Introduction
While acute rejection of solid organ allografts has become a rare clinical condition, long-term graft survival is threatened by the development of fibrosis. Such fibrosis is seen in two forms in the heart, one being an accelerated process of atherosclerosis termed transplant vasculopathy, the second a parenchymal response to the ensuing ischemia, low-grade cellular or humoral allorejection, as well as other factors.
The transplant vasculopathy (TV) of cardiac allografts (also known as graft vascular disease, chronic allograft vasculopathy or graft vascular sclerosis) is defined as a diffuse proliferative process that causes obstruction of the coronary vasculature, impairment of vascular flow and secondary myocardial ischemic injury (Mitchell, 2009). Whereas atherosclerosis of non-transplanted hearts mainly affects the proximal part of the coronary arteries, TV affects the arteries down to the level of small intramyocardial branches. Moreover, similar but less extensive changes have been observed in coronary veins (Mitchell, 2009), whereas changes in capillary basement membrane structure, as seen in chronic kidney rejection biopsies, have not been identified as a feature of chronic rejections in hearts.
The TV lesion consists of concentric intimal thickening that contains modified smooth muscle cells that have most likely migrated from the media, macrophages that contain intracellular lipids, collagen and glycosoaminoglycans (Winters & Schoen, 2001). Cholesterol clefts in a band-like distribution and lesions resembling typical atheromatous plaques may arise in the advanced form of the disease. The endothelium and internal elastic lamina are nearly always intact. The vascular media may have normal thickness or may be thinned. Cellular infiltrates, including T-lymphocytes, both CD4+ and CD8+, and macrophages are frequently present as a superficial band in the subendothelium and deeper within the intima and media.
On the other hand, interstitial fibrosis is also a feature of transplanted hearts, partly a consequence of the ischemia associated with TV, but also total ischemic time, rejection episodes and donor cause of death. Despite these different origins of graft fibrosis, the fibrosis lesion (including intimafibrosis in TV) appears to have the occurrence of myofibroblasts or myofibroblast-like cells in common. Myofibroblasts are contractile cells thought to partly derive from fibroblasts and to share characteristics with smooth muscle cells, including expression of a-smooth muscle actin (SMA). They play an important role in physiologic wound healing by synthesizing collagens and exerting strong contraction forces to minimize wound areas (comprehensive reviews of myofibroblast origins, differentiation and functions can be found in (Hinz, 2010; Wipff & Hinz, 2009). Their recruitment is thought to be mediated by cellular damage, the release of inflammatory mediators including TLR agonists (Wynn, 2008).
Matricellular proteins – between cell and matrix
The ECM consists of a complex network of structural proteins (collagens, elastin), multidomain adhesive glycoproteins (fibronectin, vitronectin, and laminin), glycosaminoglycans (GAGs) such as hyaluronan and proteoglycans (versican, syndecans, glypicans, perlecan), as well as the matricellular proteins that we shall focus on in this topic.
Although the main role of ECM is to act as a structural scaffold for tissue and a compression buffer when tissues are subjected to stress, its ability to also provide the contextual information responsible for controlling cellular behaviour has been increasingly recognized in recent years. The biophysical properties of the matrix can regulate cellular mechanosensory pathways – through global substrate rigidity or extracellular tension. Specific domains and motifs embedded in the ECM proteins act as ligands for cellular receptors, such as integrins and discoidin domain tyrosine kinase receptors. In addition, the ECM also sequesters and hence acts as a reservoir for a wide range of growth factors and cytokines (for reviews see Cox & Erler, 2011; Hynes, 2009; Rhodes & Simons, 2007; Schultz & Wysocki, 2009).
Matricellular protein is a term originally proposed by Bornstein (Bornstein, 1995) to describe secreted extracellular matrix proteins that function more as regulators of cell-matrix interactions than as structural proteins. The group of matricellular proteins includes amongst others thrombospondins, tenascins, osteopontin, periostin, osteonectin or CCN proteins. A common property of matricellular proteins is their high expression level during embryogenesis, which is strongly reduced after birth and becomes low to absent in adult life (although there are organ specific differences). Their expression re-appears at high levels in response to tissue injury (Bornstein & Sage, 2002).
