Introduction
The aim of this topic is to describe cardiomyopathies associated with myofibrillar myopathies (MFM, OMIM 601419). Myofibrillar myopathies are a group of heterogeneous neuromuscular disorders usually characterized by a severe myopathy, and generally associated with cardiomyopathy in 15% to 30% of the affected individuals. These familial or sporadic muscle disorders are characterized morphologically by focal disintegration of the myofibrils and abnormal ectopic accumulation of multiple proteins due to their degradation. The six genes that are held responsible so far for this clinically heterogenous, genetically heterogenous and morphologically homogeneous disorders are desmin, aB-crystallin, myotilin, LDB3 (ZASP), FLNC and BAG3. In the first part of the topic the normal function in skeletal and cardiac muscles of the six genes will be discussed as well as physiopathological consequences of their mutations. The second part will describe how proteins encoded by these genes, together with main contractile proteins such as actin, tropomyosin, myosin, troponin, integrate into functional sarcomeric structures, which in turn determine the main cardiac functions : force generation, force transmission, nervous influx conduction, energy metabolism. Special emphasis will be put on a dynamic point of view, including protein turnover, protein quality control, with the involvement of ubiquitin-proteasome and autophagic systems. The third part gives a view of the latest insight of the clinical and therapeutic perspectives.
Clinical manifestations of myofibrillar myopathies
Myofibrillar myopathies represent a group of muscular dystrophies, generally associated with cardiomyopathy. They present specific but not always identical morphologic features. Because aggregates present desmin with other proteins, it has been called desmin-related myopathy.
Clinical and histopathological features of DRMs
Diagnosis is based on clinical observations of patients and histologic studies using histochemistry and electron microscopy. Common symptoms of the disease are weakness and atrophy of the distal muscles of the lower limbs which progress to the hands and arms, then the trunk, neck and face. Wasting, muscle stiffness, cramps can also be found. The myopathy may progress to facial, cervical, velopharyngeal, truncal and respiratory muscles. The vast majority of MFM patients have an adult onset of their progressive muscle symptoms (Goldfarb et al., 2004). Cardiomyopathy is associated in 15 to 30 % of the affected individuals. However, in some patients, the cardiomyopathy may precede the muscle weakness. Therefore, distal muscle involvement, cardiomyopathy and peripheral neuropathy are important clinical clues, although they are not present in all patients (Bar et al., 2004; Finsterer & Stollberger, 2008; Schroder et al., 2007).
Skeletal
The abnormal size of muscular fibers, with few atrophic fibers, are the characteristics of the disorder. These fibers present amorphous, granular or hyaline deposits that vary in shape and size. As abnormal fibers can be focally distributed, the symptomatic changes may be missed in small samples. Many abnormal fibers show alteration in oxidative enzymes activity, which are diminished or absent. Reduced oxidative activity is often associated with the presence of hyaline structures, and conversely, enhanced activity around the larger inclusions. Some muscular fibers harbor small to large vacuoles containing membranous materials. Many hyaline structures are stained blue or blue-red with Trichome or intensively stained with Congo red, which is an important diagnostic feature of MFM biopsies (Schroder & Schoser, 2009).
Immunohistochemical studies reveal accumulations of desmin, myotilin, dystrophin, sarcoglycans, actin, plectin, gelsolin, filamin C, syncoilin, Bag3, synemin, aB-crystallin, Hsp27 and DNAJB2. Additional pathologic markers may be observed, including phosphorylated tau proteins, P amyloids, ubiquitin, glycoxidation and lipoxidation can be found, more specifically in desmin- and myotilin-opathies (Selcen, 2011). Electron microscopy shows a marked disorganization of the myofibrils ultrastructure, beginning at the Z-disc. Accumulation of dense materials is found in the close proximity of the Z-disc. In patients with desmin, aB-crystallin or Bag3 mutations, small pleiomorphic dense structures or granulofilamentous materials are found between the myofibrils. At later stages, Z-disc are disintegrated and sarcomeres disorganized, a prelude to myofibrils dislocation (Goldfarb & Dalakas, 2009). Electromyogram (EMO) studies of affected muscles reveal myopathic motor units potentials and abnormal electrical irritability, often with myotonic discharges (Schroder & Schoser, 2009).
