Recipient preparation and transplantation
For the recipient operation narcosis, fixation on the corkboard, and median laparotomy from the symphysis to the lower margin of the left hepatic lobe are performed as described above during the donor operation. After opening the abdominal cavity and fixation of the peritoneum with two needles (alternatively a self-retaining retractor can be used) the bowel is enveloped into a moist compress and put away to the right side outside the mouse.
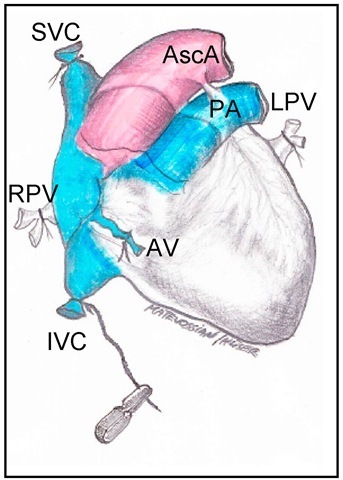
Fig. 2. Depiction of vascular ligatures at the donor’s heart for organ harvesting
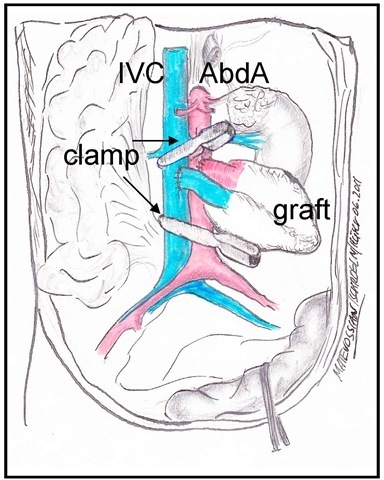
Repetitive soaking of the compress with sterile saline guarantees a humid environment for the bowel during the entire procedure. The retroperitoneal space can be opened easily by gentle rotary motions with two cotton swabs. An additional cotton swab fixed in a needle holder can be used for permanent dislocation of the sigma and the left kidney outside the operating area after dividing the meso-sigmoid. Now the large vessels – Aorta abdominalis and IVC – are accessible and have to be separated from surrounding fatty tissue and adjacent lymph nodes. The preparation of the vessels starts directly below the outlet of the renal arteries and has to be performed to the aortic bifurcation. In case of lumbal veins in this segment, they have to be exposed and coagulated to assure that both the aorta and the IVC lie unfettered. Two vessel clamps have to be positioned at the ends of the prepared segment, respectively, to interrupt the flow in both the aorta and the IVC. The distal clamp has to be put first, to assure partially filled vessels for aortotomy and venotomy in the next step (see fig.3).
Fig. 3. Recipient preparation
After opening of the retroperitoneal space and positioning of the bowel which is enveloped into a moist compress and put away to the right side outside the mouse the colon sigmoideum and the left kidney are pulled away with a cotton swab fixed in a needle holder to guarantee free access to the Aorta abdominalis (AbdA) and the vena cava inferior (IVC). Two vascular clamps are positioned first just below the renal vessels and second at the proximal side of the bifurcation.
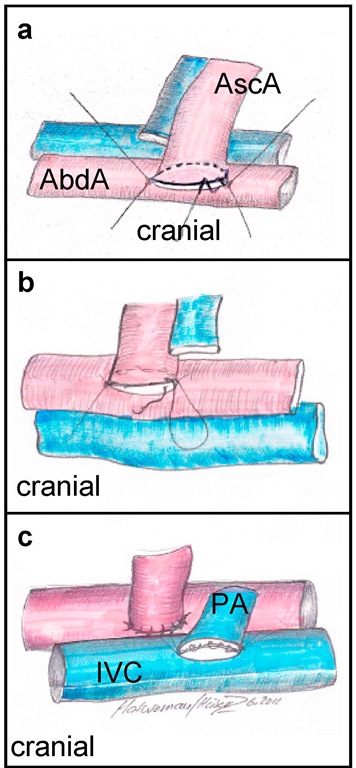
In the following step, the aorta has to be opened by a short longitudinal section using micro-scissors. This can be done easily without prior arteriotomy by a 25 or 30 gauge needle and is extended to a length of equal to the donor’s ascending aorta. After flushing the vascular lumen with saline, an end-to-side anastomosis of the pars ascendens aortae of the cardiac graft and the abdominal aorta has to be performed. The silk thread that has been left long at the IVC during the donor operation can now be inserted in a small clamp and therefore helps to move the heart without any injuring touch into the right position in relation to the recipient’s vessels.