Osteopontin
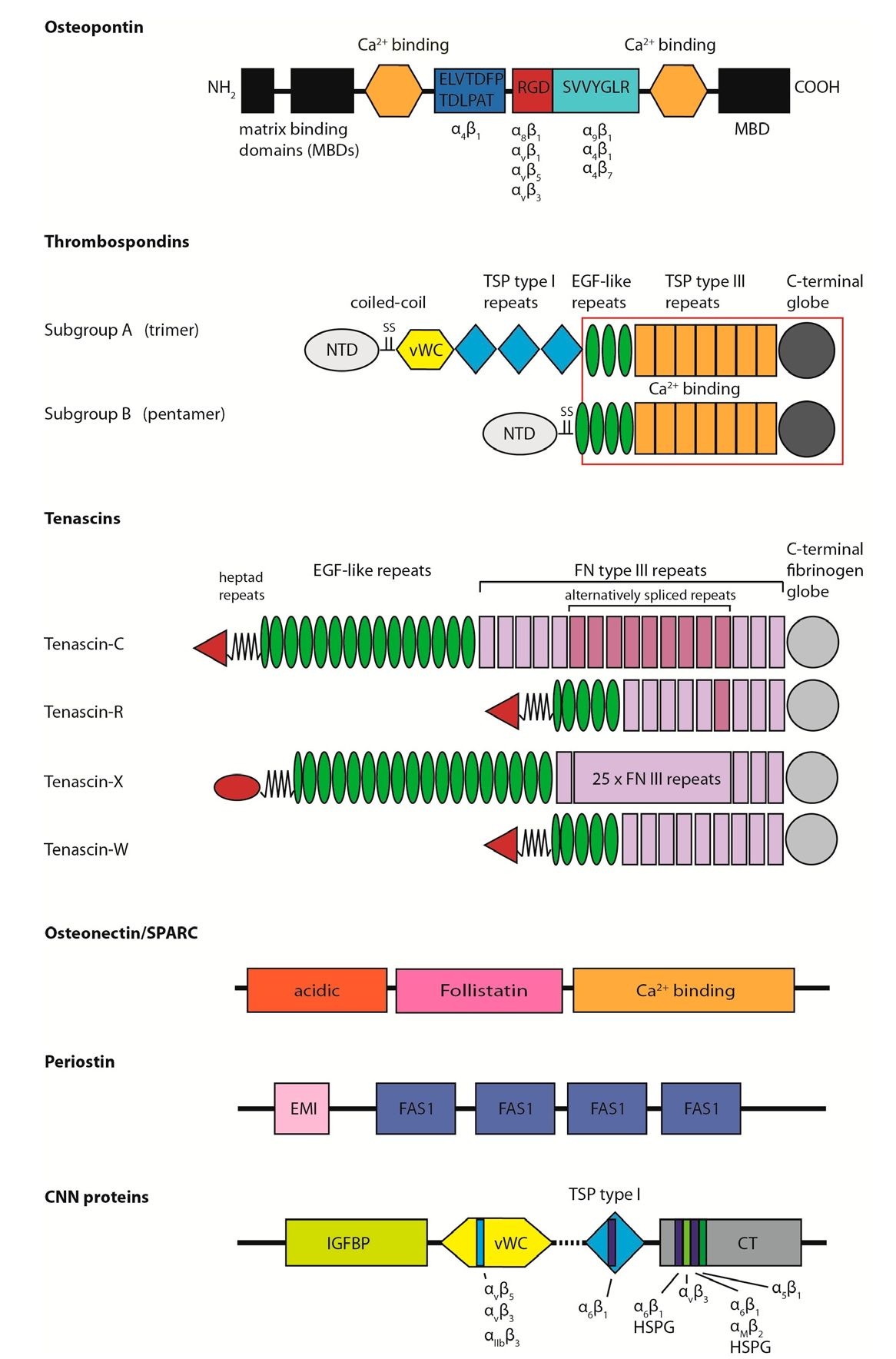
Osteopontin (also known as 44kDa bone phosphoprotein, sialoprotein I, secreted phosphoprotein I, uropontin, and early T lymphocyte activation-1) is a multifunctional protein thought to play a significant role in a variety of biological processes, including bone resorption, immune cell activation, atherosclerosis and ECM remodeling (Scatena et al., 2007). It belongs to the small integrin binding ligand N-linked glycoprotein (SIBLING) family-related proteins. It is composed of 314 amino acid residues and is subject to profound posttranslational modifications such as phosphorylation and glycosylation (Waller et al., 2010). Osteopontin has several molecular domains of established or putative function (see Firgure), among them several integrin binding domains: an arginine-glycine-aspartic acid (RGD) cell binding sequence interacts with integrins avp3, avp1, avp5 and a8p1, a serine- valine-valine-tyrosine- glutamate-leucine-arginine (SVVYGLR)-containing domain that interacts with integrins a9p1, a4p1 and a4p7 and a ELVTDFPTDLPAT domain is also reported to bind to a4p1 (Waller et al., 2010). Several cell types have the capacity to synthesize osteopontin, including bone cells, macrophages, endothelial cells, smooth muscle cells and fibroblasts (Schellings et al., 2004). In healthy adult organs osteopontin expression is low except for the kidney, bone, and in epithelial linings of several tissues (Schellings et al., 2004). By contrast, osteopontin expression is upregulated in pathological conditions, such as atherosclerosis (Giachelli et al., 1993) and ischemic injury (Ellison et al., 1998) including myocardial infarction (MI) (Tamura et al., 2003).