Cardiac
All the genes cited in paragraph 2.3 cause cardiomyopathy. In 15 to 30 % of patients, the disorder presents with cardiomyopathy. There are also cases with only cardiomyopathic signs without skeletal muscle involvement. In advanced stages of the disease, cardiomyopathies develop in up to 60 % of the patients. MFM-associated atrioventricular conduction blocks can be associated with dilated (17 %), restricted (12 %), or hypertrophic (6 %) cardiomyopathy (van Spaendonck-Zwarts et al., 2010). When the cardiac muscle is involved, impaired conduction, arrhythmia, cardiac hypertrophy or dilation, secondary valvular insufficiency, intracardial formation of thrombi and heart failure can be observed. The least severe cases are caused by myotilin mutations. Atrioventricular conduction abnormalities may occur, and require urgent implantation of permanent pacemaker. This feature of MFMs can be attributed to the fact that the conduction system is rich in desmin (Finsterer & Stollberger, 2008; Goldfarb & Dalakas, 2009).
Respiratory
As the diaphragm is the main muscle involved in the respiratory cycle, progressive respiratory muscle impairment can occur, sometimes at early stages. Respiratory insufficiency can therefore be a major cause of disability and death with hypoventilation and ultimately respiratory failure, caused for example by mutations A357P, L370P in the desmin gene, or P209L in the BAG3 gene. Respiratory muscle weakness leads to a restrictive ventilatory failure when there is a mutation on the genes causing any of the myopathies except on ZASPopathy in which respiratory muscle involvement has not yet been described (Goldfarb & Dalakas, 2009).
Inheritance
The majority of cases follow an autosomal dominant mode of inheritance, and very few an autosomal recessive pattern. However, a significant number of MFMs shows sporadic disease manifestation.
Autosomal dominant mode
80 % of families with MFMs present an autosomal dominant pattern of inheritance, mostly with full penetrance (Goldfarb & Dalakas, 2009). Depending on the mutation, however, these facts can be modulated: for example, in families with the I451M mutation in the desmin gene, incomplete penetrance was demonstrated for the first time (Li et al., 1999). It is not possible, however, to link a specific mutation of the affected genes to clinical signs, although certain mutations are more frequently associated with specific signs. For example, the desmin A350P mutation predisposes male patients to higher risks of sudden cardiac death (Walter et al., 2007), as it is also the case for men and women in mutations p.E114del and N116S of the segment 1A of the desmin gene (Klauke et al., 2010; Vernengo et al., 2010).
Autosomal recessive mode
In a restricted number of families (6%), mutations are autosomal recessive (Goldfarb & Dalakas, 2009). The disease generally develops in childhood with severe clinical symptoms (Goldfarb et al., 2004). This is the case, for example, of the deletion A173_G179del of 21 nucleotides in the 1B helical segment of desmin (Munoz-Marmol et al., 1998). Another intriguing report indicate two mutations A360P and N393I in the desmin protein, which are not pathogenic in heterozygous state, but give rise to a highly aggressive cardioskeletal myopathy when combined in the same child (Goldfarb et al., 1998). There is also a case reported for aB-crystallin (mutation S21AfsX24) (Selcen, 2011).
Modifying genes
Lamin A/C mutations have been involved in muscular dystrophies but can also lead to completely different pathologies, depending on the mutations involved. A patient with a combination of Lamin A/C A644C and desmin V469M mutations developed severe muscle weakness and complete heart block, requiring heart transplantation (Muntoni et al., 2006). Lamin A/C and desmin networks are supposed to be indirectly connected (Costa et al., 2004), and therefore may interact in the development of the disease. As individuals from the same family are diversely affected by the disease, one can suspect their individual history (practice of sport) or differential genetic background. The question of the identity of modifying genes remains, however, largely unresolved.
Molecular genetics
So far, six genes have been formally identified and are held responsible for MFMs associated with cardiomyopathy, but for around 80 % of the patients, the disease still awaits a molecular diagnosis (Selcen, 2011). Schroder et al. include FHL1 and plectin in MFM-causing genes (Schroder, 2009). The knowledge of the structure and function of the already identified genes is, therefore, a prerequisite for the understanding of human MFMs.