Fig. 4. Implantation of the vascularized graft a) After flushing the vascular lumen with saline, the end-to-end anastomosis of the pars ascendens aortae of the cardiac graft (AscA) and the recipient’s abdominal b) The corkboard is turned and now the arterial "back-wall" becomes the "front-wall" which can be sutured continuously with 4 stitches (direction of stitches: outside-inside / inside-outside). The knot is done with the initially performed edge stitch and cut. c) After venotomy again 2 edge stitches are necessary to adapt the pulmonary artery (PA) to the inferior vena cava (IVC). It is important to fix the "back wall" with slightly more tension compared to the "front wall" to obtain best possible exposition of the operation area. The first continuous suture is now performed inside the lumen (4 stitches, direction of stitches: inside-outside / outside-inside). Therefore the first stitch has to be performed from the outside to the inside and at the end of the suture the thread has to be stitched outside before knotting outside the vein’s lumen. The last suture then can be performed continuously outside the vessel with 5 stitches at the "front wall" (direction of stitches: outside-inside / inside-outside).
Finally, after knotting, the threads have to be cut to a length of approximately 2 mm. The next step is the venotomy. The incision with the micro scissors is performed just as the aortic incision and proportionately to the lumen of the pulmonary trunk of the donor. Again two edge stitches have to be done. Hereby it is important to fix the "back wall" with slightly more tension compared to the "front wall" to obtain best possible exposition of the operation area. This is due to the fact, that the end-to-side anastomosis of the opened IVC and the pulmonary artery of the donor has to be closed with a running suture in the inside of the IVC. For this purpose the suture must be stitched from the outside to the inside of the vessel lumen at the beginning and vice versa at the end of the suture before the knot can be done outside the lumen. For the continuous suture again 4 stitches are necessary (see figure 4c.)
After the knot with the proximal suture, the right wall of the IVC and the pulmonary artery of the donor are closed with another 5 stitches of a continuous suture outside the IVC. After knotting with the thread from the distal edge stitch both threads are cut to a length of approximately 2mm. Finally, the heart can be reperfused by opening the clamps after a cold ischemia period of approximately 25 minutes. The distal clamp is removed first to check the anastomosis of the IVC. Rapid stain towards bright red, refilling of the coronary vessels, and time-displaced beginning of cardiac contractions indicate the successful performance of the operation. After cleaning the heart from residual adjacent tissue particles and repositioning of the bowel into the abdominal cavity, the abdomen is closed by means of a continuous suture of the musculature and the peritoneum, respectively, and completing closure of the skin with single stitches (all using 5/ 0 monofil non-resorbable thread). Then the Isoflurane leading tube is removed from the animal’s head. Within the next minutes the mouse awakes from narcosis. The recipient’s operation takes approximately 35 minutes. The cage is put under a heating lamp for another 20 minutes and the recipient gets tramadol drops for postoperative analgesia.
Potential of heterotopic heart transplantation in mice for elucidating molecular mechanisms of graft rejection
Graduation of rejection after solid organ transplantation is primarily based on histopathological changes. Detection of immunological effector mechanisms display only a minor role in this field and cannot necessarily be correlated to the severity of graft rejection. Hyperacute, acute, and chronic rejections can be discriminated, but the impression must not be created that they are chronologically consecutive phases of rejection. Whereas hyperacute rejection emerges quite rapidly after transplantation, it is entirely possible, that acute and chronic rejection occur simultaneously. Therefore, each rejection episode is designated to a macroscopic and a histopathological image rather than to a concrete point in time (see also fig.5).
