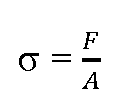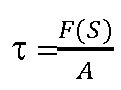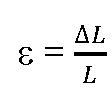Introduction
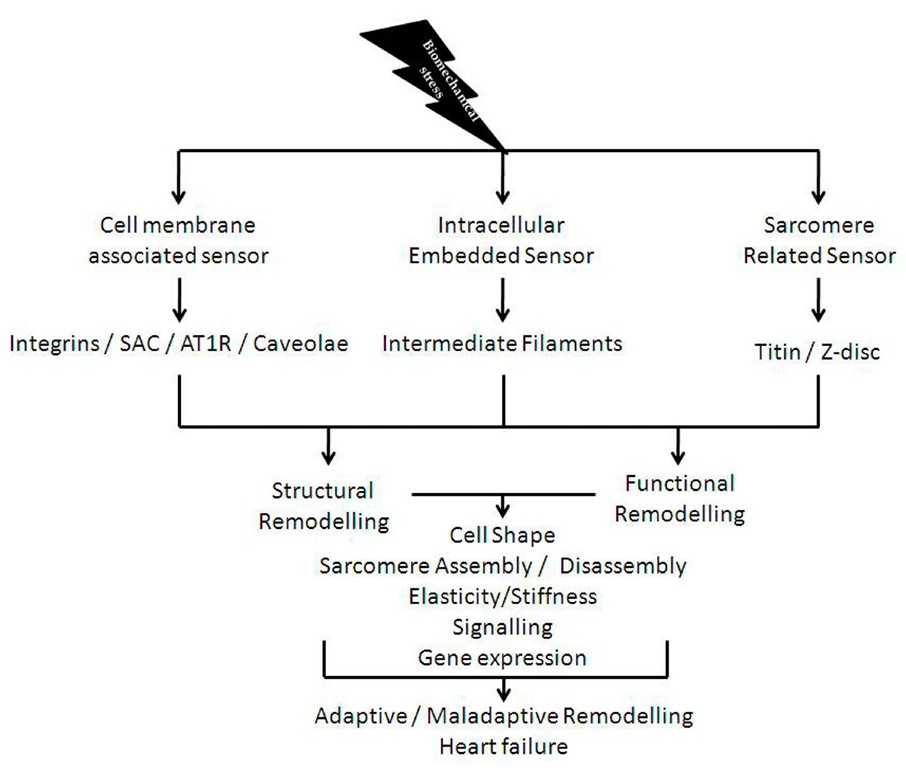
Mechanosensation is a fundamental process in biology and may have been developed by the early cells in response to hypo-osmotic stress [1]. With the evolution of different cell types and the appearance of multi-cellular organisms the mechanisms of mechanosensation and the corresponding transmission of signals became more complex and evolved in different cell types differently [2]. Particularly in cardiac myocytes different mechanosensory protein – complexes can be found: i) cell membrane associated ii) intracellular embedded iii) sarcomere related (figure 1). All these various signalosomes are sensitive to different types of mechanical signals. For example, a deformation of the cell membrane may be detected by cell membrane associated signalosomes, such as stretch activated channels (SAC), angiotensin receptors, the caveolae, and integrin mediated signalling. Depending on severity and duration, these events may also be sensed by intermediate filaments (IF) and or even by the sarcomere associated signalosomes. However it is important to differentiate between different types of stresses, such as the normal "stress" (ct) which is physically defined by:
(where F is the applied force per unit area (A), dimension: N/ m2)
And "shear stress![]() where the applied force (F(S) = shear force) acts parallel to the area (A) (dimension: N/m2):
where the applied force (F(S) = shear force) acts parallel to the area (A) (dimension: N/m2):
Other types of physical stresses such as compression and torsion may also occur and are equally important. Distinct from stress is "strain"![]() which is physically defined by:
which is physically defined by:
(where L is the initial length and .![]() is the change in length, dimensionless) Importantly different types of stress do cause strain or any type of deformation, or in other words, strain is the consequence of stress. Cells are able to detect strain via changes in conformation of proteins or macromolecular protein complexes, but the precise molecular mechanisms remains often unclear. In this regard two different models have been developed to explain mechanosensory behaviour: i) the localized and ii) the decentralized model. The localized model proposes that changes at the cell membrane are sensed immediately and are transmitted from there to other parts of the cell. In contrast the decentralized model proposes that any force applied at the cell surface will cause deformations of elastic cytoskeletal components and as such can be sensed far away from the area of impact. The latter model is also called the "tensegrity" model (derived from: tensional integrity) based on Buckminster Fuller’s geodesic dome.
