Introduction
Cardiomyopathies are an important and heterogeneous group of diseases. The awareness and knowledge of these diseases in both the public and medical communities historically has been impaired by persistent confusion surrounding definitions and nomenclature. Classification schemes, of which there have been many, (Thiene et al., 2000, 2004; Richardson et al., 1996) are potentially useful in drawing relationships and distinctions between complex disease states for the purpose of promoting greater understanding; indeed, the precise language used to describe these diseases are profoundly important. Cardiomyopathies are diseases of the heart muscle, characterized by abnormality in chamber size and wall thickness, or functional contractile dysfunctions mainly systolic or diastolic dysfunction in the absence of coronary artery disease, hypertension, valvular disease, or congenital heart disease (Elliott et al., 2008). These diseases are classified as either primary or secondary. Primary cardiomyopathies consist of disorders solely or predominantly confined to the heart muscle, which have genetic, non-genetic, or acquired causes. Secondary cardiomyopathies are disorders that have myocardial damage as a result of systemic or multiorgan disease (Maron et al., 2006). Cardiomyopathies are classified traditionally according to morphological and functional criteria into four categories: dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM) and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). These cardiomyopathies can be primary myocardial disorders or develop as a secondary consequence of a variety of conditions, including myocardial ischemia, inflammation, infection, increased myocardial pressure or volume load and toxic agents.
The definitions of cardiomyopathies presented here are in concert with the molecular era of cardiovascular disease and have direct clinical applications and implications for cardiac diagnosis. However, the classification of cardiomyopathies presented herein is not intended to provide precise methodologies or strategies for clinical diagnosis. Rather, the classification of cardiomyopathies represents a scientific presentation that offers new perspectives to aid in understanding this complex and heterogeneous group of diseases and basic disease mechanisms.
Definition
The term cardiomyopathy was used for the first time in 1957. Over the next 25 years, a number of definitions for cardiomyopathies were advanced. Indeed, in the original 1980 WHO classification, cardiomyopathies were defined only as "heart muscle diseases of unknown cause," reflecting a general lack of available information about basic disease mechanisms. In 1968, the WHO defined cardiomyopathies as "diseases of different and often unknown etiology in which the dominant feature is cardiomegaly and heart failure." The final WHO classification published in 1995 proposed "diseases of myocardium associated with cardiac dysfunction" and included for the first time ARVC/D, as well as primary RCM. The American Heart Association (AHA) expert consensus panel proposed definition of cardiomyopathies is as follows: "Cardiomyopathies are a heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction, which usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilatation, due to a variety of etiologies that frequently are genetic. Cardiomyopathies are either confined to the heart or are part of generalized systemic disorders, and often lead to cardiovascular death or progressive heart failure-related disability." This definition of cardiomyopathies, similar to that reported by the European Society of Cardiology (ESC), under the auspices of the Working Group on Myocardial and Pericardial Diseases, excludes myocardial involvement secondary to coronary artery disease, systemic hypertension, and valvular and congenital heart disease.
Classifications of cardiomyopathies
Cardiac diseases can have an external cause, such as coronary artery disease, valve disease or hypertension, or may involve cardiomyopathies, in which the heart muscle itself is abnormal (i.e. an intrinsic cause of the disease is present in the heart muscle). The distinction between different classes of cardiac diseases are an important one to make, as cardiac diseases with similar phenotypes can have a diverse origin and may need different types of management. However, classification of cardiomyopathies is difficult, as the origin or pathophysiology is not always understood. Furthermore, at present there is no consensus on how to classify cardiomyopathies (e.g., based on origin, physiology or treatment) among clinicians. In order to promote a uniform nomenclature and well-defined clinical patient groups, recent knowledge on underlying causes and pathophysiology of cardiomyopathies has been implemented in a cardiomyopathy classification system both on behalf of the American Heart Association (AHA) and of the European Society of Cardiology (ESC). The AHA divided cardiomyopathies into 2 major groups based on predominant organ involvement. Primary cardiomyopathies (genetic, nongenetic, acquired) are those solely or predominantly confined to heart muscle and are relatively few in number (Fig. 1). Secondary cardiomyopathies show pathological myocardial involvement as part of a large number and variety of generalized systemic (multiorgan) disorders (Table 1). The frequency and degree of secondary myocardial involvement vary considerably among these diseases, some of which are exceedingly uncommon and for which the evidence of myocardial pathology may be sparse and reported in only a few patients. Because many cardiomyopathies may predominantly involve the heart but are not necessarily confined to that organ, some of the distinctions between primary and secondary cardiomyopathy are necessarily arbitrary and inevitably rely on judgment about the clinical importance and consequences of the myocardial process (Maron, 2008; Maron et al., 2006).