The molecular and cellular mechanisms of the innate and the adaptive immune system responsible for acute and chronic graft rejection have been studied in the experimental setting of heterotopic cardiac transplantation in detail. In vertebrates, innate immunity mediates early host defence and comprises inflammatory cells that express receptors detecting conserved pathogen associated molecular patterns (PAMPs). Beside non-cellular mediators capable of microbial recognition (e.g. the complement system) especially natural killer (NK) cells constantly participate in surveillance for intact self by sensing the presence of autologous major histocompatibility complex molecules. In this context we could demonstrate the importance of NK cells during rejection of cardiac transplants. Acute graft rejection includes massive NK and NKT cell infiltration into the transplanted organ. We found that NK and NKT cells, contribute to the activation of alloreactive T cells. Furthermore, inhibition of NK and/or NKT cells in absence of the co-stimulatory receptor CD28 induced long-term acceptance of semi-allogeneic grafts transplanted to CD28-deficient recipients [Maier et al., 2001]. In the same situation, fully allogeneic grafts were rejected in CD28 deficient hosts, although at later time point compared to wild type controls. These findings clearly point out the advantage of the in vivo model of cardiac transplantation allowing to create an either completely or semi-allogeneic constellation between donor and recipient. Importantly, the generation of allogeneic response against grafts involves numerous activation pathways and the impact of single components is often not visible unless this complexity is reduced. Moreover, NK1.1 positive (NK/NKT) cells could not only be assigned to have relevant influence on rejection of cardiac grafts, these data also hint to the important and close interaction between the innate and adaptive immune system.
The advances in immunosuppressive therapy have made severe acute cellular rejection of organ grafts uncommon. This has revealed to the form of acute antibody-mediated rejection – now widely accepted as a distinct clinicopathologic entity [Colvin, 2007] – that is resistant to current immunosuppression and acts through graft rejection by activation of complement, vascular endothelial and smooth muscle cells, and by activation of macrophages, neutrophils or NK cells, respectively [Wehner et al., 2009]. More recent data have highlightened antibody-mediated rejection as a cause for chronic rejection [Colvin, 2007]. A detailed knowledge of the underlying mechanism could provide insights for effective therapeutic interventions, e.g. monoclonal antibodies to C5 [Wang et al., 2007]. However, the use of the murine heart transplant model to determine whether antibodies contribute to the rejection process had only limited success so far [Wehner et al., 2009]. Anatomical differences of the cardiac vascular bed between humans and mice might be a limiting factor in addressing this particular question. In contrast to mice, human coronaries contain vasa vasorum, and coronaries of the murine heart do not pass the surface but rather directly enter the myocardium after their origin. Thus, investigating humoral rejection processes, the model of heterotopic heart transplantation in mice is only applicable to a limited range.
The adaptive immune response as an essential element of the rejection process is mainly characterized by an engagement of T cells. Genetically non-identical transplanted organs display peptides recognizes as foreign to host T cells. Helper T cells of the recipient become activated by foreign peptides that are displayed on antigen presenting cells (APC) in the donor organ. Interaction of activated helper T cells with B cells results in production of antibodies against the graft and activation of other immune cells, e.g. macrophages. In addition, allogen-specific CD8 cytotoxic T cells are known to be important for promoting transplant rejection in humans. This is in line with events during heterotopic allograft rejection in mice where we and others found a predominant activation of CD8 T cells in the transplanted heart of recipients [Huser et al., 2010; Schnickel et al., 2004]. In vitro both, CD4 and CD8 T cells, proliferate in response to allogeneic stimuli. However, we could not detect activated CD4 T cells within cardiac grafts. In contrast, CD8 T cells were recruited in high numbers into the allograft and showed a phenotype of activated effector T cells [Huser et al., 2010]. Again this indicates that the in vivo model of heterotopic heart transplantation in mice has great over in vitro studies and closely resembles the events taking place in humans.
Both, B and T lymphocytes use antigen receptor engagement to initiate distinct signal transduction pathways that affect cellular responses. It is well established that adaptor molecules regulate signaling of receptor proximal events inducing gene expression or cytoskeletal rearrangement. Beer et al. identified the putative adaptor protein SLY1, that is preferentially expressed in T and B lymphocytes and is defined as target for antigen receptor signal transduction with an important role in the adaptive immunity. It was shown that SLY mutant mice reveal impaired lymphoid organ development and antigen receptor mediated lymphocyte activation. Using the model of heterotopic cardiac transplantation, we were able to show extended allograft survival. Thus, signaling events mediated by SLY1 protein in cells of the adaptive immune system appear to be of importance for mechanisms inducing transplant rejection [Beer et al., 2005].