is the change in length, dimensionless) Importantly different types of stress do cause strain or any type of deformation, or in other words, strain is the consequence of stress. Cells are able to detect strain via changes in conformation of proteins or macromolecular protein complexes, but the precise molecular mechanisms remains often unclear. In this regard two different models have been developed to explain mechanosensory behaviour: i) the localized and ii) the decentralized model. The localized model proposes that changes at the cell membrane are sensed immediately and are transmitted from there to other parts of the cell. In contrast the decentralized model proposes that any force applied at the cell surface will cause deformations of elastic cytoskeletal components and as such can be sensed far away from the area of impact. The latter model is also called the "tensegrity" model (derived from: tensional integrity) based on Buckminster Fuller’s geodesic dome.
Legend to figure 1: The figure summarizes the most important stress and strain sensors present in cardiac myocytes. All sensors affect cell shape, sarcomere assembly and disassembly, elasticity and stiffness as well as gene expression which will finally decide whether adaptive or maladaptive remodelling will take place (abbreviations: SAC: stretch activated channels, AT1R: angiotensin II type 1 receptor).
Fig. 1. Summary of cardiac myocyte stress and strain sensors
Here we shall introduce the reader into different concepts of cardiac mechanosensation:
1. Receptor / cell membrane mediated mechanosensation (centralized models):
i. Integrin mediated effects
ii. Stretch activated channels
iii. Angiotensin receptor mediated mechanosensation and other receptors
iv. Caveolae
2. Intracellular stretch sensors: i. Intermediate filaments
3. Intrasarcomeric mechanosensors
i. Z-disc associated mechanosensor complex
ii. N2A and N2B – titin mechanosensor complex
iii. Titin kinase mechanosensor complex
These different mechanosensory signalosomes integrate a variety of mechanical stimuli such as mechanical stress, shear stress, torsion and compression as well as the resulting strains into electrochemical and biochemical signals. They are translated into short term effects (i.e. changes in ion concentrations may lead to changes in action potential durations or changes in calcium concentration which may lead to changes in kinase and phosphatase activities) and long term effects via changes in gene expression.
Integrin mediated effects
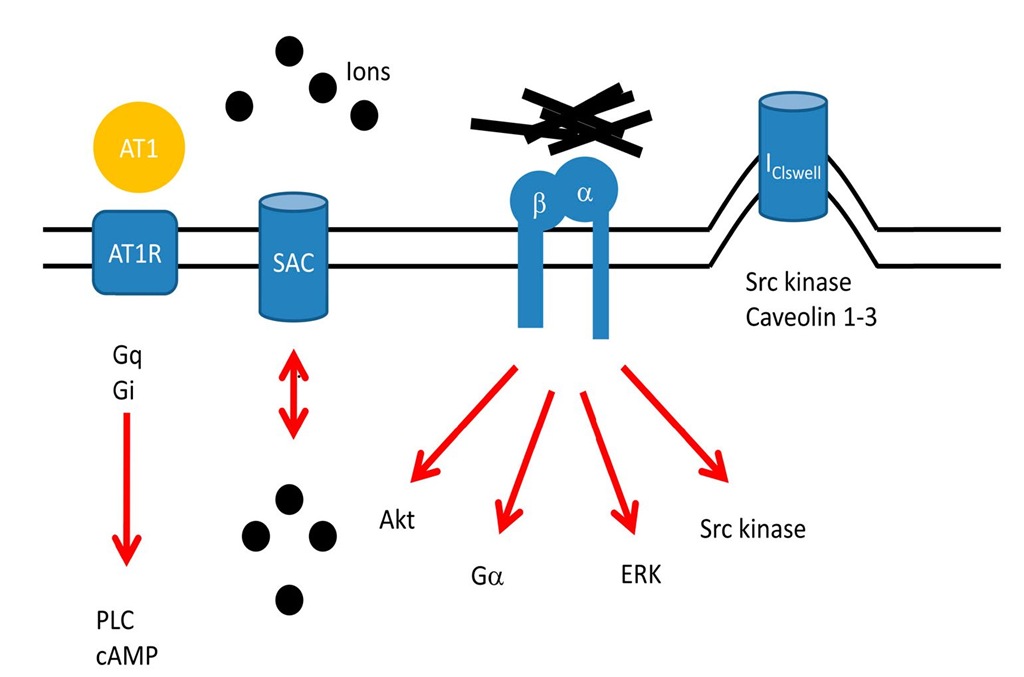
Integrins are large heterodimeric transmembrane proteins, consisting of a and p subunits. They act as receptors and are enriched at focal adhesions or costameres, sites where the Z-discs become attached to the cell membrane. The extracellular part of the molecule interacts with fibronectin, laminin or collagen, whereas the intracellular domains interact with signalling proteins such as integrin linked kinase (ILK), focal adhesion kinase (FAK), or cytoskeletal components such as actin, talin and vinculin. As such, integrins link the extracellular matrix (ECM) to the cytoplasm and are able to respond to changes in the composition of the ECM as well as with regard to forces transmitted via the ECM and vice versa (inside out and outside in signalling). They are linked via Ga proteins to cAMP and protein kinase A (PKA) mediated effects, they activate via FAK and SH2 phospholipase C (PLC) as well as phosphatidyl inositol 3 kinase (PI3K) and Akt mediated survival pathways (figure 1, 2). Integrins activate as well Src kinase which phosphorylates particularly p130 CAS, which is an important mechanosensory element [3]. Indeed tyrosine kinase activation, such as Src activation, has been observed as early as one minute after stretch and as such is one of the earliest observable effects following mechanical stimuli [4]. It has been postulated, although not yet shown, that orphan tyrosine kinases such as Src might become activated via conformational changes upon membrane stretch. If verified, this could be another mechanism whereby stress is directly translated into enzyme activity.Integrins are also linked via Ras mediated signalling to mitogen activated protein kinases (MAPK) such as ERK and as such to serum response factor (SRF) mediated transcriptional events.
In this regard, it is no surprise that loss of integrins in genetically altered animals is associated with severe heart failure [5] and that human mutations in laminin alpha 4 (LAMA4) and ILK are associated with dilated cardiomyopathy (DCM) [6]. Although integrins are meanwhile well established mechanosensors, other transmembrane protein systems such as the dystrophin associated glycoprotein complex (DAG) are certainly as well important, but they are less well studied with regard to mechanosensation and mechanotransduction.
Legend to figure 2: The figure depicts four major membrane associated mechanosensory pathways, namely the angiotensin receptor (AT1R) mediated pathway, stretch activated channels (SAC), integrins and caveolae (abbreviations: Gq, Gi, Ga – Gq, Gi, and Ga mediated effects, PLC – phospholipase C, ERK – extracellular regulated kinase, Akt – Akt kinase, black dots indicate ions such as K+, Na+, Ca++, Cl-, etc.).
Fig. 2. Membrane associated mechanosensory pathways
Stretch activated channels (SAC)
Stretch activated or stretch gated ion channels respond to strain by opening or closing their pores. They were first found in skeletal muscle [7] and since then have been identified in every living cell of every kingdom, including Archaea, Bacteria, Plant, Fungi and Eukaryote (figure 1, 2). These channels open, or even close, upon membrane stretch and allow ions such as chloride, calcium, potassium, and sodium which are permeable to this channel, to follow the electrochemical gradient and to change the membrane potential. However mechanosensitivity is not restricted to a small subset of ion channels, in fact many proteins and voltage gated channels are mechanosensitive. The difficulty is to identify whether or not the mechanosensitivity of a single protein or channel is biologically relevant [8]. At least two different mechanisms can be made responsible for the opening mechanism:
i. stretch from the plasma membrane is transferred directly to the channel resulting in a conformational change (lipid bilayer tension or stretch model) and
ii. a spring like tether, connecting ECM, channel and intracellular space, responds to changes by opening the channel (spring like tether model).
Mechanosensitive channels such as the L-type calcium channel are particularly important in cardiac myocytes where they have been made at least partially responsible for post-ischemic arrhythmias. Other effects include their ability to respond to a stretch early in the action potential and to produce a repolarizing tendency whereas if stretched late, the channel causes depolarization, an effect called: "reversal potential" [9-10]. Although it is a general principle in biology to amplify a signal via changes in ion concentrations, which supports a role for SAC in mechanosensation, inhibition of SAC by using Gadolinium is unable to inhibit major stretch induced features such as immediate early gene expression or the increase in protein synthesis [11]. Therefore additional effects must be at play.
