2001). Mutation induced changes in a-helical content and Ca2+ binding properties of RLC (Szczesna et al., 2001). Increase in Ca2+ sensitivity of force (Levine et al., 1998; Roopnarine, 2003; Szczesna-Cordary et al., 2004a) and decrease in maximal ATPase and force in skinned fibers from Tg-E22K mice (Szczesna-Cordary et al., 2007). No effect on cross-bridge kinetics (Szczesna-Cordary et al., 2007; (Dumka et al., 2006). Enlarged inter-ventricular septa and papillary muscles (Szczesna-Cordary et al., 2005). No hypertrophy detected in Tg-E22K mice (Sanbe et al., 2000). No changes in ECG (Szczesna-Cordary et al., 2005). |
|
N47K |
Pronounced interventricular (septal) and papillary musle hypertrophy; relatively high midventricular flow gradient, diastolic filling abnormalities; late onset disease with a rapidly progressing phenotype |
Danish (Andersen et al., 2001; Hougs et al., 2005) |
Abolished Ca2+ binding to RLC (Szczesna-Cordary et al., 2004a). Increased Ca2+ sensivity of myofibrillar ATPase activity (Szczesna-Cordary et al., 2004a). No change in pCa50 of force (Szczesna-Cordary et al., 2004a). Prolonged Ca2+ transient with no change in force transients in intact papillary muslces (Wang et al., 2006). Decreased isometric force in N47K-myosin based in vitro motility assays (Greenberg et al., 2009). Reduction in force and power output under loaded conditions (Greenberg et al., 2010). Decreased cardiac function in isolated perfused working hearts (Abraham et al., 2009). |
|
Mutation in RLC |
Clinical phenotype |
Population |
Major findings |
|
R58Q |
Malignant FHC phenotype, early onset of clinical manifestation and high incidence of premature SCD; classic form of HCM – increased left ventricular wall thickness and abnormal ECG findings |
German (Kabaeva et al., 2002), French (Flavigny et al., 1998; Richard et al., 2003), Swedish ( Morner et al., 2003) |
Abolished Ca2+ binding to RLC, which was restored upon RLC phosphorylation. (Szczesna et al., 2001). Mutation induced increase in a-helical content of RLC (Szczesna et al., 2001). Increased Ca2+ sensitivity of force (Szczesna-Cordary et al., 2004a), and Ca2+ and force transient in intact papillary muscles (Wang et al., 2006). Higher ATPase rate and increased activation at submaximal Ca2+ (Greenberg et al., 2009). Decreased skewness and kurtosis of fluctuations during contraction (Borejdo et al., 2010). Decreased force (Abraham et al., 2009; Greenberg et al., 2010; Greenberg et al., 2009; Wang et al., 2006). Reduced level of endogenous RLC phosphorylation (Abraham et al., 2009). Decreased cardiac function in isolated perfused working hearts (Abraham et al., 2009). Alterations in diastolic transmitral velocities and increased deceleration time, indicative of diastolic dysfunction (Abraham et al., 2009). |
|
P95A |
Rare clinical phenotype, similar to E22K and A13T, of midventricular obstruction |
American (Poetter et al., 1996) |
Mutation-induced decrease in Ca2+ binding to RLC (Szczesna et al., 2001). No significant effect on tension, Ca2+ sensitivity, or cooperativity in P95A-reconstituted fibers (Roopnarine, 2003). |
|
K104E |
Pronounced septal hypertrophy and diastolic filling abnormalities |
Danish (Andersen et al., 2001) |
Impaired interaction with IQ-MHC peptide (Szczesna- Cordary et al., 2004b). Slight decrease in binding to RLC-depleted porcine myosin (Huang et al., 2011). |
|
D166V |
Malignant FHC phenotype – poor prognosis and SCD at young age |
French (Richard et al., 2003; 2004) |
Decrease in maximal force and large increase in Ca2+ sensitivity in papillary muscle fibers from Tg-D166V mice (Kerrick et al., 2009). Decrease in Ca2+ sensitivity of force upon phosphorylation (Muthu et al., 2011). Slower cross bridge kinetics (Borejdo et al., 2010; Mettikolla et al., 2009; Muthu et al., 2010). Reduced level of endogenous RLC phosphorylation (Kerrick et al., 2009). Severe fibrotic lesions in older Tg-D166V mouse hearts (Kerrick et al., 2009). |
|
IVS6-1 |
Pronounced proximal septal hypertrophy |
Danish (Andersen et al., 2001) |
G>C transversion in Intron 6 (Andersen et al., 2001). |
|
IVS5-2 |
Malignant prognosis |
French (Richard et al., 2003) |
Donor-site splice mutation (A>G) in Intron 5 predicted to lead to a premature termination codon (Richard et al., 2003). |
Table 1. Summary of clinical and functional phenotypes of FHC mutations in the regulatory light chain (RLC).