Chemokine receptors and their ligands, expressed by responding leukocytes and the inflamed transplant tissue, are responsible for the recruitment of alloreactive immunocytes into the graft. According to Hancock, several important points have emerged so far from murine in vivo transplant studies. (1) Targeting a single chemokine is in most cases ineffective in prolonging allograft survival, (2) chemokine receptors differ in their importance as targets in alloresponses, and (3) effects of concomitant immunosuppression can modulate the outcome of chemokine receptor targeting in otherwise untreated mice [Hancock, 2002]. The innate and adaptive immune reactions are initiated before, at the time of transplantation (e.g. by ischemia-reperfusion injury), and after transplantation by non-immunological and immunological factors, leading to graft vasculopathy with a diffuse narrowing of the coronary arteries and an adventitial fibrosis as common signs of chronic graft rejection. Chronic rejection is the response of the recipient’s organism towards the cumulative injury to the transplanted graft over time, with involvement of cellular and humoral (antibody mediated) components [Chapman et al., 2005]. Best established model of chronic rejection in the mouse is the heterotopic cardiac allograft [Cornell et al., 2008]. On the basis of this in vivo model we could characterize the role of chemokine receptor CCR4 in chronic transplant failure.
Fig. 5. Macro- and microscopy of transplanted hearts at different time points a) syngeneic hearts 7 days post transplant and tissue section stained with haematoxylin and eosin, showing no signs of rejection, whereas in b) allografts 7 days post transplant display myocyte destruction by invading mononuclear cells, interstitial edema and necrosis (inflammatory lesion is highlighted within the dashed line), and macroscopically massive haemorrhage lesions, c) syngeneic grafts 100 days post transplant with slight adhesion to the surrounding tissue but microscopically normal vascular anatomy and d) chronic rejection in allografts with exhibition of transplant vasculopathy caused by excessive hyperplasia in the intima 100 days post transplant; a-b) HE staining at magnification x40, inflammatory lesion is highlighted in b, c-d) van Gieson staining at magnification x60. donor hearts in CCR4-deficient recipients and only marginal prolongation of heart survival was noticed. In addition, no significant immunohistological difference in cellular infiltration comparing wild type and CCR4-deficient mice could be observed [Huser et al., 2005]. In contrast, injection of Gallium nitrate, known to delay graft rejection, resulted in a significantly prolonged persistence of heart action in CCR4-deficient mice compared to wild type controls [Huser et al., 2005]. These results could be confirmed by findings in CCL17-deficient mice, CCL17 being the specific ligand of CCR4 [Alferink et al., 2003]. Besides constitutively expressed chemokines like CCL19 and CCL21 that mainly recruit naive T cells in the lymph node, inflammatory chemokines like CCL2, CCL3, CCL17 and CCL22 mainly cause accumulation of activated T cells and T memory cells in inflamed organs. Inhibition of several of these chemokines have been shown to be beneficial for graft survival [Tan et al., 2005].
Recruitment of activated lymphocytes into inflamed tissue is of major importance for the control of immune responses. Beside chemokines, the role of the leukocyte integrins in the immunological reactions of organ transplant rejection is essential. The integrin LFA-1 is expressed on all leukocytes as an adhesion molecule and has a significant role not only in lymphocyte migration but also in mediating T cell interaction with APC and consequently T cell priming [Hogg et al., 2003]. Function of LFA-1 is tightly controlled by regulating its activity state [Evans et al., 2009]. Generating a mouse mutant that expresses constitutively active LFA-1 (LFA-1d/d) [Semmrich et al., 2005] we could point out for the first time in vivo the importance of integrin deactivation on immune response inducing allograft rejection [Huser et al, 2010]. We demonstrated that regulating LFA-1 activity from an active to an inactive state promotes successful activation, clonal expansion, and generation of effector T cells in response to allogeneic stimuli in vivo. Defective LFA-1 deactivation furthermore negatively affects recruitment of all major leukocytes from the innate and the adaptive immune system known to be involved in transplant rejection. By using the model of heterotopic cardiac transplantation we were able to provide direct in vivo evidence that regulating LFA-1 deactivation might be as important as regulating LFA-1 activation for effective immune responses during allograft rejection.
Those examples for studies in gene knock-out and transgenic mice using organ allografts identified the central role of a variety of target cells, chemotactic mediators, signalling components, and adhesion molecules on lymphocytes. As such, the described model of herterotopic cardiac transplantation in mice can be considered as a valid animal model and serves as an important achievement for clinical development of strategies that interfere with immune responses inducing transplant rejection.