Angiotensin receptor mediated mechanosensation and other receptors
While mechanical activation affects directly transmembrane proteins and causes via direct or indirect effects conformational changes which elicit profound intracellular signaltransduction cascades, another mechanism proposes that mechanical stimulation leads to autocrine angiotensin II release which activates the angiotensin II type 1 (AT1) receptor [12]. As such Gq/11 and Gi mediated effects, which lead to phospholipase C activation and increased intracellular calcium concentrations and/or a decrease in cAMP via adenylyl cyclase inhibition, may cause long term effects such as cardiac myocyte hypertrophy. However an even more important mechanism has been discovered only recently when it was demonstrated that the AT1 receptor, even without binding to its ligand, can be activated by mechanical stimulation [13]. In addition, beta receptors have also been implicated in mechanosensation, although evidence for their role here is available, but they are less well studied with regard to mechanosensation (please see for a brief overview [14]). Angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers and beta blockers belong to the most powerful therapeutic tools in cardiovascular medicine. Based on the direct involvement of the AT1 receptor in mechanosensation, their actions might at least be partially attributable to their effects here.
Caveolae
Caveolae are small (50 – 100 nm) invaginations of the plasma membrane found in a variety of different cell types, including cardiac myocytes. They may represent a cellular compartment where ion channels such as the mechanosensitive IClswell, signal transduction components such as Src kinase and caveolins 1-3, among others, can be found enriched (figure 2). IClswell channels are important for intracellular homeostasis, particularly during hypo-osmotic conditions. Only recently it was shown that caveolae provide significant membrane reserve [15] and that caveolae are important for proper activation of IClswell channel and as such can be seen as a mechanosensitive structure [16].
Intracellular stretch sensors
Intermediate filaments
Intermediate filaments (IF) are a group of related proteins that share common structural and sequence features, such as amino and carboxy-terminus globular parts which surround the alpha helical rod domain. They were initially named after their diameter which is with ~ 10 nm in the between of actin filaments and myosins and were subdivided into types I – VI. Most types of IFs are cytoplasmic, except for the lamins which are present in the nucleus or in the nuclear membrane. At least 91 different diseases are associated with mutations in these genes and as such a comprehensive discussion of all of them within the context of this topic is impossible. However desmin is a major IF and present in almost every cell type. In cardiac myocytes desmin connects desmosomes with other organelles such as the Z-disc or the nucleus. Mutations in this gene result in a variety of different cardiac diseases such as desmin related myopathy [17-18], limb girdle muscular dystrophy [19], dilated cardiomyopathy (DCM) [20], arrhythmogenic right ventricular cardiomyopathy (ARVC) [21], cardiomyopathy with advanced AV block and arrhythmia [22], familial restrictive cardiomyopathy [23] and DCM with conduction system defects [24]. IFs interact with a variety of different proteins and because of their elasticity they are able to sense any deformation of cellular structure. As such, IFs have been linked to mechanosensation and might well have a function via "tensegration", i. e. their elasticity may enable them to change their conformation in response to any type of mechanical stimulation. As such, lamin A/C knockout animals develop severe heart failure most probably due to defects in mechanosensation. In this regard, lamin A/C mutations are one of the major causes of DCM and associated arrhythmia and it is interesting to note, that treatment of animals carrying human LMN A/C mutations with carvedilol an agent with alpha and beta receptor blocking properties improves heart failure significantly [25].
Intrasarcomeric mechanosensors
Z-disc associated mechanosensor complex (MLP/Telethonin)
While all other so far discussed mechanosensors are associated with cell structures found in almost every other cell types, the intrasarcomeric mechanosensors are skeletal and cardiac myocyte specific (figure 3). Any pharmacological intervention at this level might offer the possibility of targeting cross striated myocytes specifically.
Moreover, all other mechanosensors are probably able to sense primarily "external stimuli", whereas the sarcomere associated signalosomes are able to sense force, stress and strain produced primarily within the cell. In addition, all sarcomere associated sensors are directly or indirectly associated with titin, the giant molecular ruler which spans half the sarcomere from the Z-disc to the M-band.