Desmin
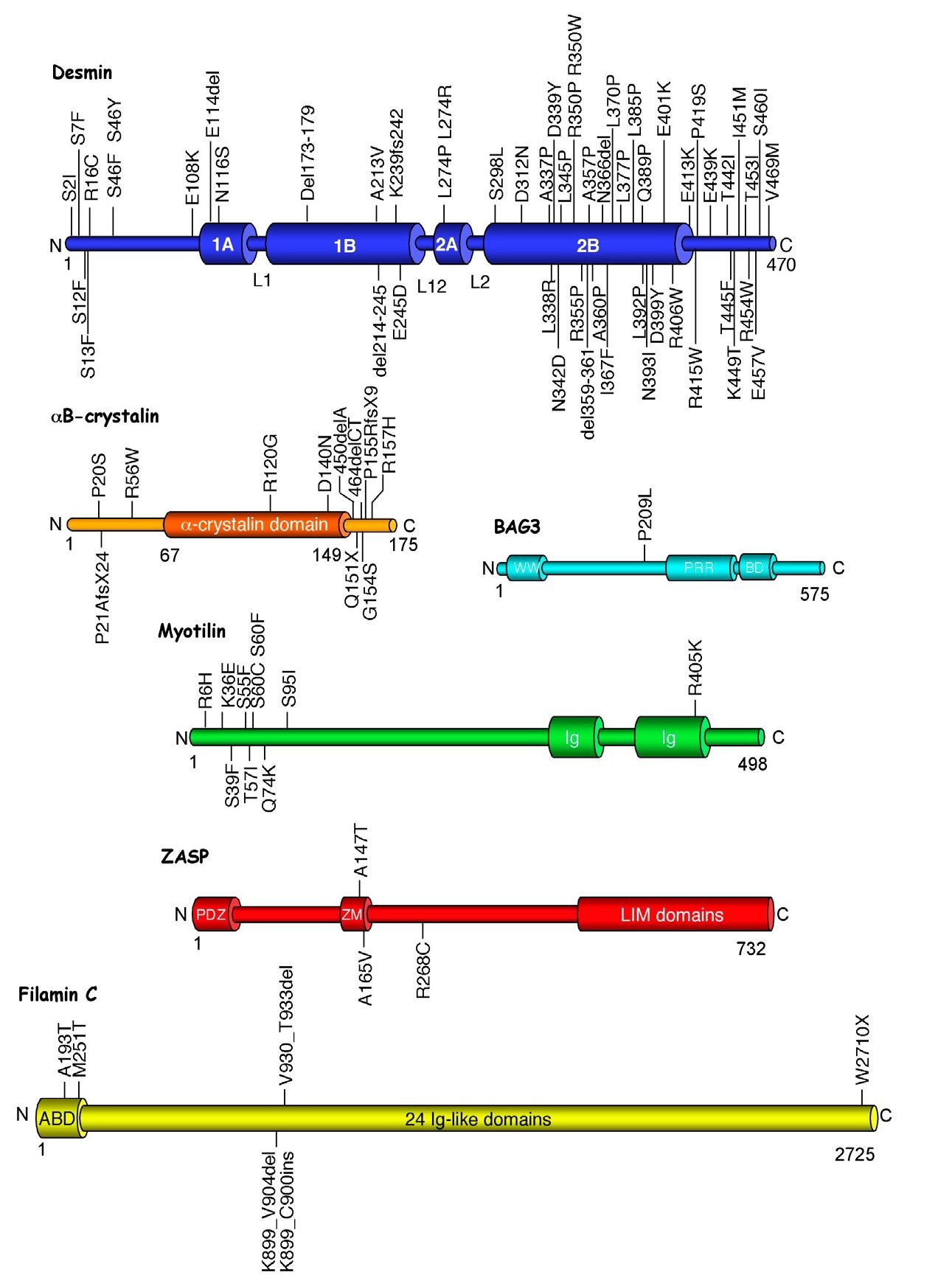
The human DES gene (NM_001927.3), on chromosome 2q35, comprises nine exons within an 8.4 kb region that encodes a 470 amino acids (53 kDa) muscle-specific protein (Li et al., 1989). Desmin belongs to the family of type III intermediate filaments (IF) proteins, which polymerize into 10 nm filaments, a size intermediate between thick (15 nm) and thin (5-6 nm) filaments. Desmin is synthetized only in cardiac, skeletal and smooth muscles (Lazarides & Hubbard, 1976 ; Paulin & Li, 2004). It is organized into three domains, a highly conserved a helical core of 303 amino acids residues flanked by globular N- and C- terminal structures. The helical structure, called the rod domain, is interrupted by three short polypeptide linkers (L1, L12, L2), which determine four consecutive helical segments (1A, 1B, 2A, 2B). Desmin is more abundant in heart muscle (2% of total proteins) of mammals than in their skeletal muscle (0.35%) (Paulin et al., 2004). It forms a three-dimensional scaffold around the myofibrillar Z-disc, and interconnects the entire contractile apparatus with the subsarcolemmal cytoskeleton and the nuclei (Lazarides & Hubbard, 1976). Desmin also forms longitudinal connections between the peripheries of successive Z-discs and along the plasma membrane. In addition, desmin IFs bind and participate to the location of mitochondria. In the heart, desmin is particularly abundant in Purkinje conduction fibers, and at intercalated discs, where it forms a double-banded structure (Thornell & Eriksson, 1981). Since the first description of desminopathy by Goldfarb et al. (Goldfarb et al., 1998) and Munoz-Marmol et al. (Munoz-Marmol et al., 1998), more than 50 mutations (45 missenses, 4 in frame deletions, 1 exon skipping, 2 single nucleotide insertion with premature termination) have been described (Klauke et al., 2011; Selcen, 2011; van Spaendonck-Zwarts et al., 2010).