Conclusion
Advances in the field of immunosuppression and better understanding of immunological courses in the last years contributed to establish organ transplantation as regularly performed clinical therapy and helped patients with severe renal, hepatic, pulmonary, and cardiac diseases to achieve longer survival and better quality of life. Short-term outcome after organ transplantation is impressive, whereas long-term graft survival remains to display a crucial problem. Induction of donor-specific tolerance towards transplanted tissue remains to bet the aim.
Heterotopic heart transplantation in mice is a vascularized immunological model and the recipient is not dependent on maintenance of circulation and cardiac functioning. As an in vivo model it has been shown to be an ideal compromise between clinical reality and experimental reproducibility of underlying essentials that peak in acute rejection or chronic transplant failure over time, however immunological differences between mice and human beings must be kept in mind. And, if so, mice as an in vivo model of first choice will continue to push forward our immunological understanding of immune reactions in transplantation.
![Macro- and microscopy of transplanted hearts at different time points a) syngeneic hearts 7 days post transplant and tissue section stained with haematoxylin and eosin, showing no signs of rejection, whereas in b) allografts 7 days post transplant display myocyte destruction by invading mononuclear cells, interstitial edema and necrosis (inflammatory lesion is highlighted within the dashed line), and macroscopically massive haemorrhage lesions, c) syngeneic grafts 100 days post transplant with slight adhesion to the surrounding tissue but microscopically normal vascular anatomy and d) chronic rejection in allografts with exhibition of transplant vasculopathy caused by excessive hyperplasia in the intima 100 days post transplant; a-b) HE staining at magnification x40, inflammatory lesion is highlighted in b, c-d) van Gieson staining at magnification x60. donor hearts in CCR4-deficient recipients and only marginal prolongation of heart survival was noticed. In addition, no significant immunohistological difference in cellular infiltration comparing wild type and CCR4-deficient mice could be observed [Huser et al., 2005]. In contrast, injection of Gallium nitrate, known to delay graft rejection, resulted in a significantly prolonged persistence of heart action in CCR4-deficient mice compared to wild type controls [Huser et al., 2005]. These results could be confirmed by findings in CCL17-deficient mice, CCL17 being the specific ligand of CCR4 [Alferink et al., 2003]. Besides constitutively expressed chemokines like CCL19 and CCL21 that mainly recruit naive T cells in the lymph node, inflammatory chemokines like CCL2, CCL3, CCL17 and CCL22 mainly cause accumulation of activated T cells and T memory cells in inflamed organs. Inhibition of several of these chemokines have been shown to be beneficial for graft survival [Tan et al., 2005]. Macro- and microscopy of transplanted hearts at different time points a) syngeneic hearts 7 days post transplant and tissue section stained with haematoxylin and eosin, showing no signs of rejection, whereas in b) allografts 7 days post transplant display myocyte destruction by invading mononuclear cells, interstitial edema and necrosis (inflammatory lesion is highlighted within the dashed line), and macroscopically massive haemorrhage lesions, c) syngeneic grafts 100 days post transplant with slight adhesion to the surrounding tissue but microscopically normal vascular anatomy and d) chronic rejection in allografts with exhibition of transplant vasculopathy caused by excessive hyperplasia in the intima 100 days post transplant; a-b) HE staining at magnification x40, inflammatory lesion is highlighted in b, c-d) van Gieson staining at magnification x60. donor hearts in CCR4-deficient recipients and only marginal prolongation of heart survival was noticed. In addition, no significant immunohistological difference in cellular infiltration comparing wild type and CCR4-deficient mice could be observed [Huser et al., 2005]. In contrast, injection of Gallium nitrate, known to delay graft rejection, resulted in a significantly prolonged persistence of heart action in CCR4-deficient mice compared to wild type controls [Huser et al., 2005]. These results could be confirmed by findings in CCL17-deficient mice, CCL17 being the specific ligand of CCR4 [Alferink et al., 2003]. Besides constitutively expressed chemokines like CCL19 and CCL21 that mainly recruit naive T cells in the lymph node, inflammatory chemokines like CCL2, CCL3, CCL17 and CCL22 mainly cause accumulation of activated T cells and T memory cells in inflamed organs. Inhibition of several of these chemokines have been shown to be beneficial for graft survival [Tan et al., 2005].](http://what-when-how.com/wp-content/uploads/2012/04/tmp5324_thumb2_thumb.jpg)