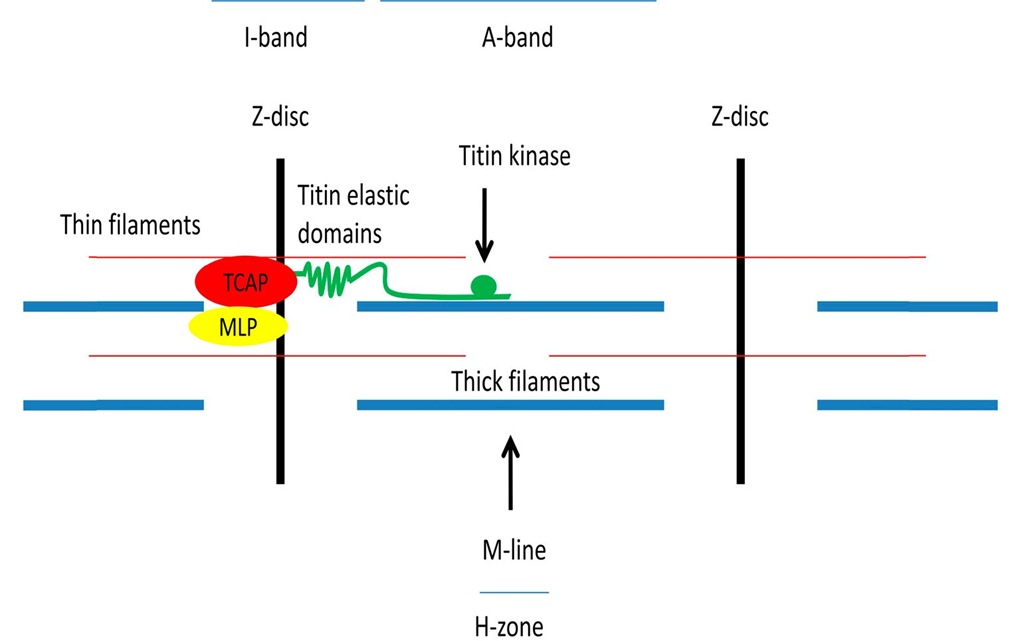
In this regard, the sarcomeric Z-disc which is probably one of the most complex macromolecular structures in biology contains at its periphery a variety of small molecules, namely muscle LIM protein (MLP, CSRP3) and telethonin (TCAP), which interact with the very aminoterminus of titin [26]. Interestingly MLP deficient papillary muscles develop a defect in passive elasticity and isolated cardiac myocytes have a defect in their BNP response following stretch whereas other signal transduction pathways, such as Gq mediated effects are still able to induce this gene. A human mutation in the MLP gene (W4R-MLP), significantly associated with DCM, was also identified and shown to lead to a significant loss of affinity between MLP and telethonin (TCAP). In comparison to the MLP knockout animals, W4R-MLP knock in mice develop a similar phenotype, for example they develop myocardial hypertrophy followed by heart failure, their papillary muscles develop less stiffness when stretched and isolated cardiac myocytes exhibit a similar defect in BNP response [27-28], albeit the effects are smaller and are gene dosage and age dependent. In this regard, MLP was also shown to shuttle into the nucleus and to be necessary for myocardial hypertrophy [29,28].
Legend to figure 3: The figure shows a schematic diagram of a sarcomere and depicts major structural elements. Please note the green titin molecule, spanning from the Z-disc to the M-line. At the aminoterminus TCAP (Telethonin) and MLP (muscle LIM protein) are localized. Titin’s elastic domains are localized within the I-band and the kinase domain is localized close to the M-line.
Fig. 3. Sarcomere associated mechanosensors
Moreover, it was also shown that MLP interacts with and is necessary for the activation of the serine threonine phosphatase calcineurin (PP2A), which is an important link to myocardial hypertrophy via transcription factors such as nuclear factor of activated T-cells (NFAT) [30].However, in addition to MLP mutations, telethonin mutations have also been shown to be associated with types of muscular dystrophy as well as with hypertrophic cardiomyopathy (HCM) and DCM [31-32,27,33-34].
In summary, MLP and telethonin are likely to be involved in Z-disc mediated stress sensation, and mutations in these genes are involved in the pathogenesis of various diseases, but the precise molecular mechanism remains to be defined [35].