Studies of DES-knockout mice have shown that defects develop in skeletal, smooth and cardiac muscle after birth, principally characterized by a loss of lateral alignment and anchorage of myofibrils, swollen mitochondria and loss of nuclear shape. The hearts develop a myopathy with impaired force generation, increased diastolic pressure with thicker ventricle walls (Li et al., 1996; Milner et al., 1996). Few transgenic mice have been described. With the Arg173_Glu179del desmin mutant transgene, aggregates containing desmin and other cytoskeletal proteins have been found in the heart (Wang et al., 2001a). A clear explanation of the molecular pathogenesis remains to be found.
Misfolded desmin molecules escape regular degradation mechanisms and accumulate with other proteins as aggregates. Cellular transfection studies have demonstrated that aggregates inhibit the proteasome system (Liu et al., 2006). In general, aggregates may accumulate through an active transport mechanism into perinuclear bodies called aggresomes (Johnston et al., 1998). Aggregates and proteasome impairement trigger autophagy (macroautophagy) as a mechanism of cellular cleaning (Tannous et al., 2008a), but recent studies have shown that this process is stalled at least with the desmin S13F mutant used in these studies (Wong et al., 2008).
aB-crystallin
AlphaB-crystallin is a small heat shock protein (sHSP) of 20 kDa that assembles into 500 -800 kDa homo and heterodimers with other sHSPs. It is encoded by the CRYAB gene (NM_001885.1), a three-exon gene on chromosome 11 (11q21-23) in human beings. aB-crystallin proteins contain a conserved a crystallin domain (residues 67 to 149), surrounded by a N-terminal domain and a C-terminal extension (residues 149 – 175) (Ganea, 2001; MacRae, 2000). aB-crystallin is abundantly expressed, together with aA-crystallin and other similar sHSPs in the lens where it prevents cataract formation (Horwitz, 2003). It is also found in other tissues, with the highest level in cardiac and skeletal muscles (Iwaki et al., 1990; Sax & Piatigorsky, 1994). In these tissues, aB-crystallin is localized to the Z-disc, and its expression is induced after stress (Golenhofen et al., 2004; Lutsch et al., 1997). aB-crystallin is known to act as a molecular chaperone of desmin, actin, tubulin and several other soluble molecules (Goldfarb & Dalakas, 2009). aB-crystallin expression reduces aggregate formation, both in vitro and in vivo, and is supposed to help neosynthetized desmin proteins by avoiding their aggregation (Bennardini et al., 1992).