Fig. 1. Structural models of matricellular proteins.
Osteopontin: MBD = matrix binding domain, the integrin binding specificity is indicated below corresponding domains; Thrombospondins: NTD = N-terminal domain, vWC = von Wiilebrand factor type C domain, TSP = thrombospondin, red box indicates conserved domains, Tenascins: in red = N-terminal oligomerisation domain, FN = fibronectin; Osteonectin with the three characteristic domains of SPARC-like proteins; Periostin: FAS1 domain = fasciclin-like domain 1; CCN proteins:; IGFBP = insulin-like growth factor binding protein homology domain, CT = C-terminal domain, HSPG = heparan sulfate proteoglycan, integrin and HSPG binding sites are indicated in colored bars, dashed line indicates the hinge region (adapted from publications Adams & Lawler, 2004; Brekken & Sage, 2000; C. C. Chen & Lau, 2009; Chiquet-Ehrismann & Chiquet, 2003; Conway & Molkentin, 2008; Lund et al., 2009; Scatena et al., 2007).
Thrombospondins
The known vertebrate thrombospondins fall into two subgroups, termed A (thrombospondin-1 and -2) and B (thrombospondin-3, -5, and -5/COMP) according to their oligomerization status. Hence, members of subgroup A form homotrimers and members of subgroup B homopentamers. Each thrombospondin contains multiple domains and a coiled-coil oligomerisation region (Figure). The hallmark of all thrombospondins is the presence in the carboxy-terminal half of each polypeptide of a variable number of EGF-like domains that are contiguous with seven so-called thrombospondin type 3 repeats and a C-terminal region of globular character (Adams & Lawler, 2004).
The most well described members of the family are thrombospondin-1 and -2. Important functions of thrombospondin-1 are activation of the TGF-P (transforming growth factor-p, see below), inhibition of angiogenesis and de-adhesion of cells. The anti-angiogenic effect of thrombospondin-1 is caused mostly by induction of apoptosis and inhibition of endothelial cell migration (Nor et al., 2000). Thrombospondin-1 also induces de-adhesion , defined as transition to an earlier stage of adhesion process (Greenwood & Murphy-Ullrich, 1998), in a variety of cells, by stimulating loss of focal adhesions and actin stress fibers (Greenwood & Murphy-Ullrich, 1998; Murphy-Ullrich & Hook, 1989). Likewise, thrombospondin-2 has been shown also to inhibit angiogenesis and cause de-adhesion by inhibiting focal adhesion in endothelial cells (Murphy-Ullrich et al., 1993, for review see Schellings et al., 2004).
Tenascins
The tenascin family includes: tenascin-C, -R, -X, -Y and -W. They are all built from a common set of structural motifs: heptad repeats, EGF-like repeats, fibronectin type III repeats and a fibrinogen globe domain (FBG, see Figure). At the N-terminus each tenascin has an oligomerisation domain that in the case of tenascin-C and -W leads to the formation of hexamers while tenascin-R has been isolated as a trimeric molecule (Racanelli et al., 1992).
Tenascin-C expression is very low after birth and in the normal adult heart its expression is limited to the chorda tendinae of papillary muscles (Sato & Shimada, 2001). However, the protein reappears under pathological conditions, such as infection, vascular hypertension, myocardial infarction and experimental autoimmune myocarditis (Imanaka-Yoshida et al., 2001; Imanaka-Yoshida et al., 2002). Tenascin-C has adhesive and de-adhesive properties, depending on ECM composition and cell surface receptor binding. De-adhesion facilitates cell migration and tissue remodeling during wound healing. In contrast to tenascin-C, tenascin-X expression remains high after birth (Geffrotin et al., 1995) and has been shown to mediate cell adhesion but not spreading (Elefteriou et al., 1999) Moreover, tenascin-X modulates collagen fibrillogenesis and is therefore described in more detail below.