N2A and N2B – Titin mechanosensor complexes (FHL1/MARP)
With a molecular mass of up to 4.2 MDa, titin is the largest molecule in biology and well known for its multiple functions such as serving as a molecular ruler, its importance during embryonic development, and for its role in providing mechanical stability – just to name a few. However the molecule contains at its I-band region several elastic domains, such as the distal and proximal Immunoglobulin (Ig) domains, the N2B and N2BA domains, the N2A domain, which is embedded within the N2BA domain, as well as the PEVK domain. All of these domains unfold upon stretch and release and/or store energy during every cycle of contraction and relaxation (i. e. entropic springs [36]). Differential splicing particularly of the N2B and the more compliant N2BA domains add an additional level of complexity, which is of course species specific, depends on the developmental stage, the environment as well as on different states of disease, where DCM and hypothyroidism lead to increased stiffness [37]. Moreover, PKA and PKG mediated phosphorylation causes the elastic domains to "soften" whereas PKC mediated effects causes them to "stiffen" (figure 3). The N2B domain binds specifically to four and a half LIM protein 1 (FHL1), which in turn is the core of a signalosome consisting of RAF, MEK1/2, and ERK2, thus connecting growth factor mediated Gq signalling to titin extensibility and finally to changes in gene expression. Interestingly, loss of FHL1 blunts pathologic hypertrophy and as such inhibition of this pathway might be beneficial [38].
Another important pathway is linked to the N2A elastic titin domain, were the muscle ankyrin repeat proteins (MARP) including cardiac ankyrin repeat protein (CARP), ankrd2/Arpp and DARP interact to constitute a signalosome which responds to passive stretch in vitro [39].
Titin kinase mechanosensor complex
While titin’s elastic I-band domains may be able to sense strain, titin’s amino-terminus, which is anchored within the Z-disc and its carboxy-terminus, anchored within the M-line, may well be able or may at least be involved in the sensation of stress. Interestingly titin’s M-line (or better H-band) domain contains a mechanically modulated kinase able to bind and to phosphorylate nbr1 and p62 (SQSTM1) in vitro. MURF1 and 2 (and probably MURF3 which has not been analyzed yet) also bind to this complex and will translocate into the nucleus upon mechanical inactivity, where they downregulate and or induce the nuclear export of SRF and as such aggravate the transcriptional atrophy programme [40]. This is supported by the R279W-Titin kinase mutation which is associated with hereditary myopathy with early respiratory failure (HMERF) and which leads to a dramatic loss of affinity to nbr1 [41]. Additional evidence for this model is supported by in vitro experiments whereby stretching of the kinase domain leads to activation of the kinase, thus effectively linking mechanosensation to kinase activity ("mechanozymatics") [42]. Moreover titin’s kinase domain is linked via nbr1 and p62 to autophagy, a process of regulated protein and or organelle turnover [43].
Summary
Every cell is capable of mechanical stress sensation either via local or decentralized molecular mechanisms and to transform these signals into electrochemical and biochemical mediators. Because of their force generating ability cardiac myocytes developed additional sarcomere titin I-band related strain and Z-disc as well as M-line related stress sensors. It is a general principle in biology to amplify a signal via an increase in local ion concentrations as such SAC play certainly a major role in nerve and muscle cells. Mechanical stimulation also might lead via conformational changes to the direct activation of tyrosine kinases and or the titin kinase – an effect which might be called "mechanozymatics". Direct activation of AT1 receptors via mechanical stimuli has been shown and in this context it might well be possible that other receptors, such as P-receptors, play a role in mechanosensation as well.
Mutations in components of any of the above mentioned systems have been found to cause muscle and or heart failure phenotypes (figure 1, 2) [44-45].
Abbreviations
Cardiovascular disease: CVD Dilated cardiomyopathy: DCM Extracellular matrix: ECM Focal adhesion kinase: FAK Hypertrophic cardiomyopathy: HCM Immunoglobulin: Ig Integrin linked kinase: ILK Muscle Ankyrin Repeat Proteins: MARP Muscle LIM protein: MLP, CSRP3 Nuclear factor of activated T-cells: NFAT Protein kinase A, C, G: PKA/C/G Sarcomere length: SL Serum response factor: SRF Telethonin: TCAP