The first identification of a MFM case due to a heterozygous missense mutation in the aB-crystallin gene (R120G) was reported in 1998 (Vicart et al., 1998). Since then 9 other mutations have been discovered. Some patients develop also a familial cataract. The only knock-out model is deleted for both aB-crystallin and HspB2 (MKBP) genes because of their close proximity on the chromosome (Brady et al., 2001). CRYAB/HspB2 null mouse heart display poorer functional recovery, high cell death rate, increased stiffness and poor relaxation of myocardium following ischemia / reperfusion. In these mice, mitochondrial permeability transition and calcium uptake were increased in cardiomyocytes (Morrison et al., 2004). In contrast, overexpression of WT aB-crystallin delays or suppresses cardiac hypertrophic response to pressure overload (Kumarapeli et al., 2008). In addition, transgenic mice with cardiac-specific expression of R120G mutant aB-crystallin develop cardiomyopathy in three months and die of heart failure in six – seven months. Just as it is the case of the desmin mutations causing MFMs, aB-crystallinopathies present cytoplasmic aggregates that include desmin, aB-crystallin and several other proteins (Wang et al., 2001b).
Myotilin
Myotilin is a 57 kDa protein that is predominantly expressed in skeletal muscle and more weakly in the heart (Salmikangas et al., 1999). The human gene (NM_001135940) is located at the locus 5q31. The N-terminal region contains serine-rich and hydrophobic stretches, and the C-terminal half two immunoglobulin-(Ig)-like domains. The Ig-like domains are required for the formation of antiparallel myotilin dimers. Myotilin is located at the Z-disc where it binds to a-actinin, the main component of the Z-disc, and to filamin C at the periphery. Myotilin also cross-links actin filaments and plays a role in the alignment of myofibrils (Salmikangas et al., 2003). The involvement of myotilin was detected in the year 2000 as a missense mutation (T57I) and was identified as limb-girdle muscular dystrophy 1A (LGMD1A) (Hauser et al., 2000). Since then, six new myotilin mutations were identified in eight unrelated patients of the Mayo Clinic MFM cohort. The LGDM1A pathology is therefore a MFM (Selcen & Engel, 2004). Cardiac involvement was found in a subset of patients. While myotilin deletion in mice does not lead to obvious abnormalities, transgenic mice expressing the T57I mutant reproduce morphological and functional features of human myotilinopathies (Garvey et al., 2006). As for desmin, abnormal accumulation of many proteins occur in myotilinopathies.
ZASP
ZASP (Z band Alternatively Spliced PDZ motif-containing protein), also called Oracle or Cypher, is expressed predominantly in cardiac and skeletal muscles (Faulkner et al., 1999). The ZASP gene, called LDB3 (NM_001080114), situated on chromosome 10 (10q22.3-q23.2), encompasses 16 exons, and splice variants exist in cardiac and skeletal muscles, each expressing 3 distincts variants. All ZASP isoforms have a N- terminal PDZ (PSD-95/SAP90, ZO-1 proteins) domain important for interaction with other proteins, and a ZASP-like motif (ZM) needed for the interaction with a-actinin. The largest isoforms have three C-terminal LIM (LIN-11, Isl1m, MEC-3 proteins) domains that interact with Protein kinases C (PKCs) (Zhou et al., 1999). ZASP proteins were shown to localize at the Z disc (Klaavuniemi & Ylanne, 2006). The first case of ZASPopathy causing MFM was described in 2005 in 11 MFM patients carrying heterozygous missense mutations (Selcen & Engel, 2005). There was a cardiac involvement in 3 of these 11 patients. Mutations in ZASP was also shown to be responsible for dilated cardiac myopathy, and left-ventricle non compaction (Vatta et al., 2003). Knockout mice for ZASP develop skeletal and cardiac myopathy with fragmented Z-discs (Zheng et al., 2009).