Osteonectin/SPARC
Osteonectin (also known as SPARC (Secreted Protein Acidic and Rich in Cysteine) or BM-40) is a 32-kDa glycoprotein characterized by three modular domains (Figure): (1) an N-terminal acidic and low-affinity calcium-binding domain; (2) a disulfide-bonded, copper-binding follistatin domain (homologous to the TGF|3-inhibitors activin and inhibin), and (3) the C-terminal extracellular calcium-binding domain (Clark et al., 1997; Schellings et al., 2004).
Tissue expression of osteonectin in healthy adult organs is very low except in epithelia that exhibit high turnover rates (gut, skin, and glandular tissue) (Sage et al., 1989). However, like the preceding matricellular proteins, osteonectin expression re-appears in lesions of injury such as myocardial infarction (Komatsubara et al., 2003). Osteonectin has been reported to bind to thrombospondin-1, vitronectin, entactin, fibrillar collagens and collagen type IV (Schellings et al., 2004). Additionally, it inhibits endothelial cell adhesion and proliferation (Rosenblatt et al., 1997). The de-adhesive effect is mediated through a tyrosine phosphorylation-dependent pathway, whereas its antiproliferative function is dependent, in part, on signal transduction via a G protein-coupled receptor (Motamed & Sage, 1998). Absence of osteonectin in mice during embryogenesis resulted in pups with curly tails and reduced tensile strength of the skin. Smaller collagen fibrils that were more uniform in diameter were seen at the ultrastructural level (Schellings et al., 2004).
Periostin
Periostin (osteoblast specific factor-2, OSF-2) (reviewed in refs. Conway & Molkentin, 2008; Norris et al., 2009) has a molecular weight of ~90 kDa and consists of), four-coiled fasciclin-like repeats, an aminoterminal cysteine-rich region (EMI domain), and heparin binding domains present in the carboxyl tail (Figure). In the heart, periostin is expressed at the very early stages of embryogenesis; however, it is not detected in the normal adult myocardium, except in the valves (Kruzynska-Frejtag et al., 2001; Norris et al., 2007).
Periostin can directly interact with other ECM proteins such as fibronectin, tenascin-C, collagen I, collagen V, and heparin and serve as a ligand for integrins av33, av35 and a436. Recently, it was demonstrated that periostin plays a crucial role in activation of fibroblasts and smooth muscle cells (SMC) through FAK-integrin signaling (Li et al., 2010; Shimazaki et al., 2008). It also promotes collagen fibril formation (Maruhashi et al., 2010; Norris et al., 2007) and incorporation of tenascin-C, organizing a meshwork architecture of the ECM (Kii et al., 2010).
CCN protein family
CCN (an acronym formed from names of first three members) proteins family (reviewed in Chen & Lau, 2009; Kular et al., 2011) includes 6 members: CYR61 (cysteine-rich 61; CCN1), CTGF (connective tissue growth factor; CCN2), NOV (nephroblastoma over-expressed; CCN3), CCN4 (WISP1), CCN5 (WISP2), and CCN6 (WISP3). All members share a multimodular structure, with an N-terminal secretory signal sequence followed by four conserved domains with homology to insulin-like growth factor binding proteins (IGFBPs), von Willebrand factor type C repeat (VWC), thrombospondin type I repeat (TSP) and a carboxyterminal domain (CT) containing a cystein knot (with the exception of CCN5, see figure). A multimodular structure of the CCN proteins allows them to bind and interact with a broad range of partners including integrins, heparan sulfate proteoglycans (HSPGs), ECM components such as fibronectin and fibulin 1C, receptors like Notch1, TrkA, low-density lipoprotein receptor-related proteins (LRPs), growth factors including BMPs, TGF-p and VEGF, as well as gap junction protein connexin 43. CCN proteins regulate cellular functions such as adhesion, migration, proliferation, differentiation, survival, apoptosis or extracellular matrix remodelling in a cell-type specific manner and they play crucial roles in vascular and skeletal development, angiogenesis, wound healing, fibrosis, vascular disease or cancer.