Fig. 1. Classification model for Primary cardiomyopathies (disease processes solely or predominantly involves the myocardium). The conditions have been segregated according to their genetic or nongenetic etiologies. *Predominantly nongenetic; familial disease with a genetic origin has been reported in a minority of cases. ARVC/D indicates arrhythmogenic right ventricular cardiomyopathy/dysplasia; CPVT, catecholaminergic polymorphic ventricular tachycardia; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LQTS, long QT syndrome; LVNC, left ventricular noncompaction; SQTS, short QT syndrome; and SUNDS, sudden unexplained nocturnal death syndrome.
|
Infiltrative* |
|
|
|
Amyloidosis (primary, familial autosomal dominantj", senile, secondary forms) |
|
Gaucher diseaset |
|
|
Hurler’s diseaset |
|
|
Hunter’s diseaset |
|
|
Storage* |
|
|
Hemochromatosis |
|
|
Fabry’s diseaset |
|
|
Glycogen storage diseaset (type II, Pompe) |
|
|
Niemann-Pick diseasef |
|
|
Toxicity |
|
|
Drugs, heavy metals, chemical agents |
|
|
Endomyocardial |
|
|
Endomyocardial fibrosis |
|
|
Hypereosinophilic syndrome (Loeffler’s endocarditis) |
|
|
Inflammatory (granulomatous) Sarcoidosis |
|
|
Endocrine |
|
|
Diabetes mellitust |
|
|
Hyperthyroidism |
|
|
Hypothyroidism |
|
|
Hyperparathyroidism |
|
|
Pheochromocytoma |
|
|
Acromegaly |
|
|
Cardiofacial |
|
|
Noonan syndromet |
|
|
Lentiginosist |
|
|
Neuromuscular/neurological |
|
|
Friedreich’s ataxiat |
|
|
Duchenne-Becker muscular dystrophyt |
|
|
Emery-Dreifuss muscular dystrophyt |
|
|
Myotonic dystrophyt |
|
|
Neurofibromatosist |
|
|
Tuberous sclerosist |
|
|
Nutritional deficiencies |
|
|
Beriberi (thiamine), pallaera, scurvy, selenium, carnitine, kwashiorkor |
|
|
Autoimmune/collagen |
|
|
Systemic lupus erythematosis |
|
|
Dermatomyositis |
|
|
Rheumatoid arthritis |
|
|
Scleroderma |
|
|
Polyarteritis nodosa |
|
|
Electrolyte imbalance Consequence of cancer therapy |
|
|
Anthracyclines: doxorubicin (adriamycin), daunorubicin |
|
|
Cyclophosphamide |
|
|
Radiation |
|
*Accumulation of abnormal substances between myocytes (i.e., extracellular). tGenetic (familial) origin.
Table 1. Important secondary cardiomyopathies
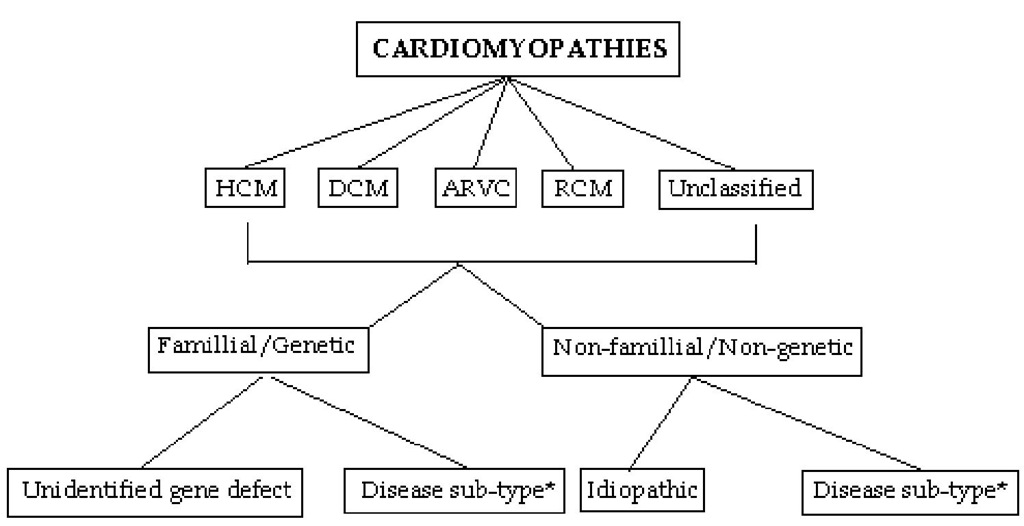
The ESC guidelines are more clinically orientated, which is appealing as this circumvents the complex pathophysiology of cardiomyopathies, which is not always comprehended upon presentation of the patient. According to the ESC guidelines cardiomyopathies are grouped into specific morphological and functional phenotypes; each phenotype is then sub-classified into familial and non-familial forms (Fig. 2). In this context, familial refers to the occurrence, in more than one family member, of either the same disorder or a phenotype that is (or could be) caused by the same genetic mutation and not to acquired cardiac or systemic diseases in which the clinical phenotype is influenced by genetic polymorphism. Most familial cardiomyopathies are monogenic disorders (i.e., the gene defect is sufficient by itself to cause the trait). A monogenic cardiomyopathy can be sporadic when the causative mutation is de novo, i.e. has occurred in an individual for the first time within the family (or at the germinal level in one of the parents). In this classification system, patients with identified de novo mutations are assigned to the familial category as their disorder can be subsequently transmitted to their offspring (Elliott et al., 2008). Non-familial cardiomyopathies are clinically defined by the presence of a cardiomyopathy in the index patient and the absence of disease in other family members (based on pedigree analysis and clinical evaluation). They are subdivided into idiopathic (no identifiable cause) and acquired cardiomyopathies in which ventricular dysfunction is a complication of the disorder rather than an intrinsic feature of the disease (Elliott et al., 2008).