Filamin C
Filamin C (y-filamin or Filamin 2) belongs to a family of high molecular weight cytoskeletal proteins, expressed in skeletal and cardiac muscle, in contrast to an ubiquitous expression of filamin A and B. Filamin C (NM_001127487) is a 48-exon gene (280 aminoacids) on chromosome 7 (7q32) which belongs to the filamin family of actin-binding proteins that are involved in the reshaping of the actin cytoskeleton and it is associated to myotilin. The amino terminal domain contains an actin-binding domain, followed by a semiflexible rod comprising 24 Ig-like folds, serving as interface for interaction with numerous filamin-binding proteins (van der Flier & Sonnenberg, 2001). Homodimers of Filamin C are involved in the organization of actin filaments and serve as a scaffold for signaling proteins. They link the Z-disc to the sarcolemma by interacting with Z-disc proteins and sarcoglycans in costameres (Thompson et al., 2000). The Ig-like domain 20 also binds to myotilin, and may represent a Z disc targeting motif (van der Ven et al., 2000). The nonsense mutation W2710X was identified in 2005 in patients presenting MFM signs, associated with cardiomyopathy, respiratory insufficiency and peripheral neuropathy (Vorgerd et al., 2005). Life expectancy is shortened in patients who have mutations in the filamin C gene because of cardiomyopathy and the involvement of the respiratory muscles. Cataract and peripheral neuropathy can also occur thus demonstrating that there is a multisystem involvement. Filamin C is expressed before formation of myotubules and is required for a proper muscle development (van der Ven et al., 2000).
BAG3
BAG3 (Bcl-2 associated athanogen 3), which gene (NM_004281) is situated on chromosome 10 (10q25.2-q26.2), encodes a 535 aminoacid protein. It is a complex cochaperone which principally mediates interaction with Hsp70, Hsc70 and Bcl-2, an antiapoptotic protein, through its C-terminal BAG domain. The proline-rich domain interacts with the WW-domain (~35-40 amino acid residues including two highly conserved tryptophan (W) residues separated by 20-23 amino acids) that interacts with proteins implicated in signal transduction (Takayama & Reed, 2001). BAG3 forms a stable complex with HspB8 (Hsp22) and therefore participates to the degradation, via autophagy, of misfolded and aggregated proteins (Carra et al., 2008a). The first case of BAG3 mutation causing MFM was described in 2009 (P209L) in exon 3 (Selcen et al., 2009). All patients presented a childhood onset with severe progressive muscle weakness and atrophy, associated with large left atrium, pulmonary and mitral regurgitation with a restrictive cardiomyopathy pattern. There is also bilateral diaphragm paralysis, reduced forced vital capacity and respiratory insufficiency. Patients have a rigid spine and scapular winging. The progression of illness was found rapid when compared to other MFM mutations, and was linked to a significant level of apoptosis (8 % of nuclei). The function of BAG3 is to stabilize myofibril structure through F-actin. When it is mutated there is myofibril disruption and destabilization of the Z-disk structure under mechanical stress. Knockout mice for BAG3 results in a rapidly-developing myopathy with early lethality and apoptotic features, suggesting a role for BAG3 in supporting cytoskeletal connections between the Z-disc and myofibrils under mechanical stress (Homma et al., 2006).
Conclusion
MFMS are muscular dystrophies with specific, but not always identical morphologic features. All six genes causing MFMs with cardiac involvement identified so far encode proteins (Figure 1) that are related to the Z-disc. It is therefore important to study the Z-disc, which appears increasingly more complex. In fact, it is subjected to an exquisitely fine-tuned process of proteins quality control and protein turnover, and is involved in a mechanism of mechanosensing and signaling. These two important functions will be detailed in the following part.
Integrative biology of myofibrillar myopathies-involved genes
To understand how MFMs develop, it is necessary to describe how muscles are depending on the optimal functioning of the products of the genes described above. For that purpose, this part will develop how the different partners of the muscular structure interact with each others, in a static as well as in a dynamic point of view.
Fig. 1. Schematic representation and localization of mutations in the six proteins involved in myofibrilar myopathies.
N, C: respectively aminoterminal and carboxyterminal extremities. Numbers indicate the aminoacid position in the molecule. 1A, 1B, 2A, 2B are the helical domains of desmin. L1, L12, L2 are the non-helical linkers. WW: tryptophan-conserved domain interacting with proline-rich regions. PRR: Proline-rich region, interacting with WW domains. BD: BAG-domain. Ig: immunoglobulin-like domain. PDZ: PSD-95 / SAP90 / ZO-1 proteins common domain. ZM: ZASP-like motif. LIM domains: LIN-11 / Isl1m / MEC-3 proteins common domain. ABD: Actin-binding domain. Ig-like domains: immunoglobulin-like domain.