Therefore, on the basis of all these considerations, cardiomyopathies can be most effectively classified as primary: genetic, mixed (genetic and nongenetic), acquired; and secondary.
Fig. 2. Summery of proposed classification. HCM, hypertrophic cardiomyopathy; DCM, dilated cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy; RCM, restrictive cardiomyopathy (*see table 2).
|
HCM |
DCM |
|
|
Famillial |
Familial, unknown gene |
Familial, unknown gene |
|
Sarcomeric protein mutations |
Sarcomeric protein mutations |
|
|
^-myosin heavy chain |
(see HCM) |
|
|
Cardiac myosin binding protein C |
Z-band |
|
|
Cardiac troponin I |
Muscle LIM protein |
|
|
Troponin T |
TCAP |
|
|
a-tropomyosin |
Cytoskeletal genes |
|
|
Essential myosin light chain |
Dystrophin |
|
|
Regulatory myosin light chain |
Desmin |
|
|
Cardiac actin |
Metavinculin |
|
|
a-myosin heavy chain |
Sarcoglycan complex |
|
|
Titin |
CRYAB |
|
|
Troponin C |
Epicardin |
|
|
Muscle LIM protein |
Nuclear membrane |
|
|
Glycogen storage disease |
Lamin A/C |
|
|
(e.g. Pompe; PRKAG2, Forbes’, Danon) |
Emerin |
|
|
Lysosomal storage diseases |
Mildly dilated CM |
|
|
(e.g. Anderson-Fabry, Hurler’s) |
Intercalated disc protein mutations |
|
|
Disorders of fatty acid metabolism |
(see ARVC) |
|
|
Carnitine deficiency |
Mitochondrial cytopathy |
|
|
Phosphorylase B kinase deficiency |
||
|
Mitochondrial cytopathies |
||
|
Syndromic HCM |
||
|
Noonan’s syndrome |
||
|
LEOPARD syndrome |
||
|
Friedreich’s ataxia |
||
|
Beckwith-Wiedermann syndrome |
||
|
Swyer’s syndrome |
||
|
Other |
||
|
Phospholamban promoter |
||
|
Familial amyloid |
||
|
Non-familial |
Obesity |
Myocarditis (infective/toxic/immune) |
|
Infants of diabetic mothers |
Kawasaki disease |
|
|
Athletic training |
Eosinophilic (Churg Strauss syndrome) |
|
|
Amyloid (AL/prealbumin) |
Viral persistence Drugs Pregnancy Endocrine Nutritional – thiamine, carnitine, selenium, hypophosphatemia, hypocalcaemia Alcohol Tachycardiomyopathy |
Table 2.Examples of different disease that cause cardiomyopathies
|
ARVC |
RCM |
Unclassified |
|
Familial, unknown gene |
Familial, unknown gene |
Left ventricular |
|
Intercalated disc protein |
Sarcomeric protein mutations |
non-compaction |
|
mutations |
Troponin I (RCM +/- HCM) |
Barth syndrome |
|
Plakoglobin |
Essential light chain of myosin |
Lamin A/C |
|
Desmoplakin |
Familial amyloidosis |
ZASP |
|
Plakophilin 2 |
Transthyretin (RCM + |
a-dystrobrevin |
|
Desmoglein 2 |
neuropathy) |
|
|
Desmocollin 2 |
Apolipoprotein (RCM + nephropathy) |
|
|
Cardiac ryanodine receptor |
Desminopathy |
|
|
(RyR2) |
Pseuxanthoma elasticum |
|
|
Transforming growth |
Haemochromatosis |
|
|
factor-[}3 (TGFP3) |
Anderson-Fabry disease Glycogen storage disease |
|
|
Inflammation |
Amyloid (AL/prealbumin) |
Tako Tsubo cardiomyopathy |
|
Scleroderma |
||
|
Endomyocardial fibrosis |
||
|
Hypereosinophilic syndrome |
||
|
Idiopathic |
||
|
Chromosomal cause |
||
|
Drugs (serotonin, methysergide, |
||
|
ergotamine, mercurial agents, busulfan) |
||
|
Carcinoid heart disease |
||
|
Metastatic cancers |
||
|
Radiation |
||
|
Drugs (anthracyclines) |
||